Misreported Cancer Deaths in PLATO Trial
Abstract
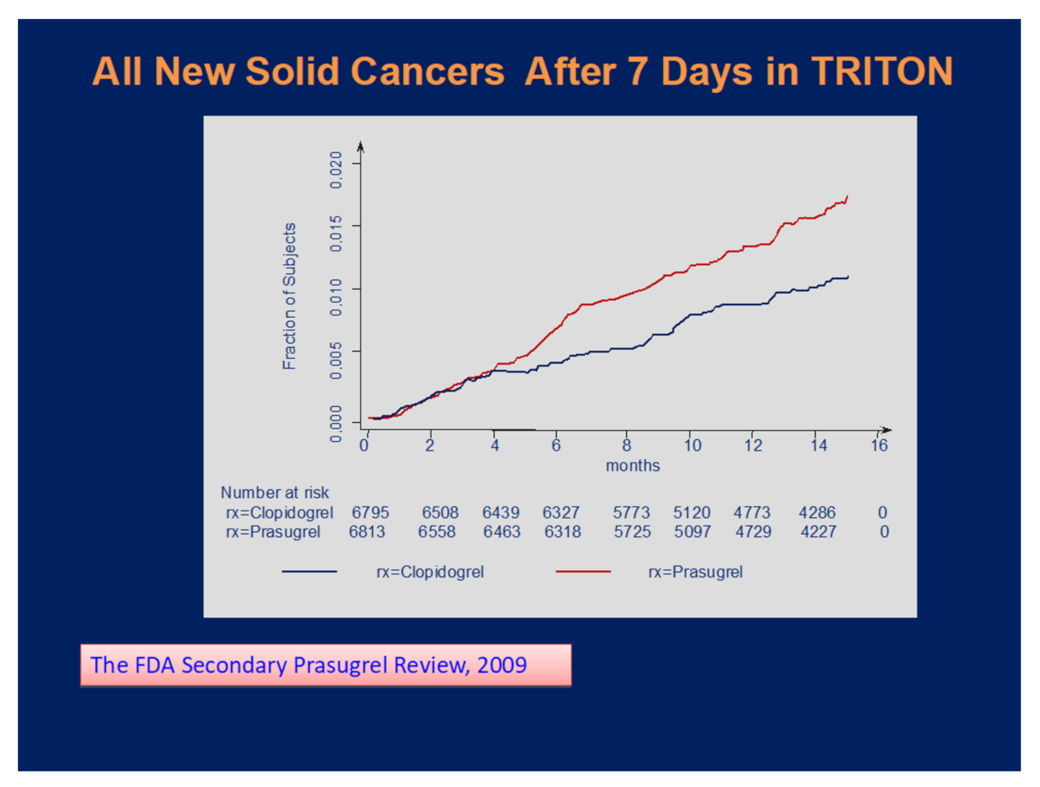
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Disclosures
References
- Coupland, L.; Parish, C.R. Platelets, Selectins, and the Control of Tumor Metastasis. Semin. Oncol. 2014, 41, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Clinical Review on Cancer Risk after Antithrombotic Drugs. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206316Orig1Orig2s000MedR.pdf (accessed on 20 August 2020).
- Serebruany, V.L. Platelet Inhibition with Prasugrel and Increased Cancer Risks: Potential Causes and Implications. Am. J. Med. 2009, 122, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serebruany, V.; Tanguay, J.-F.; Benavides, M.A.; Cabrera-Fuentes, H.; Eisert, W.; Kim, M.H.; Yoon, J.; Lee, C.-W.; Jang, K.; Swan, J.; et al. Verifying Death Reports in the Platelet Inhibition and Patient Outcomes (PLATO) Trial. Am. J. Ther. 2020, 27, e563–e572. [Google Scholar] [CrossRef] [PubMed]
- Serebruany, V.L.; Tanguay, J.; Marciniak, T.A. The FDA and PLATO Investigators death lists: Call for a match. Int. J. Clin. Pr. 2021, e14105. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Braunwald, E.; Cohen, M.; Steg, P.G.; Storey, R.; Held, P.; Jensen, E.C.; Sabatine, M.S. Design and rationale for the Prevention of Cardiovascular Events in Patients With Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial. Am. Hear. J. 2014, 167, 437–444.e5. [Google Scholar] [CrossRef] [PubMed]
- Mauri, L.; Kereiakes, D.J.; Yeh, R.W.; Driscoll-Shempp, P.; Cutlip, D.E.; Steg, P.G.; Normand, S.-L.; Braunwald, E.; Wiviott, S.D.; Cohen, D.J.; et al. Twelve or 30 Months of Dual Antiplatelet Therapy after Drug-Eluting Stents. N. Engl. J. Med. 2014, 371, 2155–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The FDA Ticagrelor Review of Complete Response. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022433Orig1s000MedR.pdf (accessed on 20 August 2020).
- DiNicolantonio, J.J.; Serebruany, V.L. Comparing the safety of ticagrelor versus clopidogrel: Insights from the FDA reports. Ther. Adv. Cardiovasc. Dis. 2013, 7, 5–9. [Google Scholar] [CrossRef] [PubMed]
| ENTR | Age | Gender | STUDYDY | Country | TRTRTXT | NVASSCLS |
|---|---|---|---|---|---|---|
| 236 | 69 | Male | 9 | Denmark | Clopidogrel | 3 |
| 237 | 84 | Male | 7 | Denmark | Clopidogrel | 3 |
| 597 | 68 | Male | 400 | Poland | Clopidogrel | 3 |
| 598 | 52 | Male | 157 | Poland | Clopidogrel | 3 |
| 679 | 85 | Female | 302 | Romania | Clopidogrel | 3 |
| 680 | 60 | Male | 191 | Romania | Clopidogrel | 3 |
| 786 | 69 | Male | 227 | S. Africa | Clopidogrel | 3 |
| 789 ** | 80 | Male | 327 | Spain | Clopidogrel | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serebruany, V.; Tanguay, J.-F. Misreported Cancer Deaths in PLATO Trial. J. Clin. Med. 2021, 10, 3140. https://doi.org/10.3390/jcm10143140
Serebruany V, Tanguay J-F. Misreported Cancer Deaths in PLATO Trial. Journal of Clinical Medicine. 2021; 10(14):3140. https://doi.org/10.3390/jcm10143140
Chicago/Turabian StyleSerebruany, Victor, and Jean-Francois Tanguay. 2021. "Misreported Cancer Deaths in PLATO Trial" Journal of Clinical Medicine 10, no. 14: 3140. https://doi.org/10.3390/jcm10143140
APA StyleSerebruany, V., & Tanguay, J.-F. (2021). Misreported Cancer Deaths in PLATO Trial. Journal of Clinical Medicine, 10(14), 3140. https://doi.org/10.3390/jcm10143140





