Urinary Cytokines Reflect Renal Inflammation in Acute Tubulointerstitial Nephritis: A Multiplex Bead-Based Assay Assessment
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Design and Study Population
2.2. Clinical Variables
2.3. Histological Grading of Tubule-Interstitial Infiltrates
2.4. Sampling and Measurement of Urinary Cytokine Levels
3. Statistical Analysis
4. Results
4.1. Baseline Characteristics of the Population
4.2. Urinary Cytokine Levels Are Significantly Increased in ATIN Patients and Correlate with the Extend of Tubulointerstitial Infiltrate
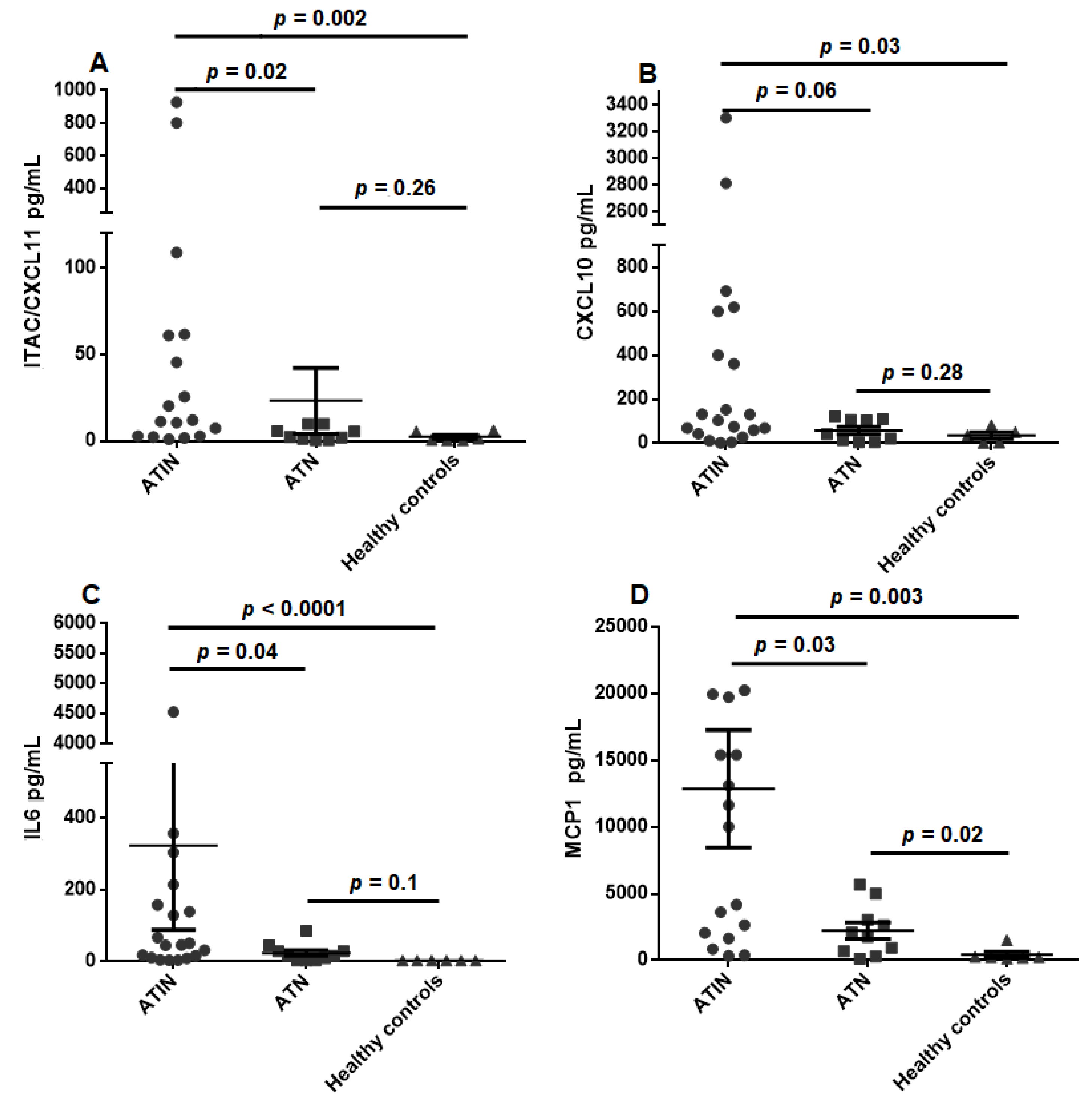
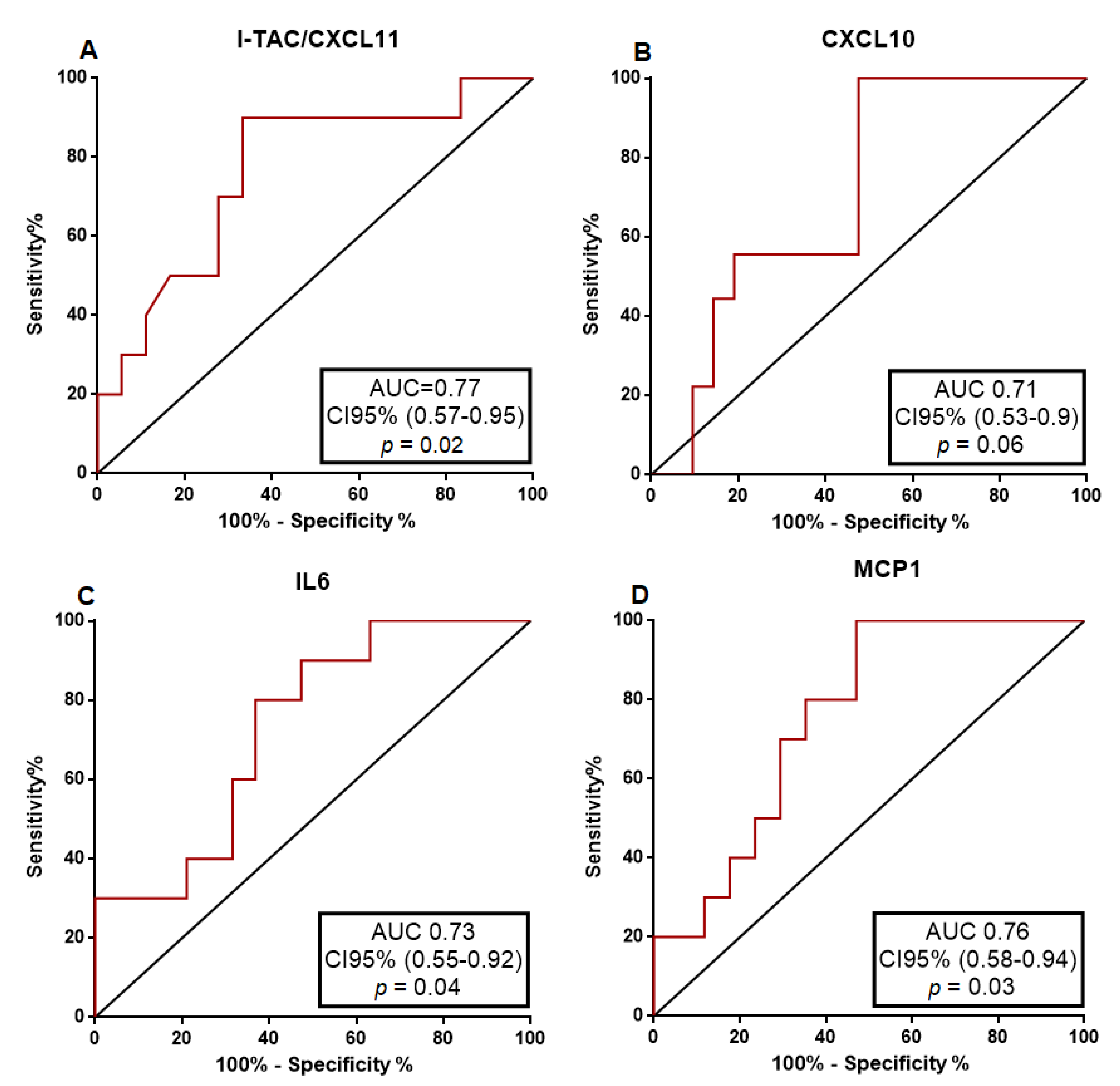
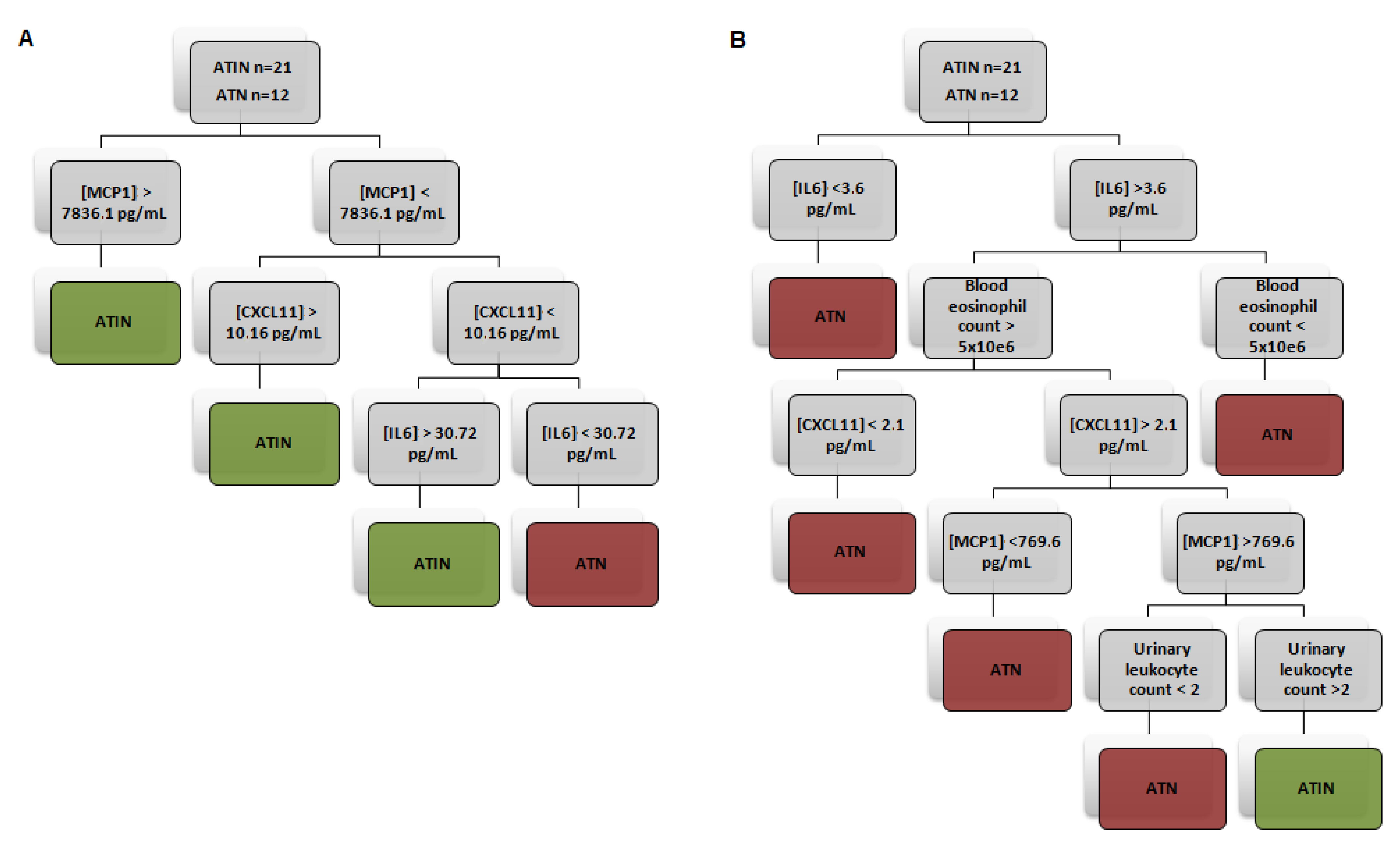
4.3. Urinary Cytokine Levels Distinguish ATIN from ATN Patients
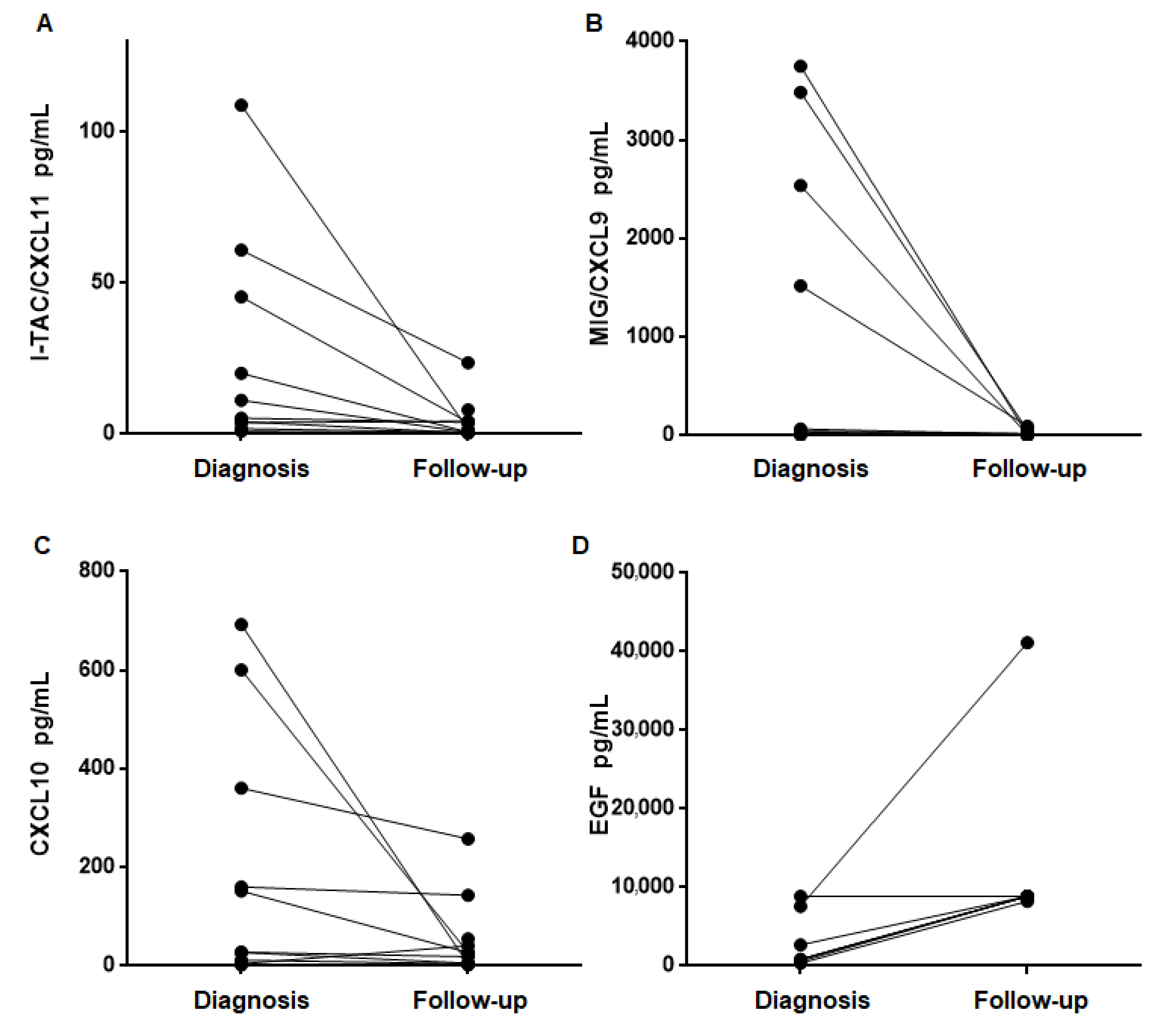
4.4. Urinary Cytokine Levels Decrease after Treatment and Recovery of Renal Function
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raghavan, R.; Eknoyan, G. Acute interstitial nephritis—A reappraisal and update. Clin. Nephrol. 2014, 82, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Shawar, S. Mechanisms of Drug-Induced Interstitial Nephritis. Adv. Chronic Kidney Dis. 2017, 24, 64–71. [Google Scholar] [CrossRef]
- Krishnan, N.; Perazella, M.A. Drug-induced acute interstitial nephritis: Pathology, pathogenesis, and treatment. Iran. J. Kidney Dis. 2015, 9, 3–13. [Google Scholar] [PubMed]
- Praga, M.; González, E. Acute interstitial nephritis. Kidney Int. 2010, 77, 956–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nussbaum, E.Z.; Perazella, M.A. Diagnosing acute interstitial nephritis: Considerations for clinicians. Clin. Kidney J. 2019, 12, 808–813. [Google Scholar] [CrossRef]
- Perazella, M.A. Diagnosing drug-induced AIN in the hospitalized patient: A challenge for the clinician. Clin. Nephrol. 2014, 81, 381–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandolfini, I.; Harris, C.; Abecassis, M.; Anderson, L.; Bestard, O.; Comai, G.; Cravedi, P.; Cremaschi, E.; Duty, J.A.; Florman, S.; et al. Rapid Biolayer Interferometry Measurements of Urinary CXCL9 to Detect Cellular Infiltrates Noninvasively After Kidney Transplantation. Kidney Int. Rep. 2017, 2, 1186–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muriithi, A.K.; Leung, N.; Valeri, A.M.; Cornell, L.D.; Sethi, S.; Fidler, M.E.; Nasr, S.H. Biopsy-Proven Acute Interstitial Nephritis, 1993-2011: A Case Series. Am. J. Kidney Dis. 2014, 64, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-Z.; Gu, Y.-H.; Su, T.; Zhou, X.-J.; Huang, J.-W.; Sun, P.-P.; Jia, Y.; Xu, D.-M.; Wang, S.-X.; Liu, G.; et al. Elevation of erythrocyte sedimentation rate and C-reactive protein levels reflects renal interstitial inflammation in drug-induced acute tubulointerstitial nephritis. BMC Nephrol. 2020, 21, 514. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.U.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Ohmori, Y.; Wyner, L.; Narumi, S.; Armstrong, D.; Stoler, M.; Hamilton, T.A. Tumor necrosis factor-α induces cell type and tis-sue-specific expression of chemoattractant cytokines in vivo. Am. J. Pathol. 1993, 142, 861–870. [Google Scholar] [PubMed]
- Su, H.; Lei, C.T.; Zhang, C. Interleukin-6 signaling pathway and its role in kidney disease: An update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Yang, L.; Su, T.; Wang, C.; Liu, G.; Li, X.-M. Pathological Significance of a Panel of Urinary Biomarkers in Patients with Drug-Induced Tubulointerstitial Nephritis. Clin. J. Am. Soc. Nephrol. 2010, 5, 1954–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, D.; Jang, M.-J.; An, J.N.; Lee, J.P.; Kim, D.K.; Chin, H.J.; Kim, Y.S.; Lee, D.-S.; Han, S.S. Effect of steroids and relevant cytokine analysis in acute tubulointerstitial nephritis. BMC Nephrol. 2019, 20, 88. [Google Scholar] [CrossRef]
- Moledina, D.G.; Wilson, F.P.; Pober, J.S.; Perazella, M.A.; Singh, N.; Luciano, R.L.; Obeid, W.; Lin, H.; Kuperman, M.; Moeckel, G.W.; et al. Urine TNF-α and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight 2019, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Moledina, D.G.; Parikh, C.R. Differentiating Acute Interstitial Nephritis from Acute Tubular Injury: A Challenge for Clinicians. Nephron 2019, 143, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liu, N.; Zhuang, S. Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney Int. 2013, 83, 804–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taira, T.; Yoshimura, A.; Lizuka, K.; Iwasaki, S.; Ideura, T.; Koshikawa, S. Urinary Epidermal Growth Factor Levels in Patients With Acute Renal Failure. Am. J. Kidney Dis. 1993, 22, 656–661. [Google Scholar] [CrossRef]
- Moran, S.M.; Monach, P.A.; Zgaga, L.; Cuthbertson, D.; Carette, S.; Khalidi, N.A.; Koening, C.L.; Langford, C.A.; McAlear, C.A.; Moreland, L.; et al. Urinary soluble CD163 and monocyte chemoattractant protein-1 in the identification of subtle renal flare in anti-neutrophil cytoplasmic antibody–associated vascu-litis. Nephrol. Dial. Transplant. 2018, 35, 283–291. [Google Scholar] [CrossRef]
- Dekkema, G.J.; Abdulahad, W.H.; Bijma, T.; Moran, S.M.; Ryan, L.; Little, M.A.; Stegeman, C.A.; Heeringa, P.; San-ders, J.S.F. Urinary and serum soluble CD25 complements urinary soluble CD163 to detect active renal anti-neutrophil cytoplasmic autoanti-body-associated vasculitis: A cohort study. Nephrol. Dial. Transplant. 2019, 34, 234–242. [Google Scholar] [CrossRef]
- Crespo, E.; Cravedi, P.; Martorell, J.; Luque, S.; Melilli, E.; Cruzado, J.M.; Jarque, M.; Meneghini, M.; Manonelles, A.; Donadei, C.; et al. Posttransplant peripheral blood donor–specific interferon-γ enzyme-linked immune spot assay differentiates risk of subclinical rejection and de novo donor-specific alloantibodies in kidney transplant recipients. Kidney Int. 2017, 92, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Hricik, D.E.; Nickerson, P.; Formica, R.N.; Poggio, E.D.; Rush, D.; Newell, K.A.; Goebel, J.; Gibson, I.; Fairchild, R.L.; Riggs, M.; et al. Multicenter Validation of Urinary CXCL9 as a Risk-Stratifying Biomarker for Kidney Transplant Injury. Arab. Archaeol. Epigr. 2013, 13, 2634–2644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| ATIN (n = 21) | ATN (n = 12) | p-Value | |
|---|---|---|---|
| Age (years) | 66.5 ± 13.52 | 63.5 ± 12.5 | 0.536 |
| Sex (%male) | 57.10% | 81.80% | 0.248 |
| Diabetes (% patients) | 33.3% | 41.66% | 0.365 |
| Hypertension (% patients) | 57.14% | 50% | 0.432 |
| Baseline creatinine (µmol/L) | 87.84 ± 19.17 | 89.58 ± 15.56 | 0.322 |
| Creatinine (µmol/L) | 324.9 ± 141.8 | 321.7 ± 176.3 | 0.956 |
| CRP (mg/L) | 74.9 ± 95.1 | 25.6 ± 59.7 | 0.129 |
| Urinary leukocyte count (leukocytes/ μL) | 400 ± 1467 | 166.5 ± 452.7 | 0.613 |
| Urinary erythrocyte count (leukocytes/ μL) | 248.6 ± 1075.5 | 46.8 ± 80.1 | 0.542 |
| Proteinuria (g/mol) | 70.49 ± 114.9 | 94.8 ± 112.5 | 0.587 |
| Eosinophil blood count (×106) | 257.2 ± 210.7 | 102.7 ± 105.4 | 0.03 * |
| ATIN (n = 21) | ATN (n = 12) | Healthy Controls (n = 6) | p-Value | |
|---|---|---|---|---|
| I-TAC/CXCL11 | 129.5 ± 64.68 | 23.07 ± 19.01 | 2.35 ± 1.07 | 0.046 * |
| MIG/CXCL9 | 1170 ± 739.5 | 1456 ± 896.2 | 43.37 ± 9.91 | 0.665 |
| IL1B | 9.08 ± 5.84 | 14.56 ± 9.73 | 2.03 ± 0.53 | 0.241 |
| CXCL10 | 909.9 ± 468.1 | 58.60 ± 16.74 | 35.31 ± 15.3 | 0.105 |
| IL6 | 323.1 ± 235.1 | 24.09 ± 8.11 | 3.2 ± 0.2 | 0.029 * |
| IL17 | 7.28 ± 5.15 | 4.67 ± 2.37 | 2.18 ± 0.65 | 0.83 |
| TNFα | 5.46 ± 1.12 | 4.17 ± 0.89 | 2.84 ± 0.27 | 0.032 * |
| IFNα | 23.04 ± 13.82 | 18.21 ± 6.99 | 27.48 ± 13.8 | 0.669 |
| MCP1 | 12848 ± 4413 | 2221 ± 603.2 | 418 ± 217.6 | 0.002 * |
| EGF | 3888 ± 851.3 | 3067 ± 1207 | 18748 ± 6454 | 0.003 * |
| ATIN (n = 21) | Healthy Controls (n = 6) | p-Value | |
|---|---|---|---|
| I-TAC/CXCL11 | 129.5 ± 64.68 | 2.35 ± 1.07 | 0.002 * |
| MIG/CXCL9 | 1170 ± 739.5 | 43.37 ± 9.91 | 0.629 |
| IL1B | 9.08 ± 5.84 | 2.03 ± 0.53 | 0.693 |
| CXCL10 | 909.9 ± 468.1 | 35.31 ± 15.3 | 0.034 * |
| IL6 | 323.1 ± 235.1 | 3.2 ± 0.2 | <0.001 * |
| IL17 | 7.28 ± 5.15 | 2.18 ± 0.65 | 0.781 |
| TNFα | 5.46 ± 1.12 | 2.84 ± 0.27 | 0.027 * |
| IFNα | 23.04 ± 13.82 | 27.48 ± 13.8 | 0.558 |
| MCP1 | 12848 ± 4413 | 418 ± 217.6 | 0.001 * |
| EGF | 3888 ± 851.3 | 18748 ± 6454 | 0.002 * |
| ATIN (n = 21) | ATN (n = 12) | p-Value | |
|---|---|---|---|
| I-TAC/CXCL11 | 129.5 ± 64.68 | 23.07 ± 19.01 | 0.022 * |
| MIG/CXCL9 | 1170 ± 739.5 | 1456 ± 896.2 | 0.945 |
| IL1B | 9.08 ± 5.84 | 14.56 ± 9.73 | 0.199 |
| CXCL10 | 909.9 ± 468.1 | 58.60 ± 16.74 | 0.060 |
| IL6 | 323.1 ± 235.1 | 24.09 ± 8.11 | 0.043 * |
| IL17 | 7.28 ± 5.15 | 4.67 ± 2.37 | 0.664 |
| TNFα | 5.46 ± 1.12 | 4.17 ± 0.89 | 0.300 |
| IFNα | 23.04 ± 13.82 | 18.21 ± 6.99 | 0.473 |
| MCP1 | 12848 ± 4413 | 2221 ± 603.2 | 0.026 * |
| EGF | 3888 ± 851.3 | 3067 ± 1207 | 0.386 |
| ATN (n = 12) | Healthy Controls (n = 6) | p-Value | |
|---|---|---|---|
| I-TAC/CXCL11 | 2 3.07 ± 19.01 | 2.35 ± 1.07 | 0.269 |
| MIG/CXCL9 | 1456 ± 896.2 | 43.37 ± 9.91 | 0.732 |
| IL1B | 14.56 ± 9.73 | 2.03 ± 0.53 | 0.110 |
| CXCL10 | 58.60 ± 16.74 | 35.31 ± 15.3 | 0.298 |
| IL6 | 24.09 ± 8.11 | 3.2 ± 0.2 | 0.105 |
| IL17 | 4.67 ± 2.37 | 2.18 ± 0.65 | 0.944 |
| TNFα | 4.17 ± 0.89 | 2.84 ± 0.27 | 0.301 |
| IFNα | 18.21 ± 6.99 | 27.48 ± 13.8 | 0.387 |
| MCP1 | 2221 ± 603.2 | 418 ± 217.6 | 0.022 * |
| EGF | 3067 ± 1207 | 18748 ± 6454 | 0.655 |
| AUC | Cut-Off | Sensitivity | Specificity | PPV | NPV | LR | |
|---|---|---|---|---|---|---|---|
| I-TAC/CXCL11 | 0.77 | 10.49 pg/mL | 66.70% | 90% | 92.30% | 60% | 6.66 |
| CXCL10 | 0.71 | 131.50 pg/mL | 53% | 100% | 100% | 47.40% | 4.71 |
| IL6 | 0.73 | 31.82 pg/mL | 63.20% | 80% | 85.70% | 53.30% | 3.15 |
| MCP1 | 0.76 | 10,000 pg/mL | 53% | 100% | 100% | 55.60% | 5.3 |
| Blood eosinophil count | 0.68 | 240 | 55% | 81.82% | 84.6% | 50% | 3.03 |
| Urinary leukocyte count | 0.47 | 26/µL | 65% | 27% | 47.2% | 43.8% | 0.9 |
| Model 1 | 84.2% | 83.3% | 88.9% | 76.9% | 5.04 | ||
| Model 2 | 81% | 91.7% | 94.4% | 73.3% | 9.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez Valenzuela, L.; Draibe, J.; Bestard, O.; Fulladosa, X.; Gómez-Preciado, F.; Antón, P.; Nadal, E.; Jové, M.; Cruzado, J.M.; Torras, J. Urinary Cytokines Reflect Renal Inflammation in Acute Tubulointerstitial Nephritis: A Multiplex Bead-Based Assay Assessment. J. Clin. Med. 2021, 10, 2986. https://doi.org/10.3390/jcm10132986
Martinez Valenzuela L, Draibe J, Bestard O, Fulladosa X, Gómez-Preciado F, Antón P, Nadal E, Jové M, Cruzado JM, Torras J. Urinary Cytokines Reflect Renal Inflammation in Acute Tubulointerstitial Nephritis: A Multiplex Bead-Based Assay Assessment. Journal of Clinical Medicine. 2021; 10(13):2986. https://doi.org/10.3390/jcm10132986
Chicago/Turabian StyleMartinez Valenzuela, Laura, Juliana Draibe, Oriol Bestard, Xavier Fulladosa, Francisco Gómez-Preciado, Paula Antón, Ernest Nadal, Maria Jové, Josep Maria Cruzado, and Juan Torras. 2021. "Urinary Cytokines Reflect Renal Inflammation in Acute Tubulointerstitial Nephritis: A Multiplex Bead-Based Assay Assessment" Journal of Clinical Medicine 10, no. 13: 2986. https://doi.org/10.3390/jcm10132986
APA StyleMartinez Valenzuela, L., Draibe, J., Bestard, O., Fulladosa, X., Gómez-Preciado, F., Antón, P., Nadal, E., Jové, M., Cruzado, J. M., & Torras, J. (2021). Urinary Cytokines Reflect Renal Inflammation in Acute Tubulointerstitial Nephritis: A Multiplex Bead-Based Assay Assessment. Journal of Clinical Medicine, 10(13), 2986. https://doi.org/10.3390/jcm10132986







