Decreased Production of TNF-α and IL-6 Inflammatory Cytokines in Non-Pregnant Idiopathic RPL Women Immunomodulatory Effect of Sildenafil Citrate on the Cellular Response of Idiopathic RPL Women
Abstract
1. Introduction
2. Material and Methods
2.1. Control Group
2.2. Study Group
2.3. Cell Preparation
Sildenafil Concentration in Cultures
2.4. Cell Cultures
Cytokine Determination
2.5. Flow Cytometry Analysis
2.5.1. NKT Cells Immunophenotyping
2.5.2. CD4+CD25+IL-17+, CD4+CD25+FOXP3+, and CD4+CD25+FOXP3+IL-17+ Cell Determination
2.6. Statistical Analysis
3. Results
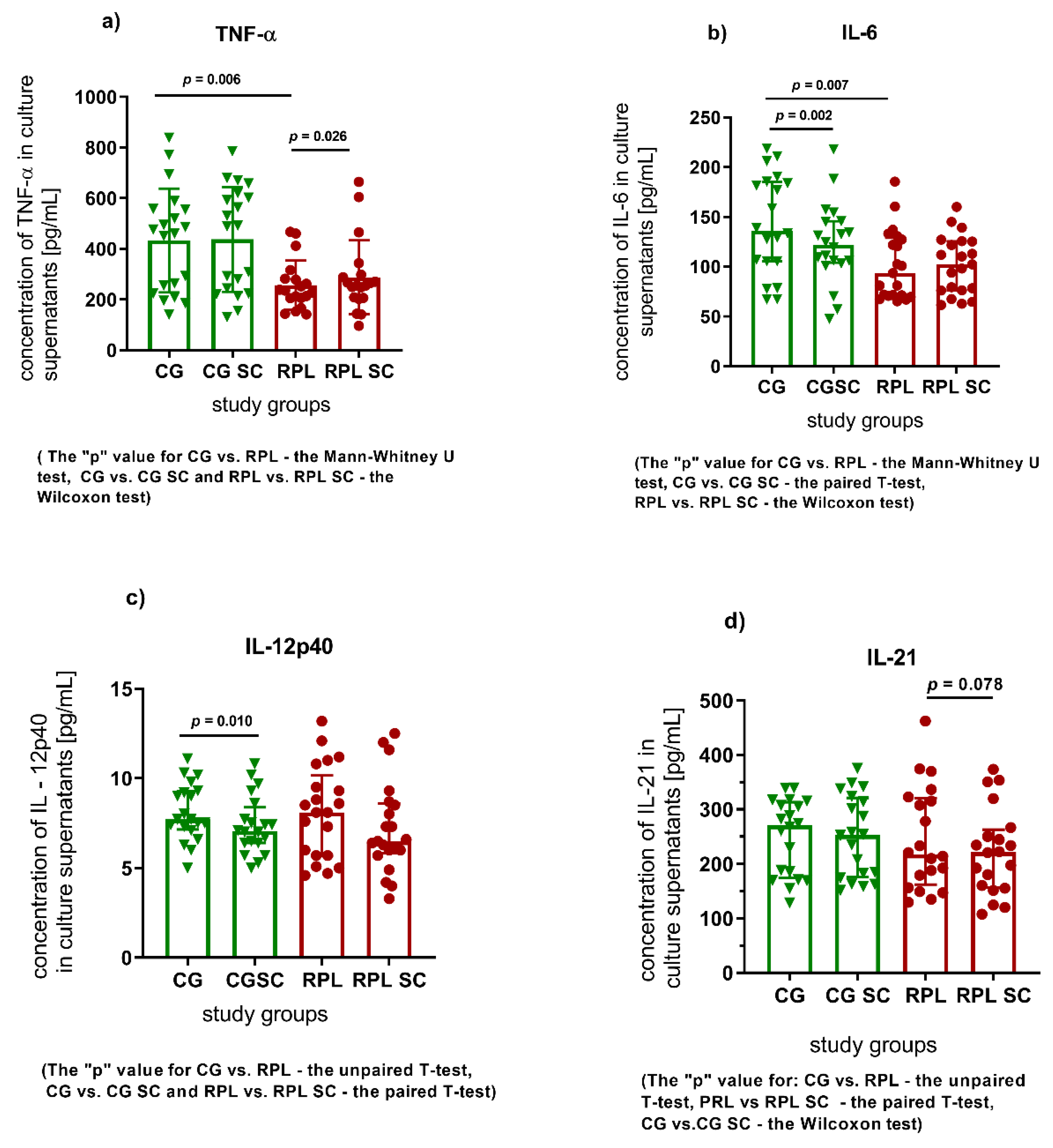
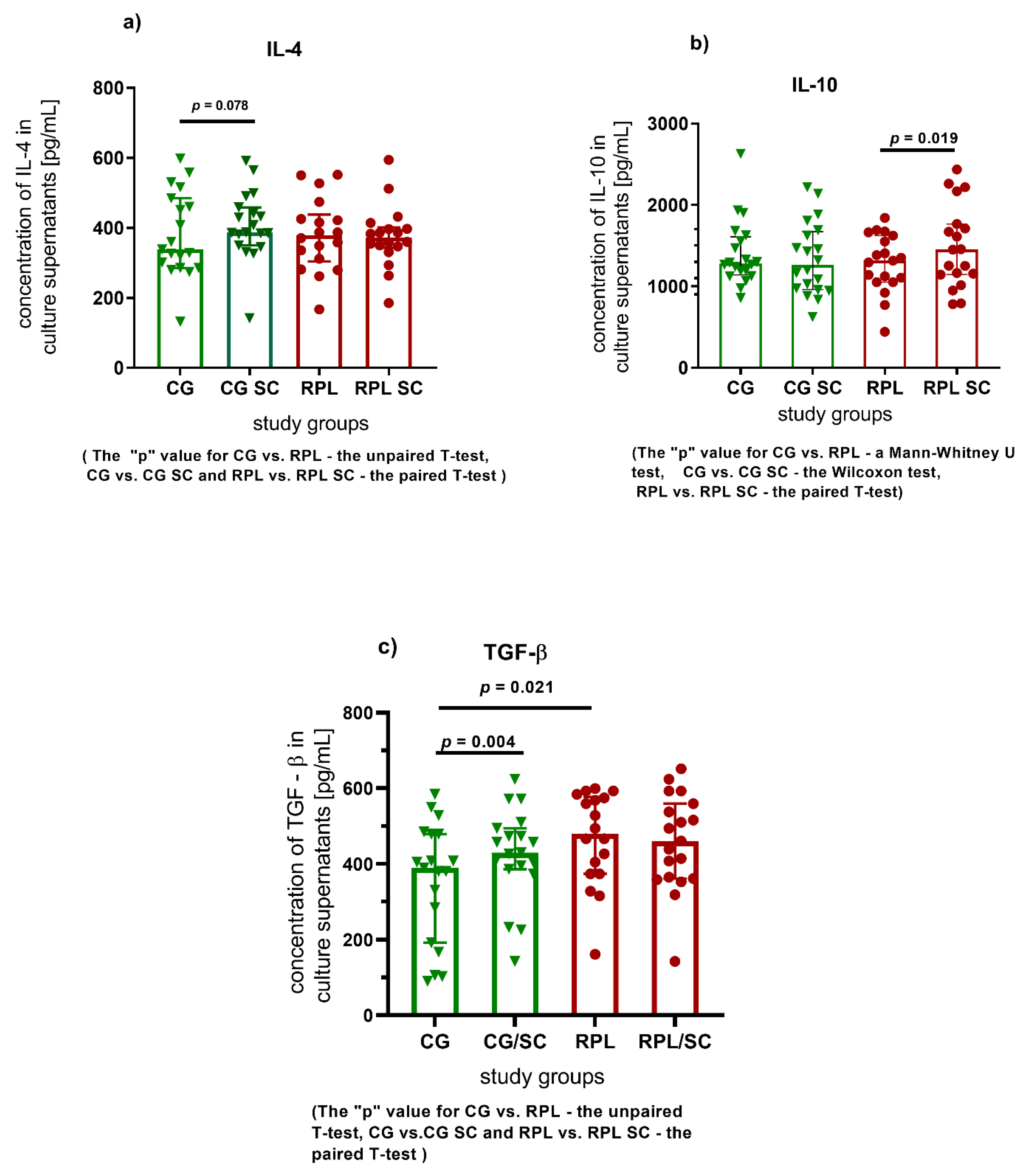
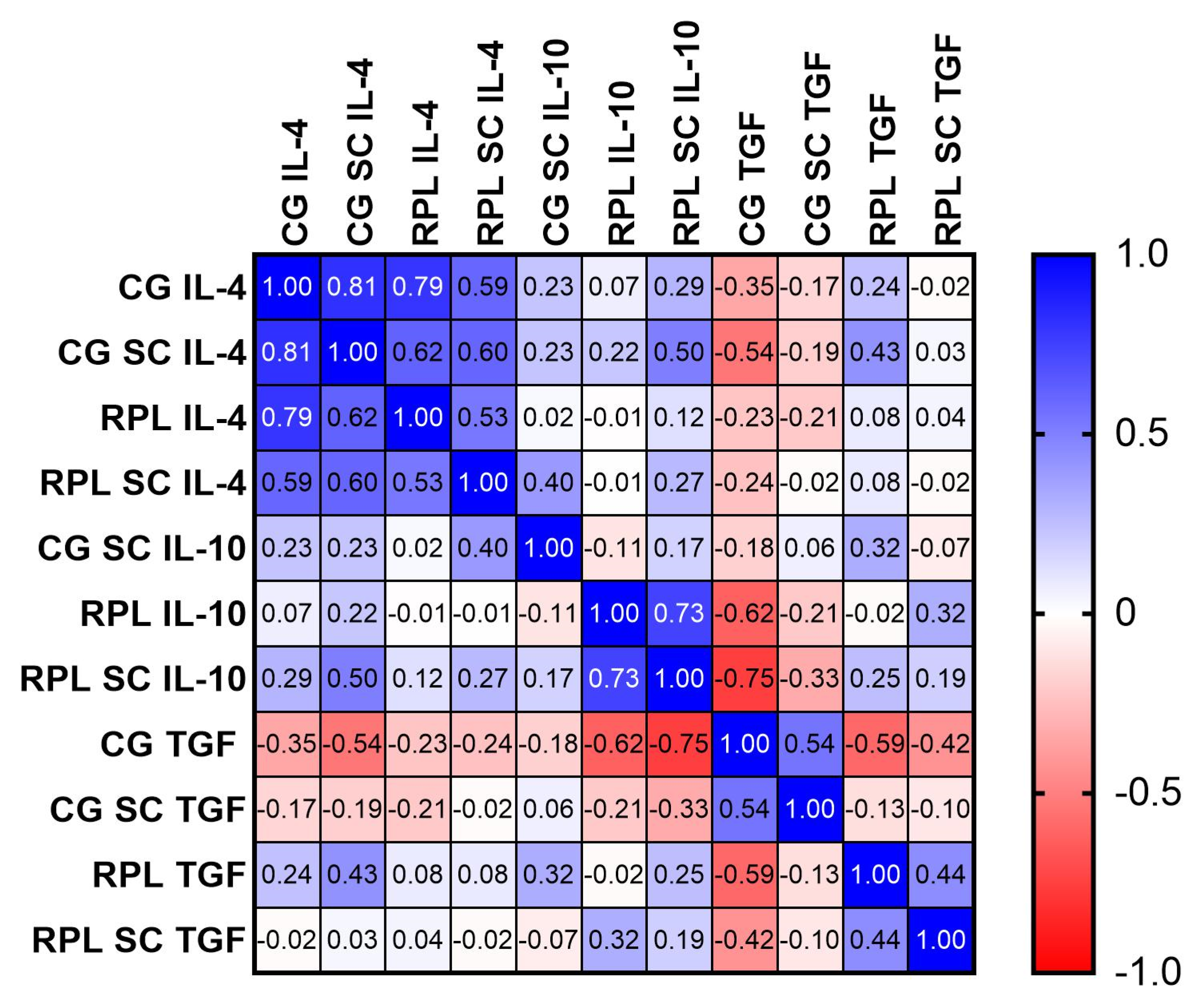
3.1. Secretion of Cytokines
3.2. The Effect of Sildenafil on Th17, Th17/Treg, Treg, and NKT Cells in PBMC Cultures
4. Discussion
The Influence of Sildenafil Citrate on PBMC Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APA | antiphospholipid antibodies |
| APS | antiphospholipid antibody syndrome |
| cGMP | cyclic guanosine monophosphate |
| CTLA–4 | cytotoxic T cell antigen 4 |
| GMP | guanosine monophosphate |
| IL | interleukin |
| IR | insulin resistance |
| MTHFR | methylenetetrahydrofolate reductase |
| NKT | Natural Killer T cell |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| PBS | phosphate-buffered saline |
| PCOS | polycystic ovary syndrome |
| PDE-Is | phosphodiesterase inhibitors |
| PDE | phosphodiesterase |
| RPL | recurrent pregnancy loss |
| SC | sildenafil citrate |
| TGF-β | transforming growth factor β |
| TNF-α | tumor necrosis factor α |
| Treg | T regulatory cell |
References
- Kwak-Kim, J.; Yang, K.M.; Gilman-Sachs, A. Recurrent pregnancy loss: A disease of inflammation and coagulation. J. Obstet. Gynaecol. Res. 2009, 35, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Saravelos, S.H.; Li, T.C. Unexplained recurrent miscarriage: How can we explain it? Hum. Reprod. 2012, 27, 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Fraccaroli, L.; Grasso, E.; Hauk, V.; Cortelezzi, M.; Calo, G.; Leiros, C.P.; Ramhorst, R. Defects in the vasoactive intestinal peptide (VIP)/VPAC system during early stages of the placental-maternal leucocyte interaction impair the maternal tolerogenic response. Clin. Exp. Immunol. 2012, 170, 310–320. [Google Scholar] [CrossRef]
- Saito, S.; Nakashima, A.; Shima, T. Future directions of studies for recurrent miscarriage associated with immune etiologies. J. Reprod. Immunol. 2011, 90, 91–95. [Google Scholar] [CrossRef]
- Fukui, A.; Funamizu, A.; Yokota, M.; Yamada, K.; Nakamua, R.; Fukuhara, R.; Kimura, H.; Mizunuma, H. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J. Reprod. Immunol. 2011, 90, 105–110. [Google Scholar] [CrossRef]
- Wallace, A.E.; Fraser, R.; Gurung, S.; Goulwara, S.S.; Whitley, G.S.; Johnstone, A.P.; Cartwright, J.E. Increased angiogenic factor secretion by decidual natural killer cells from pregnancies with high uterine artery resistance alters trophoblast function. Hum. Reprod. 2014, 29, 652–660. [Google Scholar] [CrossRef]
- Fraser, R.; Whitley, G.S.; Johnstone, A.P.; Host, A.J.; Sebire, N.J.; Thilaganathan, B.; Cartwright, J.E. Impaired decidual natural killer cell regulation of vascular remodelling in early human pregnancies with high uterine artery resistance. J. Pathol. 2012, 228, 322–332. [Google Scholar] [CrossRef]
- Saito, S.; Nakashima, A.; Shima, T.; Ito, M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 601–610. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Hafler, D.A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 2013, 25, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005, 6, 345–352. [Google Scholar] [CrossRef]
- Warning, J.C.; McCracken, S.A.; Morris, J.M. A balancing act: Mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction 2011, 141, 715–724. [Google Scholar] [CrossRef]
- Yamaguchi, K. Tacrolimus treatment for infertility related to maternal-fetal immune interactions. Am. J. Reprod. Immunol. 2019, 81, e13097. [Google Scholar] [CrossRef] [PubMed]
- Jerzak, M.; Kniotek, M.; Mrozek, J.; Gorski, A.; Baranowski, W. Sildenafil citrate decreased natural killer cell activity and enhanced chance of successful pregnancy in women with a history of recurrent miscarriage. Fertil. Steril. 2008, 90, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, N.; Gupta, M.; Kovar, A.; Meibohm, B. The role of pharmacokinetics and pharmacodynamics in phosphodiesterase-5 inhibitor therapy. Int. J. Impot. Res. 2007, 19, 253–264. [Google Scholar] [CrossRef]
- Oyston, C.; Stanley, J.L.; Oliver, M.H.; Bloomfield, F.H.; Baker, P.N. Maternal Administration of Sildenafil Citrate Alters Fetal and Placental Growth and Fetal-Placental Vascular Resistance in the Growth-Restricted Ovine Fetus. Hypertension 2016, 68, 760–767. [Google Scholar] [CrossRef]
- Cavalli, R.C.; Cerdeira, A.S.; Pernicone, E.; Korkes, H.A.; Burke, S.D.; Rajakumar, A.; Thadhani, R.I.; Roberts, D.J.; Bhasin, M.; Karumanchi, S.A.; et al. Induced Human Decidual NK-Like Cells Improve Utero-Placental Perfusion in Mice. PLoS ONE 2016, 11, e0164353. [Google Scholar] [CrossRef]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef]
- Ramos-Medina, R.; García-Segovia, Á.; León, J.A.; Alonso, B.; Tejera-Alhambra, M.; Gil, J.; Caputo, J.D.; Seyfferth, A.; Aguarón, Á.; Vicente, Á.; et al. New decision-tree model for defining the risk of reproductive failure. Am. J. Reprod. Immunol. 2013, 70, 59–68. [Google Scholar] [CrossRef]
- Yamada, H.; Morikawa, M.; Kato, E.H.; Shimada, S.; Kobashi, G.; Minakami, H. Pre-conceptional natural killer cell activity and percentage as predictors of biochemical pregnancy and spontaneous abortion with normal chromosome karyotype. Am. J. Reprod. Immunol. 2003, 50, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Li, J. Human Uterine Decidual NK Cells in Women with a History of Early Pregnancy Enhance Angiogenesis and Trophoblast Invasion. Biomed. Res. Int. 2020, 2020, 6247526. [Google Scholar] [CrossRef] [PubMed]
- Kossmann, S.; Schwenk, M.; Hausding, M.; Karbach, S.H.; Schmidgen, M.I.; Brandt, M.; Knorr, M.; Hu, H.; Kröller-Schön, S.; Schönfelder, T.; et al. Angiotensin II-induced vascular dysfunction depends on interferon-γ-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Nowak, I.; Bylinska, A.; Wilczynska, K.; Wisniewski, A.; Malinowski, A.; Wilczynski, J.R.; Radwan, P.; Radwan, M.; Barcz, E.; Ploski, R.; et al. The methylenetetrahydrofolate reductase c.c.677 C > T and c.c.1298 A > C polymorphisms in reproductive failures: Experience from an RSA and RIF study on a Polish population. PLoS ONE 2017, 12, e0186022. [Google Scholar] [CrossRef]
- The ESHRE Guideline Group on RPL; Atik, R.B.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; et al. ESHRE guideline: Recurrent pregnancy loss. Hum. Reprod. Open 2018, 2018. [Google Scholar] [CrossRef]
- Karakhanova, S.; Yang, Y.; Link, J.; Soltek, S.; von Ahn, K.; Umansky, V.; Werner, J.; Bazhin, A.V. Gender-specific immunological effects of the phosphodiesterase 5 inhibitor sildenafil in healthy mice. Mol. Immunol. 2013, 56, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Pifarré, P.; Gutierrez-Mecinas, M.; Prado, J.; Usero, L.; Roura-Mir, C.; Giralt, M.; Hidalgo, J.; García, A. Phosphodiesterase 5 inhibition at disease onset prevents experimental autoimmune encephalomyelitis progression through immunoregulatory and neuroprotective actions. Exp. Neurol. 2014, 251, 58–71. [Google Scholar] [CrossRef]
- Nichols, D.J.; Muirhead, G.J.; Harness, J.A. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: Absolute bioavailability, food effects and dose proportionality. Br. J. Clin. Pharmacol. 2002, 53 (Suppl. 1), 5S–12S. [Google Scholar] [CrossRef] [PubMed]
- Glossmann, H.; Petrischor, G.; Bartsch, G. Molecular mechanisms of the effects of sildenafil (VIAGRA). Exp. Gerontol. 1999, 34, 305–318. [Google Scholar] [CrossRef]
- Shanmugam, S.; Kim, Y.H.; Park, J.H.; Im, H.T.; Sohn, Y.T.; Kim, K.S.; Kim, Y.I.; Yong, C.S.; Kim, J.O.; Choi, H.G.; et al. Sildenafil vaginal suppositories: Preparation, characterization, in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2014, 40, 803–812. [Google Scholar] [CrossRef]
- Lebedinskaya, O.; Akchmatova, N.; Chikileva, I.; Shubina, I.; Kiselevsky, M. Natural killer T (NKT) cells: Immunophenotype, functional characteristics and significance in clinical practice. In Atlas Effectors of Anti-Tumor Immunity; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lin, S.C. Analysis of the CD161-expressing cell quantities and CD161 expression levels in peripheral blood natural killer and T cells of systemic lupus erythematosus patients. Clin. Exp. Med. 2017, 17, 101–109. [Google Scholar] [CrossRef]
- Jain, M.; Pandey, P.; Tiwary, N.K.; Jain, S. MTHFR C677T polymorphism is associated with hyperlipidemia in women with polycystic ovary syndrome. J. Hum. Reprod. Sci. 2012, 5, 52–56. [Google Scholar] [CrossRef]
- Farina, N.; Jerneren, F.; Turner, C.; Hart, K.; Tabet, N. Homocysteine concentrations in the cognitive progression of Alzheimer’s disease. Exp. Gerontol. 2017, 99, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Pereza, N.; Ostojic, S.; Kapovic, M.; Peterlin, B. Systematic review and meta-analysis of genetic association studies in idiopathic recurrent spontaneous abortion. Fertil. Steril. 2017, 107, 150–159.e2. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Morikawa, M.; Furuta, I.; Kato, E.H.; Shimada, S.; Sata, F.; Kishi, R.; Minakami, H. Circulating cytokines during early pregnancy in women with recurrent spontaneous abortion: Decreased TNF-alpha levels in abortion with normal chromosome karyotype. Hokkaido Igaku Zasshi 2004, 79, 237–241. [Google Scholar] [PubMed]
- Bates, M.D.; Quenby, S.; Takakuwa, K.; Johnson, P.M.; Vince, G.S. Aberrant cytokine production by peripheral blood mononuclear cells in recurrent pregnancy loss? Hum. Reprod. 2002, 17, 2439–2444. [Google Scholar] [CrossRef]
- Whitcomb, B.W.; Schisterman, E.F.; Klebanoff, M.A.; Baumgarten, M.; Luo, X.; Chegini, N. Circulating levels of cytokines during pregnancy: Thrombopoietin is elevated in miscarriage. Fertil. Steril. 2008, 89, 1795–1802. [Google Scholar] [CrossRef]
- Zangeneh, F.Z.; Naghizadeh, M.M.; Masoumi, M. Polycystic ovary syndrome and circulating inflammatory markers. Int. J. Reprod. Biomed. 2017, 15, 375–382. [Google Scholar] [CrossRef]
- AlJameil, N.; Tabassum, H.; AlMayouf, H.; Alshenefy, A.; Almohizea, M.; Ali, M.N. Identification of serum cytokines as markers in women with recurrent pregnancy loss or miscarriage using MILLIPLEX analysis. Biomed. Res. 2018, 29. [Google Scholar] [CrossRef]
- Hwang, E.S. Transcriptional regulation of T helper 17 cell differentiation. Yonsei Med. J. 2010, 51, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Salama, K.M.; Alloush, M.K.; al Hussini, R.M. Are the cytokines TNF alpha and IL 1Beta early predictors of embryo implantation? Cross sectional study. J. Reprod. Immunol. 2020, 137, 102618. [Google Scholar] [CrossRef]
- Ozkan, Z.S.; Deveci, D.; Simsek, M.; Ilhan, F.; Risvanli, A.; Sapmaz, E. What is the impact of SOCS3, IL-35 and IL17 in immune pathogenesis of recurrent pregnancy loss? J. Matern. Fetal Neonatal Med. 2015, 28, 324–328. [Google Scholar] [CrossRef]
- Ogasawara, M.S.; Aoki, K.; Aoyama, T.; Katano, K.; Iinuma, Y.; Ozaki, Y.; Suzumori, K. Elevation of transforming growth factor-β1 is associated with recurrent miscarriage. J. Clin. Immunol. 2000, 20, 453–457. [Google Scholar] [CrossRef]
- Zhu, L.; Aly, M.; Kuon, R.J.; Toth, B.; Wang, H.; Karakizlis, H.; Weimer, R.; Morath, C.; Ibrahim, E.; Ekpoom, N.; et al. Patients with idiopathic recurrent miscarriage have abnormally high TGFß+ blood NK, NKT and T cells in the presence of abnormally low TGFß plasma levels. BMC Immunol. 2019, 20, 10. [Google Scholar] [CrossRef]
- Li, Q. Transforming growth factor β signaling in uterine development and function. J. Anim. Sci. Biotechnol. 2014, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the Immune Response by TGF-β: From Conception to Autoimmunity and Infection. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Arruvito, L.; Sanz, M.; Banham, A.H.; Fainboim, L. Expansion of CD4+CD25+and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: Implications for human reproduction. J. Immunol. 2007, 178, 2572–2578. [Google Scholar] [CrossRef] [PubMed]
- El-Far, M.; El-Motwally, A.E.-G.; Hashem, I.A.; Bakry, N. Biochemical role of intravaginal sildenafil citrate as a novel antiabortive agent in unexplained recurrent spontaneous miscarriage: First clinical study of four case reports from Egypt. Clin. Chem. Lab. Med. 2009, 47, 1433–1438. [Google Scholar] [CrossRef]
- Boyson, J.E.; Aktan, I.; Barkhuff, D.A.; Chant, A. NKT cells at the maternal-fetal interface. Immunol. Investig. 2008, 37, 565–582. [Google Scholar] [CrossRef][Green Version]
- Paauw, N.D.; Terstappen, F.; Ganzevoort, W.; Joles, J.A.; Gremmels, H.; Lely, A.T. Sildenafil During Pregnancy: A Preclinical Meta-Analysis on Fetal Growth and Maternal Blood Pressure. Hypertension 2017, 70, 998–1006. [Google Scholar] [CrossRef]
- Jerzak, M.; Szafarowska, M.; Kniotek, M.; Gorski, A. Successful pregnancy after Intralipid addition to sildenafil and enoxaparin in woman with history of recurrent pregnancy loss (RPL). Neuro Endocrinol. Lett. 2016, 37, 473–477. [Google Scholar]
- Firouzabadi, R.D.; Davar, R.; Hojjat, F.; Mahdavi, M. Effect of sildenafil citrate on endometrial preparation and outcome of frozen-thawed embryo transfer cycles: A randomized clinical trial. Iran. J. Reprod. Med. 2013, 11, 151–158. [Google Scholar]
- Moini, A.; Zafarani, F.; Jahangiri, N.; Sadatmahalleh, S.H.J.; Sadeghi, M.; Chehrazi, M.; Ahmadi, F. The Effect of Vaginal Sildenafil on The Outcome of Assisted Reproductive Technology Cycles in Patients with Repeated Implantation Failures: A Randomized Placebo-Controlled Trial. Int. J. Fertil. Steril. 2020, 13, 289–295. [Google Scholar] [CrossRef] [PubMed]
- di Luigi, L.; Corinaldesi, C.; Colletti, M.; Scolletta, S.; Antinozzi, C.; Vannelli, G.B.; Giannetta, E.; Gianfrilli, D.; Isidori, A.M.; Migliaccio, S.; et al. Phosphodiesterase Type 5 Inhibitor Sildenafil Decreases the Proinflammatory Chemokine CXCL10 in Human Cardiomyocytes and in Subjects with Diabetic Cardiomyopathy. Inflammation 2016, 39, 1238–1252. [Google Scholar] [CrossRef]
- de Nunes, A.K.; Raposo, C.; Bjorklund, U.; da Cruz-Hofling, M.A.; Peixoto, C.A.; Hansson, E. Sildenafil (Viagra((R))) prevents and restores LPS-induced inflammation in astrocytes. Neurosci. Lett. 2016, 630, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.K.; Raposo, C.; Luna, R.L.; Cruz-Hofling, M.A.; Peixoto, C.A. Sildenafil (Viagra(R)) down regulates cytokines and prevents demyelination in a cuprizone-induced MS mouse model. Cytokine 2012, 60, 540–551. [Google Scholar] [CrossRef] [PubMed]
- West, H.M.; Jozwiak, M.; Dodd, J.M. Methods of term labour induction for women with a previous caesarean section. Cochrane Database Syst. Rev. 2017, 6, Cd009792. [Google Scholar] [CrossRef]
- Luna, R.L.; Nunes, A.K.; Oliveira, A.G.; Araujo, S.M.; Lemos, A.J.; Rocha, S.W.; Croy, B.A.; Peixoto, C.A. Sildenafil (Viagra(R)) blocks inflammatory injury in LPS-induced mouse abortion: A potential prophylactic treatment against acute pregnancy loss? Placenta 2015, 36, 1122–1129. [Google Scholar] [CrossRef]
- Ohams, M.; Jerzak, M.; Gorski, A. Effects of sildenafil citrate and etanercept treatment on TNF-alpha levels in peripheral blood of women with recurrent miscarriage. Ginekol. Pol. 2015, 86, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Kaleta, B.; Boguska, A.; Borysowski, J.; Górski, A. Sildenafil upregulates tumor necrosis factor α production in peripheral blood mononuclear cells of healthy men ñ preliminary report. Acta Pol. Pharm. Drug Res. 2019, 76, 123–128. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Azizieh, F.Y.; Raghupathy, R.G. Tumor necrosis factor-alpha and pregnancy complications: A prospective study. Med. Princ. Pract. 2015, 24, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Zinger, M.; Liu, J.H.; Thomas, M.A. Successful use of vaginal sildenafil citrate in two infertility patients with Asherman’s syndrome. J. Women’s Health 2006, 15, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, H.; Ryu, H.; Park, C.; Min, S.; Park, C.; Jee, B. Efficacy of luteal supplementation of vaginal sildenafil and oral estrogen on pregnancy rate following IVF-ET in women with a history of thin endometria: A pilot study. J. Women’s Med. 2010, 3, 155. [Google Scholar] [CrossRef]
- Chanona, J.; García, M.; Ruvalcaba, L.S.; Bermúdez, A.; Muñiz, M.; Beltrán, M.; Cuneo, S. The Mexican experience in the use of vaginal sildenafil in patients with poor endometrial response. Int. Congr. Ser. 2004, 1271, 19–21. [Google Scholar] [CrossRef]

| RPL Patients, n = 22 | Control Group, n = 21 | |
|---|---|---|
| Age (years) | 36.70 ± 4.48 | 37.40 ± 1.90 |
| Number of miscarriages | 3.66 ± 1.57 | 0 |
| PCOS | 11 | 0 |
| MTHFR variant (C677T or A1298C) | 18 | 0 |
| Mean level of homocysteine | 12.48 ± 1.63 µmol/L | - |
| Percentage of Positive Cells (%), Median, IQR1–IQR3 or Mean ±SD | Fertile Women PBMC (CG) | Fertile Women -PBMC (CG) + 400 ng/mL SC | p Value CG vs. CG SC | RPL PBMC | RPL PBMC + 400 ng/mL SC | p Value RPL vs. RPL SC | p Value CG vs. RPL |
|---|---|---|---|---|---|---|---|
| CD4+CD25+ | 2.2 (1.6–2.9) | 2.0 (1.4–2.8) | ns | 2.4 (1.8–3.2) | 2.3 (1.7–3.4) | ns | ns |
| CD4+CD25+FOXP3+ | 23.1 ± 14.8 | 21.7 ± 14.5 | ns | 31 ± 21.3 | 29.3 ± 20 | ns | 0.157 |
| CD4+CD25+IL-17+ | 3.3 (1.25–7.0) | 3.2 (1.3–9.5) | ns | 3.85 (1.95–8.9) | 3.6 (2.3–8.7) | ns | ns |
| CD4+CD25+FOXP3+IL-17+ | 0.6 (0.3–0.9) | 0.7 (0.4–1.2) | ns | 0.7 (0.4–2.0) | 1.3 (0.6–3.7) | 0.018 | ns |
| Percentage of Positive Cells (%), Median, IQR1–IQR3 or Mean ± SD | Fertile Women PBMC (CG) | Fertile Women -PBMC (CG) + 400 ng/mL SC | p Value CG vs. CG SC | RPL PBMC | RPL PBMC + 400 ng/mL SC | p Value RPL vs. RPL SC | p Value CG vs. RPL |
|---|---|---|---|---|---|---|---|
| CD3+CD56+ | 8.4 (4.8–16.0) | 6.9 (3.8–16.5) | ns | 6.1 (3.3–10.5) | 6.0 (3.6–13.2) | ns | ns |
| CD3+CD56+CD44+CD161+ | 20.4 ± 8.9 | 20.2 ± 8.0 | ns | 24.1 (16.8–34.2) | 26.7 (17.7–32.8) | ns | 0.073 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kniotek, M.; Zych, M.; Roszczyk, A.; Szafarowska, M.; Jerzak, M.M. Decreased Production of TNF-α and IL-6 Inflammatory Cytokines in Non-Pregnant Idiopathic RPL Women Immunomodulatory Effect of Sildenafil Citrate on the Cellular Response of Idiopathic RPL Women. J. Clin. Med. 2021, 10, 3115. https://doi.org/10.3390/jcm10143115
Kniotek M, Zych M, Roszczyk A, Szafarowska M, Jerzak MM. Decreased Production of TNF-α and IL-6 Inflammatory Cytokines in Non-Pregnant Idiopathic RPL Women Immunomodulatory Effect of Sildenafil Citrate on the Cellular Response of Idiopathic RPL Women. Journal of Clinical Medicine. 2021; 10(14):3115. https://doi.org/10.3390/jcm10143115
Chicago/Turabian StyleKniotek, Monika, Michał Zych, Aleksander Roszczyk, Monika Szafarowska, and Małgorzata Maria Jerzak. 2021. "Decreased Production of TNF-α and IL-6 Inflammatory Cytokines in Non-Pregnant Idiopathic RPL Women Immunomodulatory Effect of Sildenafil Citrate on the Cellular Response of Idiopathic RPL Women" Journal of Clinical Medicine 10, no. 14: 3115. https://doi.org/10.3390/jcm10143115
APA StyleKniotek, M., Zych, M., Roszczyk, A., Szafarowska, M., & Jerzak, M. M. (2021). Decreased Production of TNF-α and IL-6 Inflammatory Cytokines in Non-Pregnant Idiopathic RPL Women Immunomodulatory Effect of Sildenafil Citrate on the Cellular Response of Idiopathic RPL Women. Journal of Clinical Medicine, 10(14), 3115. https://doi.org/10.3390/jcm10143115






