A Risk Score for Predicting Long-Term Mortality Following Off-Pump Coronary Artery Bypass Grafting
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Surgery
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Risk Factors of Long-Term Mortality
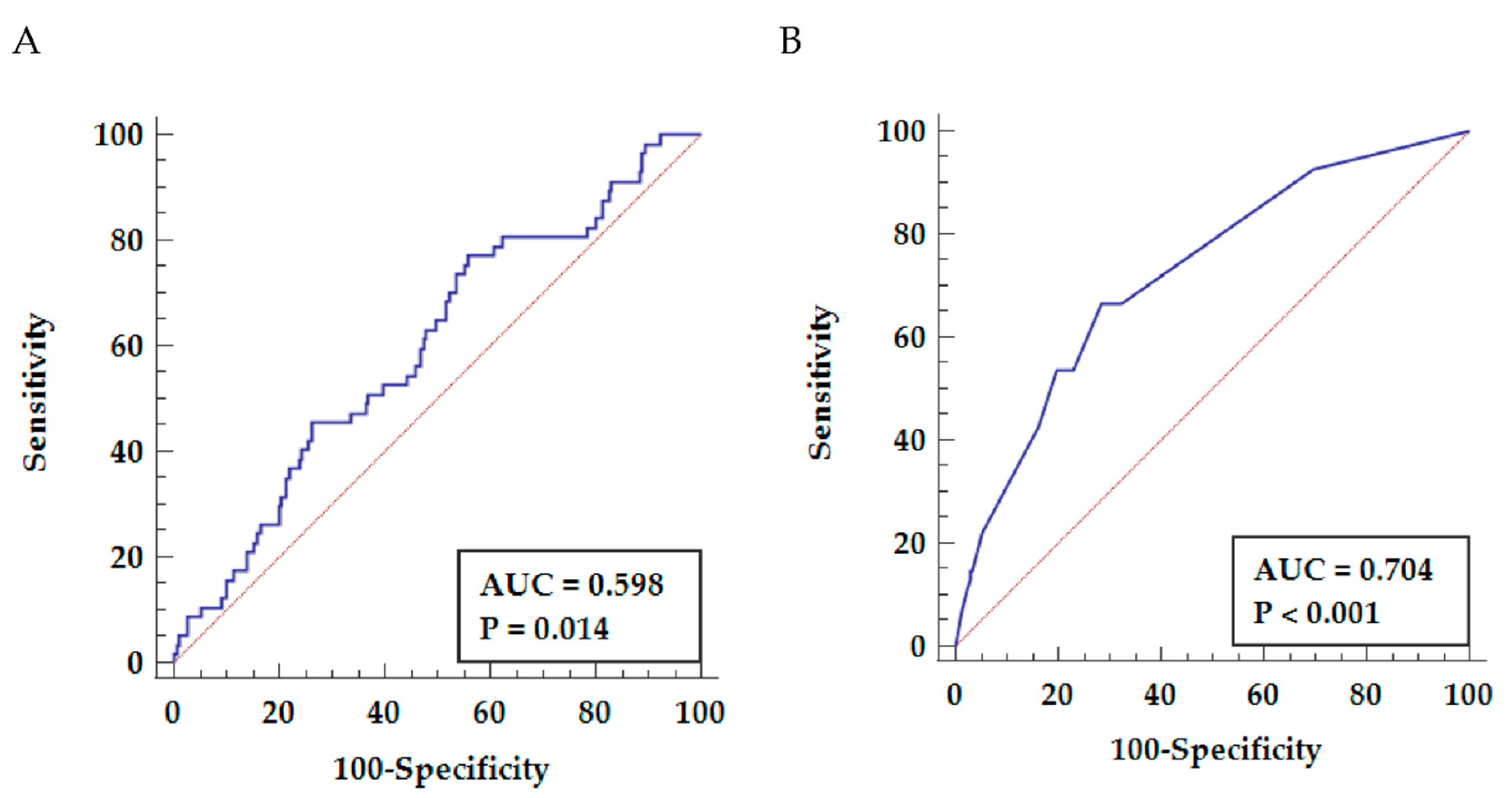
3.3. Predictive Value of NLR for Long-Term Mortality
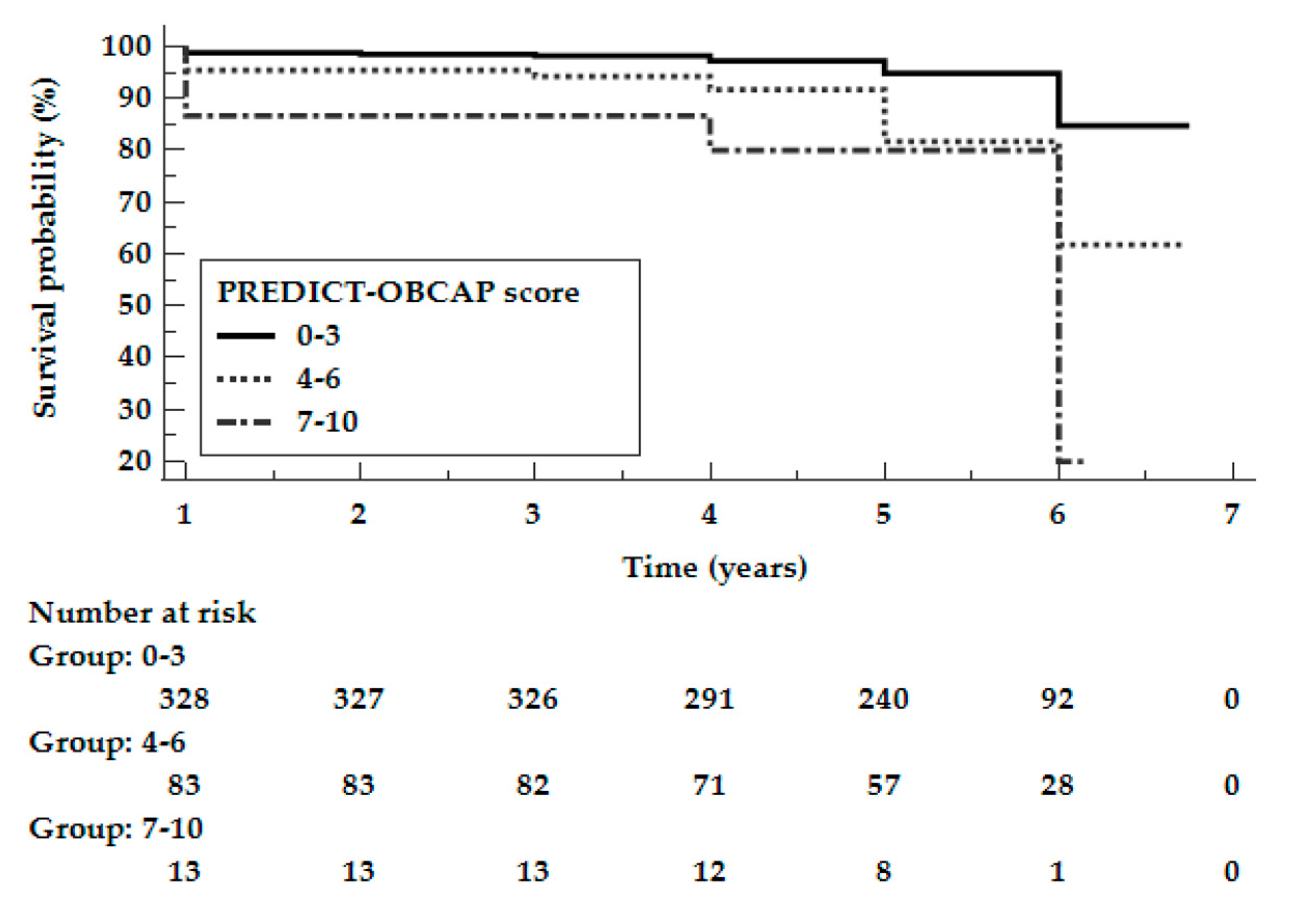
3.4. Development and Performance of the Risk Score
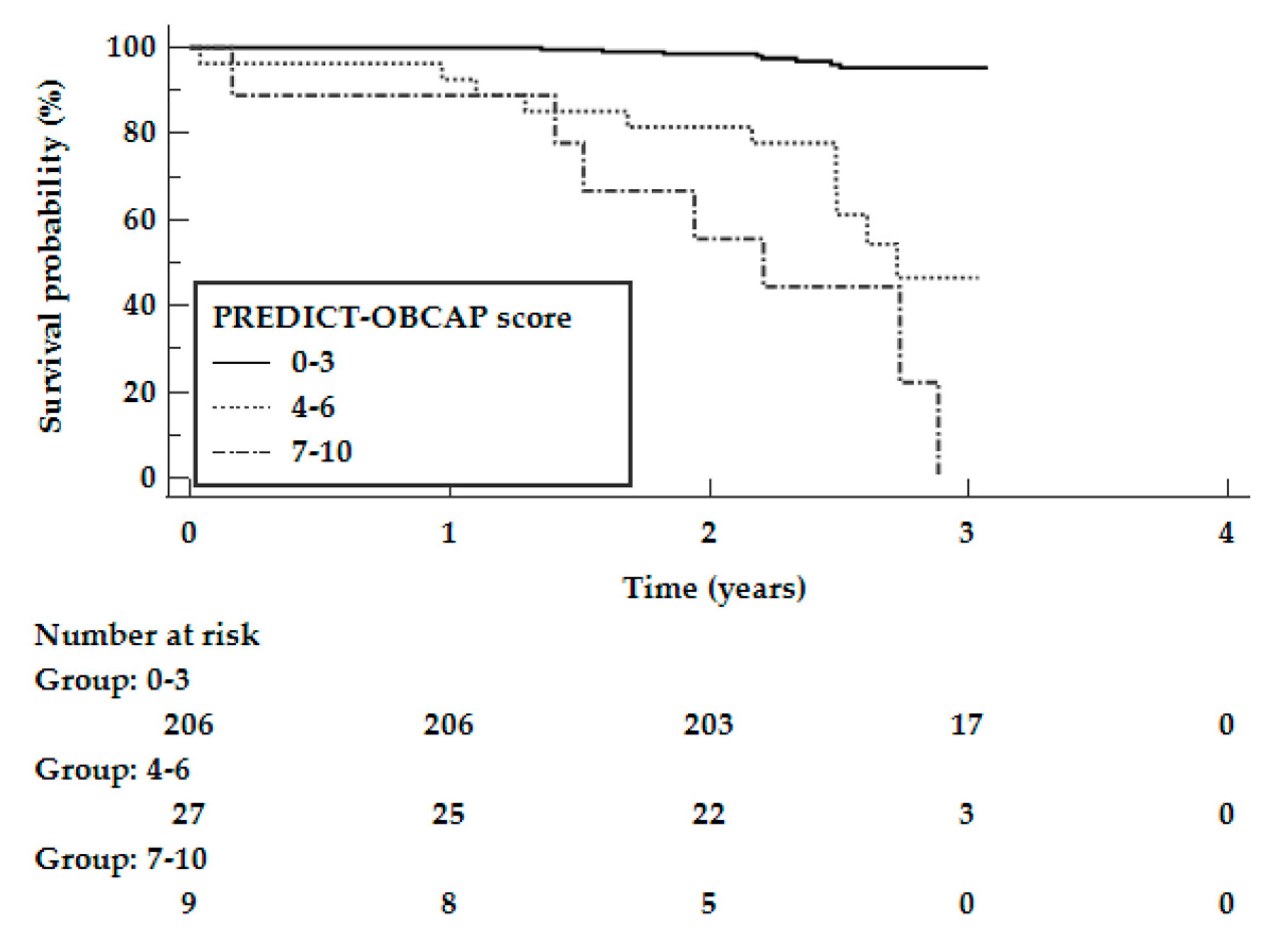
3.5. Validation of the Risk Score in an Independent Cohort
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jahangiri, M.; Mani, K.; Yates, M.T.; Nowell, J. The EXCEL Trial: The Surgeons’ Perspective. Eur. Cardiol. 2020, 15, e67. [Google Scholar] [CrossRef]
- Zubarevich, A.; Kadyraliev, B.; Arutyunyan, V.; Chragyan, V.; Askadinov, M.; Sozkov, A.; Ponomarev, D.; Zyazeva, I.; Sá, M.P.B.O.; Osswald, A.; et al. On-pump versus off-pump coronary artery bypass surgery for multi-vessel coronary revascularization. J. Thorac. Dis. 2020, 12, 5639. [Google Scholar] [CrossRef]
- Matkovic, M.; Tutus, V.; Bilbija, I.; Lazovic, J.M.; Savic, M.; Cubrilo, M.; Aleksic, N.; Atanasijevic, I.; Andrijasevic, V.; Putnik, S. Long Term Outcomes of The Off-Pump and On-Pump Coronary Artery Bypass Grafting in A High-Volume Center. Sci. Rep. 2019, 9, 8567. [Google Scholar] [CrossRef]
- Widimsky, P.; Straka, Z.; Stros, P.; Jirasek, K.; Dvorak, J.; Votava, J.; Lisa, L.; Budesinsky, T.; Kolesar, M.; Vanek, T.; et al. One-year coronary bypass graft patency: A randomized comparison between off-pump and on-pump surgery angiographic results of the PRAGUE-4 trial. Circulation 2004, 110, 3418–3423. [Google Scholar] [CrossRef] [Green Version]
- Waheed, A.; Klosterman, E.; Lee, J.; Mishra, A.; Narasimha, V.; Tuma, F.; Bokhari, F.; Haq, F.; Misra, S. Assessing the long-term patency and clinical outcomes of venous and arterial grafts used in coronary artery bypass grafting: A meta-analysis. Cureus 2019, 11, e5670. [Google Scholar] [CrossRef] [Green Version]
- Nashef, S.A.M.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thorac. Surg. 2012, 41, 734–745. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sato, K.; Narayanswami, J.; Banerjee, K.; Andress, K.; Lokhande, C.; Mohananey, D.; Anumandla, A.K.; Khan, A.R.; Sawant, A.C.; et al. Current society of thoracic surgeons model reclassifies mortality risk in patients undergoing transcatheter aortic valve replacement. Circ. Cardiovasc. Interv. 2018, 11, e006664. [Google Scholar] [CrossRef]
- Wu, C.; Camacho, F.T.; Wechsler, A.S.; Lahey, S.; Culliford, A.T.; Jordan, D.; Gold, J.P.; Higgins, R.S.D.; Smith, C.R.; Hannan, E.L. Risk score for predicting long-term mortality after coronary artery bypass graft surgery. Circulation 2012, 125, 2423–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannan, E.L.; Wu, C.; Bennett, E.V.; Carlson, R.E.; Culliford, A.T.; Gold, J.P.; Higgins, R.S.D.; Isom, O.W.; Smith, C.R.; Jones, R.H. Risk stratification of in-hospital mortality for coronary artery bypass graft surgery. J. Am. Coll. Cardiol. 2006, 47, 661–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Ridker, P.M.; Hansson, G.K.; Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [Green Version]
- Dybdahl, B.; Wahba, A.; Lien, E.; Flo, T.H.; Waage, A.; Qureshi, N.; Sellevold, O.F.M.; Espevik, T.; Sundan, A. Inflammatory response after open heart surgery: Release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation 2002, 105, 685–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadem, J.; Rossnick, R.; Hesse, B.; Herr, M.; Hansen, M.; Bergmann, A.; Kensah, G.; Maess, C.; Baraki, H.; Kümpers, P.; et al. Endothelial dysfunction following coronary artery bypass grafting. Springer. Herz 2020, 45, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Perros, A.J.; Esguerra-Lallen, A.; Rooks, K.; Chong, F.; Engkilde-Pedersen, S.; Faddy, H.M.; Hewlett, E.; Naidoo, R.; Tung, J.-P.; Fraser, J.F.; et al. Coronary artery bypass grafting is associated with immunoparalysis of monocytes and dendritic cells. J. Cell. Mol. Med. 2020, 24, 4791–4803. [Google Scholar] [CrossRef]
- Jongman, R.M.; Zijlstra, J.G.; Kok, W.F.; van Harten, A.E.; Mariani, M.A.; Moser, J.; Struys, M.M.R.F.; Absalom, A.R.; Molema, G.; Scheeren, T.W.L.; et al. Off-pump CABG surgery reduces systemic inflammation compared with on-pump surgery but does not change systemic endothelial responses: A prospective randomized study. Shock 2014, 42, 121–128. [Google Scholar] [CrossRef]
- Rimmelé, T.; Venkataraman, R.; Madden, N.J.; Elder, M.M.; Wei, L.M.; Pellegrini, R.V.; Kellum, J.A. Comparison of inflammatory response during on-pump and off-pump coronary artery bypass surgery. Int. J. Artif. Organs 2010, 33, 131–138. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, M.; Sun, J.; Ma, S. The combination of neutrophil-to-lymphocyte ratio and platelet correlation parameters in predicting the no-reflow phenomenon after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Scand. Cardiovasc. J. 2020, 54, 352–357. [Google Scholar] [CrossRef]
- Machado, G.P.; de Araujo, G.N.; Carpes, C.K.; Lech, M.; Mariani, S.; Valle, F.H.; Bergoli, L.C.C.; Gonçalves, S.C.; Wainstein, R.V.; Wainstein, M.V. Comparison of neutrophil-to-lymphocyte ratio and mean platelet volume in the prediction of adverse events after primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction. Atherosclerosis 2018, 274, 212–217. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Munoz, D.; et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, L.; Yang, G.; Gong, J.; Ye, L.; Zhi, S.; Zhang, X.; Li, J. Postoperative outcomes of patients with chronic obstructive pAulmonary disease undergoing coronary artery bypass grafting surgery: A meta-analysis. Medicine 2019, 98, e14388. [Google Scholar] [CrossRef] [PubMed]
- Medalion, B.; Katz, M.G.; Cohen, A.J.; Hauptman, E.; Sasson, L.; Schachner, A. Long-term beneficial effect of coronary artery bypass grafting in patients with COPD. Chest 2004, 125, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Viceconte, M.; Rocco, I.S.; Pauletti, H.O.; Vidotto, M.; Arena, R.; Gomes, W.J.; Guizilini, S. Chronic obstructive pulmonary disease severity influences outcomes after off-pump coronary artery bypass. J. Thorac. Cardiovasc. Surg. 2018, 156, 1554–1561. [Google Scholar] [CrossRef]
- Head, S.J.; Milojevic, M.; Daemen, J.; Ahn, J.-M.; Boersma, E.; Christiansen, E.H.; Domanski, M.J.; Farkouh, M.E.; Flather, M.; Fuster, V.; et al. Stroke rates following surgical versus percutaneous coronary revascularization. J. Am. Coll. Cardiol. 2018, 72, 386–398. [Google Scholar] [CrossRef]
- Gaudino, M.; Angiolillo, D.J.; Di Franco, A.; Capodanno, D.; Bakaeen, F.; Farkouh, M.E.; Fremes, S.E.; Holmes, D.; Girardi, L.N.; Nakamura, S.; et al. Stroke after coronary artery bypass grafting and percutaneous coronary intervention: Incidence, pathogenesis, and outcomes. J. Am. Heart Assoc. 2019, 8, e013032. [Google Scholar] [CrossRef]
- Bottle, A.; Mozid, A.; Grocott, H.P.; Walters, M.R.; Lees, K.R.; Aylin, P.; Sanders, R.D. Preoperative stroke and outcomes after coronary artery bypass graft surgery. Anesthesiology 2013, 118, 885–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalén, M.; Lund, L.H.; Ivert, T.; Holzmann, M.J.; Sartipy, U. Survival after coronary artery bypass grafting in patients with preoperative heart failure and preserved vs reduced ejection fraction. JAMA Cardiol. 2016, 1, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Neumann, A.; Serna-Higuita, L.; Detzel, H.; Popov, A.-F.; Krüger, T.; Vöhringer, L.; Schlensak, C. Off-pump coronary artery bypass grafting for patients with severely reduced ventricular function—A justified strategy? J. Card. Surg. 2021. [Google Scholar] [CrossRef]
- Maltais, S.; Ladouceur, M.; Cartier, R. The influence of a low ejection fraction on long-term survival in systematic off-pump coronary artery bypass surgery. Eur. J. Cardio-Thorac. Surg. 2011, 39, e122–e127. [Google Scholar] [CrossRef]
- Zhang, S.; Diao, J.; Qi, C.; Jin, J.; Li, L.; Gao, X.; Gong, L.; Wu, W. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: A meta-analysis. BMC Cardiovasc. Disord. 2018, 18, 75. [Google Scholar] [CrossRef] [Green Version]
- Kurtul, S.; Sarli, B.; Baktir, A.O.; Demirbas, M.; Saglam, H.; Doğan, Y.; Sahin, O.; Akpek, M.; Odabas, H.; Arinc, H.; et al. Neutrophil to lymphocyte ratio predicts SYNTAX score in patients with non-ST segment elevation myocardial infarction. Int. Heart J. 2014, 56, 18–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larmann, J.; Handke, J.; Scholz, A.S.; Dehne, S.; Arens, C.; Gillmann, H.-J.; Uhle, F.; Motsch, J.; Weigand, M.A.; Janssen, H. Preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with major adverse cardiovascular and cerebrovascular events in coronary heart disease patients undergoing non-cardiac surgery. BMC Cardiovasc. Disord. 2020, 20, 230. [Google Scholar] [CrossRef] [PubMed]
- Weedle, R.C.; Da Costa, M.; Veerasingam, D.; Soo, A.W.S. The use of neutrophil lymphocyte ratio to predict complications post cardiac surgery. Ann. Transl. Med. 2019, 7, 778. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Mesana, T.G.; Lee, D.S.; Eddeen, A.B.; Sun, L.Y. Sex differences in long-term survival after major cardiac surgery: A population-based cohort study. J. Am. Heart Assoc. 2019, 8, e013260. [Google Scholar] [CrossRef] [PubMed]
- Kytö, V.; Sipilä, J.; Rautava, P.; Gunn, J. Sex differences in outcomes following acute coronary syndrome treated with coronary artery bypass surgery. Hear. Lung Circ. 2020, 30, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, F.; Fortuna, D.; Contini, G.A.; Pacini, D.; Gabbieri, D.; Zussa, C.; De Palma, R.; Vezzani, A.; Gherli, T. The impact of age on clinical outcomes of coronary artery bypass grafting: Long-term results of a real-world registry. BioMed Res. Int. 2017, 2017, 9829487. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.P.; Parlow, J.L.; Whitehead, M.; Xu, J.; Rohland, S.; Milne, B. Body mass index, outcomes, and mortality following cardiac surgery in Ontario, Canada. J. Am. Heart Assoc. 2015, 4, e002140. [Google Scholar] [CrossRef] [Green Version]
| Survival n = 381 (87%) | Death n = 59 (13%) | p-Value | |
|---|---|---|---|
| Demographic data | |||
| Gender, male n (%) | 332 (87%) | 50 (85%) | 0.8827 |
| Age, years (mean ± SD) | 64.1 ± 7.9 | 66.6 ± 8 | 0.0351 |
| BMI, kg/m2 (mean ± SD) | 28.5 ± 3.6 | 28.8 ± 4.2 | 0.4593 |
| BSA, m2 (mean ± SD) | 1.9 ± 0.2 | 1.9 ± 0.2 | 0.9404 |
| Clinical data | |||
| Left main disease n (%) | 83 (22%) | 18 (31%) | 0.1276 |
| Arterial hypertension n (%) | 290 (76%) | 49 (83%) | 0.2349 |
| DM n (%) | 128 (34%) | 22 (37%) | 0.6517 |
| Hypercholesterolemia n (%) | 218 (57%) | 24 (41%) | 0.3829 |
| COPD n (%) | 34 (9%) | 10 (17%) | <0.0001 |
| Stroke n (%) | 29 (8%) | 10 (17%) | 0.0261 |
| PAD n (%) | 53 (14%) | 14 (24%) | 0.0473 |
| Chronic kidney disease n (%) | 25 (7%) | 8 (14%) | 0.0642 |
| Pre-operative laboratory parameters | |||
| WBC, ×109/L (mean ± SD) | 8.0 ± 2.9 | 8.1 ± 2.0 | 0.4125 |
| Neutrophils, ×109/L (mean ± SD) | 5.2 ± 2.3 | 5.6 ± 1.9 | 0.0715 |
| Hb, mmol/L (mean ± SD) | 8.7 ± 1.1 | 8.6 ± 0.9 | 0.1747 |
| Plt ×109/L (mean ± SD) | 232.8 ± 63.0 | 239.1 ± 71.7 | 0.5670 |
| NLR (mean ± SD) | 3.2 ± 2.8 | 3.7 ± 3.6 | 0.0256 |
| Pre-operative echocardiographic parameters | |||
| LVEDD, mm (mean ± SD) | 49 ± 6 | 51 ± 7 | 0.0049 |
| LVEF, (mean ± SD) | 54 ± 9 | 49 ± 11 | 0.0012 |
| Intraoperative data | |||
| Surgery time, h (mean ± SD) | 2:39 ± 0:3 | 2:36 ± 0:4 | 0.4965 |
| Anastomoses, number (mean ± SD) | 2.3 ± 0.2 | 2.2 ± 0.2 | 0.0746 |
| Blood product transfusion n (%) | 151 (40%) | 27 (46%) | 0.3829 |
| Length of stay, days (mean ± SD) | 8 ± 3 | 8 ± 2 | 1.0000 |
| Post-operative laboratory parameters | |||
| WBC, ×109/L (mean ± SD) | 8.9 ± 3.2 | 9.1 ± 3.2 | 0.7186 |
| Neutrophils, ×109/L (mean ± SD) | 5.4 ± 2.4 | 6 ± 2.9 | 0.2165 |
| Hb, mmol/L (mean ± SD) | 6.9 ± 0.8 | 7 ± 0.8 | 0.9804 |
| Plt ×109/L (mean ± SD) | 287.1 ± 106.9 | 283.4 ± 98.2 | 0.5843 |
| NLR (mean ± SD) | 3.0 ± 1.9 | 3.8 ± 3.2 | 0.0173 |
| Injury markers: | |||
| Troponin T, ng/mL (mean ± SD) | 4.33 ± 14.2 | 3.66 ± 5.9 | 0.3726 |
| CK-MB mass, ng/mL (mean ± SD) | 18.7 ± 33.4 | 19.3 ± 28.9 | 0.5293 |
| Post-operative echocardiographic parameters | |||
| LVEDD, mm (mean ± SD) | 48 ± 7 | 51 ± 7 | 0.0017 |
| LVEF, (mean ± SD) | 55 ± 9 | 49 ± 11 | 0.0010 |
| Parameter | HR | 95% CI | p-Value |
|---|---|---|---|
| Baseline parameters | |||
| Age | 1.04 | 0.98–1.08 | 0.0531 |
| Gender (male) | 0.64 | 0.26–1.54 | 0.3200 |
| Comorbidities | |||
| Arterial hypertension | 1.30 | 0.61–2.77 | 0.4838 |
| Left main disease | 1.98 | 1.12–3.49 | 0.0180 |
| Diabetes mellitus | 1.03 | 0.58–1.79 | 0.8962 |
| COPD | 2.07 | 1.43–4.11 | 0.0373 |
| Hypercholesterolemia | 0.61 | 0.35–1.04 | 0.0706 |
| Chronic kidney disease | 1.65 | 0.74–3.65 | 0.2150 |
| Stroke history | 2.40 | 1.21–4.76 | 0.0122 |
| Pre-operative laboratory parameters | |||
| WBC | 1.01 | 0.94–1.09 | 0.6143 |
| Lymphocytes | 0.76 | 0.50–1.15 | 0.2061 |
| Neutrophils | 1.08 | 0.97–1.21 | 0.1253 |
| Hemoglobin | 0.86 | 0.67–1.12 | 0.2801 |
| Platelets | 1.00 | 0.99–1.00 | 0.3800 |
| NLR | 1.07 | 0.99–1.15 | 0.0714 |
| Post-operative laboratory parameters | |||
| WBC | 1.02 | 0.96–1.08 | 0.4668 |
| Lymphocytes | 0.68 | 0.45–1.04 | 0.0827 |
| Neutrophils | 1.10 | 1.00–1.20 | 0.0323 |
| Hemoglobin | 1.05 | 0.75–1.48 | 0.7521 |
| Platelets | 0.99 | 0.99–1.00 | 0.6761 |
| NLR | 1.13 | 1.05–1.21 | 0.0011 |
| Cardiac injury markers | |||
| Troponins | 0.99 | 0.96–1.02 | 0.8615 |
| CK-MB mass | 1.00 | 0.99–1.01 | |
| Echocardiographic parameters | |||
| LVEDD preoperative | 1.05 | 1.01–1.09 | 0.0084 |
| LVEDD postoperative | 1.06 | 1.02–1.10 | 0.0031 |
| LVEF preoperative | 0.95 | 0.93–0.97 | 0.0002 |
| LVEF postoperative | 0.96 | 0.93–0.98 | 0.0015 |
| Parameter | HR | 95% CI | p-Value | Predict-Opcab Score |
|---|---|---|---|---|
| COPD | 2.19 | 1.08–4.40 | 0.0278 | 2 |
| Stroke history | 2.67 | 1.34–5.31 | 0.0053 | 3 |
| NLR after surgery ≥ 2.42 | 1.96 | 1.04–3.68 | 0.0364 | 2 |
| LVEF after surgery ≤ 45 | 2.64 | 1.53–4.56 | 0.0005 | 3 |
| Deaths | Predict-Opcab Score: 0–3 | Predict-Opcab Score: 4–6 | Predict-Opcab Score: 7–10 | Log-Rank p-Value | Log-Rank p-Value 0–3 vs. 4–6 | Log-Rank p-Value 0–3 vs. 7–10 | Log-Rank p-Value 4–6 vs. 7–10 |
|---|---|---|---|---|---|---|---|
| Study population N = 440 | 28 (8.3%) | 24 (27.0%) | 6 (40.0%) | <0.0001 | <0.0001 | <0.0001 | 0.1100 |
| Validation cohort N = 242 | 8 (3.9%) | 11 (40.7%) | 7 (77.8%) | <0.0001 | <0.0001 | <0.0001 | 0.0838 |
| Survival n= 198 (89%) | Death n= 26 (11%) | p-Value | |
|---|---|---|---|
| Demographic data | |||
| Gender, male n (%) | 174 (88%) | 24 (92%) | 0.5458 |
| Age, years (mean ± SD) | 65 ± 9 | 67 ± 9 | 0.2739 |
| BMI, kg/m2 (mean ± SD) | 22 ± 1.4 | 29 ± 5 | 0.4467 |
| BSA, m2 (mean ± SD) | 1.9 ± 0.2 | 1.9 ± 0.2 | 0.6726 |
| Clinical data | |||
| Left main disease n (%) | 41 (21%) | 5 (19%) | 0.8132 |
| Arterial hypertension n (%) | 166 (84%) | 21 (81%) | 0.6921 |
| DM n (%) | 78 (39%) | 9 (35%) | 0.6385 |
| Hypercholesterolemia n (%) | 134 (68%) | 18 (69%) | 0.8733 |
| COPD n (%) | 11 (6%) | 8 (70%) | <0.0001 |
| Stroke n (%) | 5 (3%) | 10 (38%) | <0.0001 |
| PAD n (%) | 29 (15%) | 11 (42%) | 0.0005 |
| Chronic kidney disease n (%) | 18 (9%) | 2 (9%) | 0.7341 |
| Pre-operative laboratory parameters | |||
| WBC, ×109/L (mean ± SD) | 8.4 ± 3.3 | 7.7 ± 2.0 | 0.6769 |
| Neutrophils, ×109/L (mean ± SD) | 5.3 ± 1.7 | 5 ± 1.5 | 0.7839 |
| Hb, mmol/L (mean ± SD) | 8.7 ± 0.9 | 8.7 ± 1.0 | 0.9882 |
| Plt ×109/L (mean ± SD) | 229 ± 63 | 233 ± 62 | 0.9385 |
| NLR (mean ± SD) | 3.1 ± 1.8 | 3.2 ± 1.5 | 0.7109 |
| Pre-operative echocardiographic parameters | |||
| LVEDD, mm (mean ± SD) | 47 ± 6 | 48 ± 6 | 0.5624 |
| LVEF, (mean ± SD) | 54 ± 8 | 50 ± 7 | 0.0030 |
| Intraoperative data | |||
| Surgery time, h (mean ± SD) | 2:47 ±1:36 | 2:35 ± 1:26 | 0.5154 |
| Anastomoses, number (mean ± SD) | 2.3 ± 0.6 | 2.3 ± 0.7 | 0.9999 |
| Length of stay, days (mean ± SD) | 9 ± 3 | 13 ± 10 | 0.0030 |
| Post-operative laboratory parameters | |||
| WBC, ×109/L (mean ± SD) | 9.1 ± 4.9 | 12 ± 13 | 0.0330 |
| Neutrophils, ×109/L (mean ± SD) | 5.1± 2.0 | 8.7 ± 11.6 | 0.0012 |
| Hb, mmol/L (mean ± SD) | 6.9 ± 0.6 | 7.0 ± 0.5 | 0.3563 |
| Plt ×109/L (mean ± SD) | 305 ± 92 | 354 ± 107 | 0.0157 |
| NLR (mean ± SD) | 2.8 ± 1.5 | 5.1 ± 3.6 | 0.0003 |
| Injury markers: | |||
| Troponin T, ng/mL (mean ± SD) | 3.9 ± 6 | 9.9 ± 11 | 0.0113 |
| Post-operative echocardiographic parameters | |||
| LVEDD, mm (mean ± SD) | 47 ± 6 | 50 ± 6 | 0.0205 |
| LVEF, (mean ± SD) | 56 ± 8 | 43 ± 7 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanowicz, T.K.; Michalak, M.; Gąsecka, A.; Olasińska-Wiśniewska, A.; Perek, B.; Rodzki, M.; Bociański, M.; Jemielity, M. A Risk Score for Predicting Long-Term Mortality Following Off-Pump Coronary Artery Bypass Grafting. J. Clin. Med. 2021, 10, 3032. https://doi.org/10.3390/jcm10143032
Urbanowicz TK, Michalak M, Gąsecka A, Olasińska-Wiśniewska A, Perek B, Rodzki M, Bociański M, Jemielity M. A Risk Score for Predicting Long-Term Mortality Following Off-Pump Coronary Artery Bypass Grafting. Journal of Clinical Medicine. 2021; 10(14):3032. https://doi.org/10.3390/jcm10143032
Chicago/Turabian StyleUrbanowicz, Tomasz Kamil, Michał Michalak, Aleksandra Gąsecka, Anna Olasińska-Wiśniewska, Bartłomiej Perek, Michał Rodzki, Michał Bociański, and Marek Jemielity. 2021. "A Risk Score for Predicting Long-Term Mortality Following Off-Pump Coronary Artery Bypass Grafting" Journal of Clinical Medicine 10, no. 14: 3032. https://doi.org/10.3390/jcm10143032
APA StyleUrbanowicz, T. K., Michalak, M., Gąsecka, A., Olasińska-Wiśniewska, A., Perek, B., Rodzki, M., Bociański, M., & Jemielity, M. (2021). A Risk Score for Predicting Long-Term Mortality Following Off-Pump Coronary Artery Bypass Grafting. Journal of Clinical Medicine, 10(14), 3032. https://doi.org/10.3390/jcm10143032







