Endoscopic Papillary Abnormalities and Stone Recognition (EPSR) during Flexible Ureteroscopy: A Comprehensive Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results
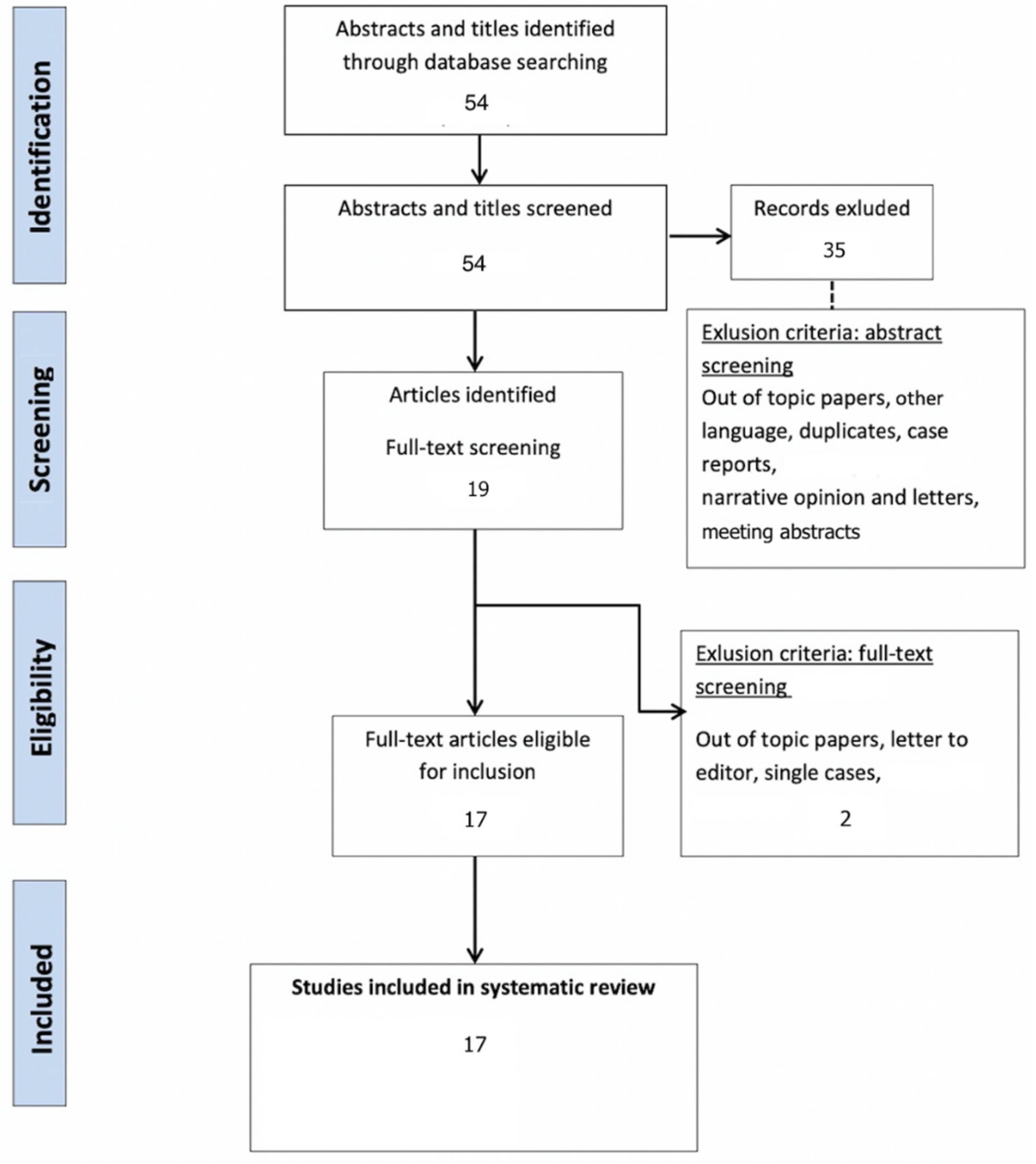
3.1. Study Selection and Characteristics
3.2. Evidence Synthesis
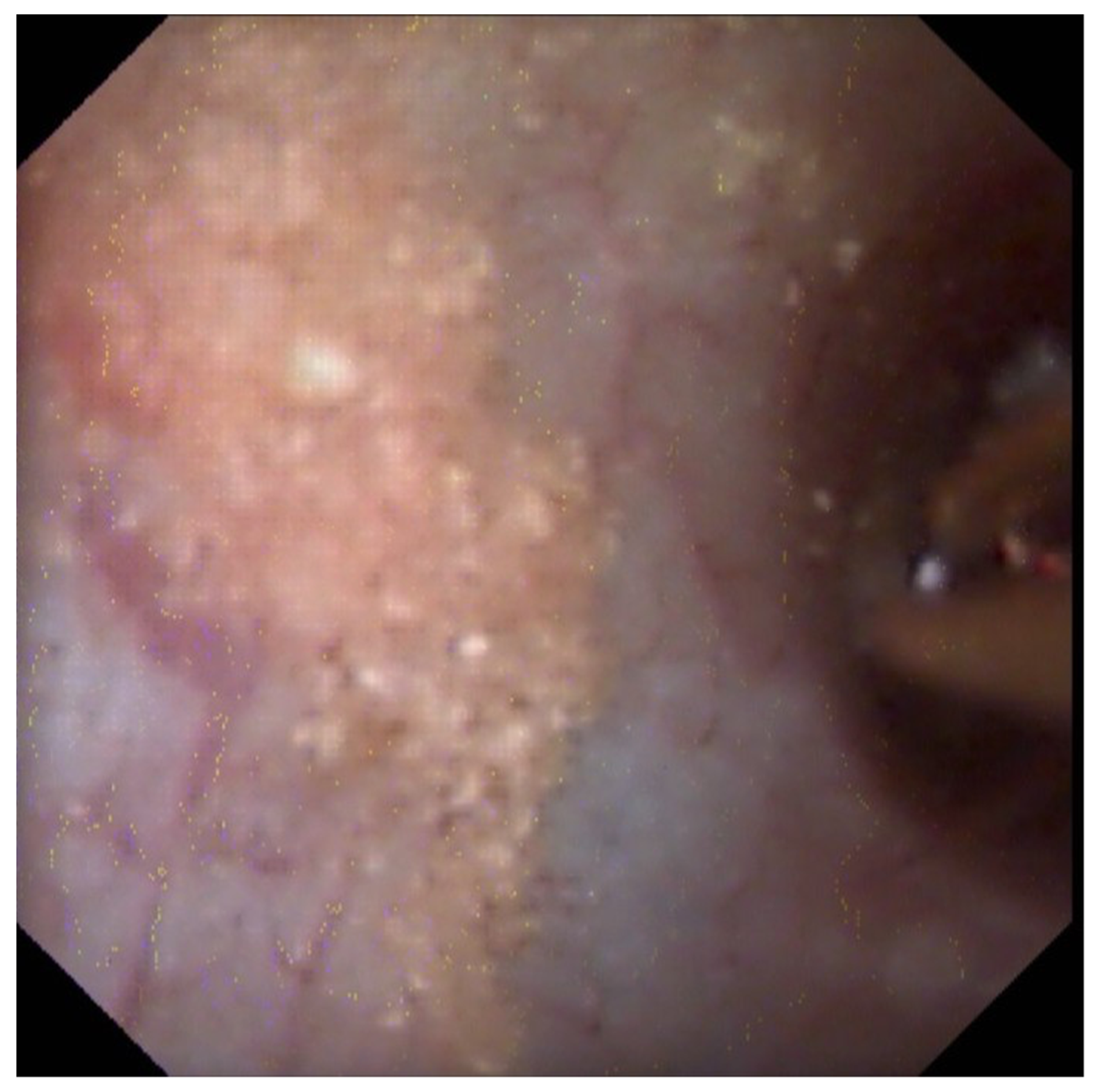
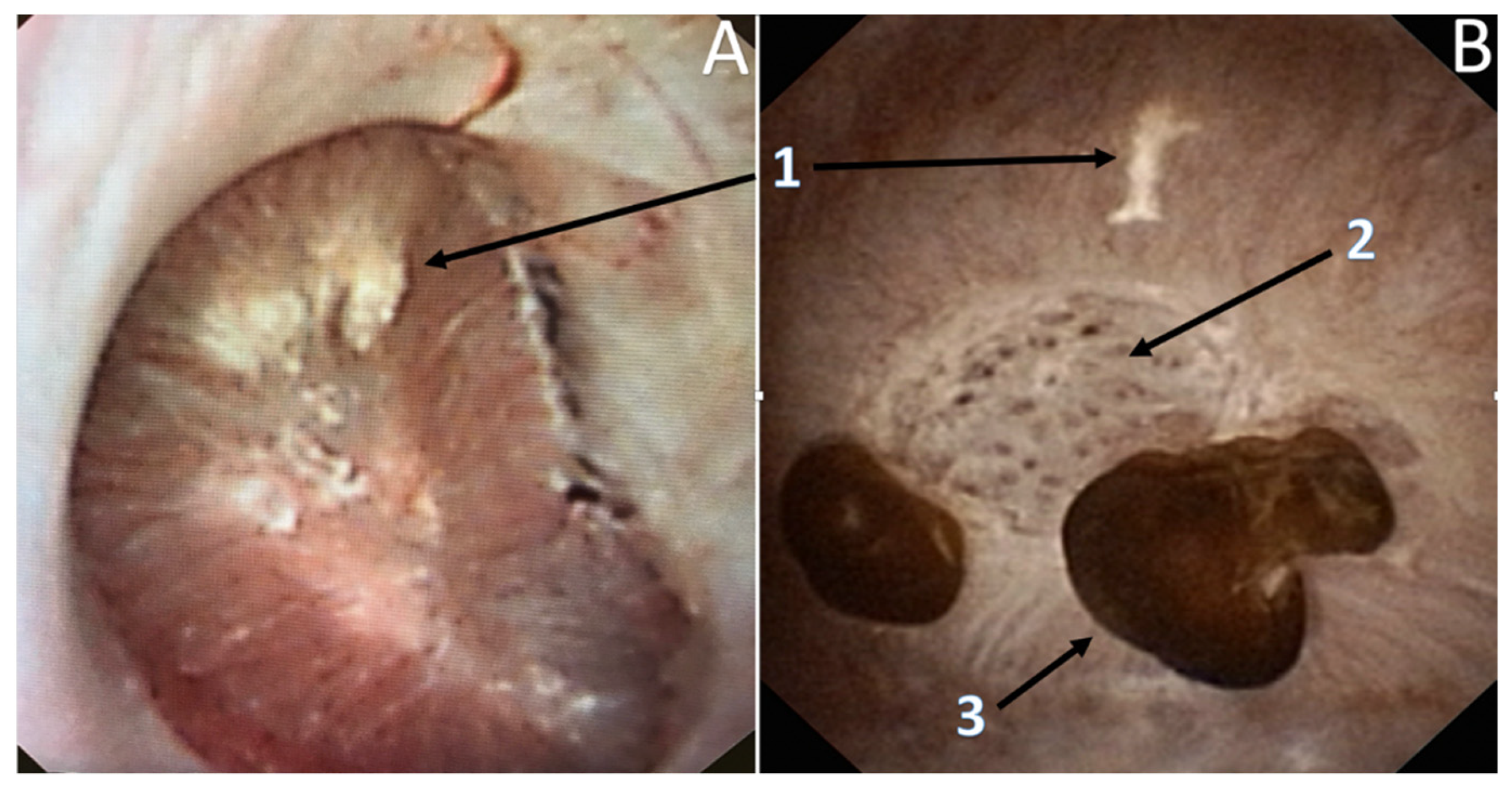
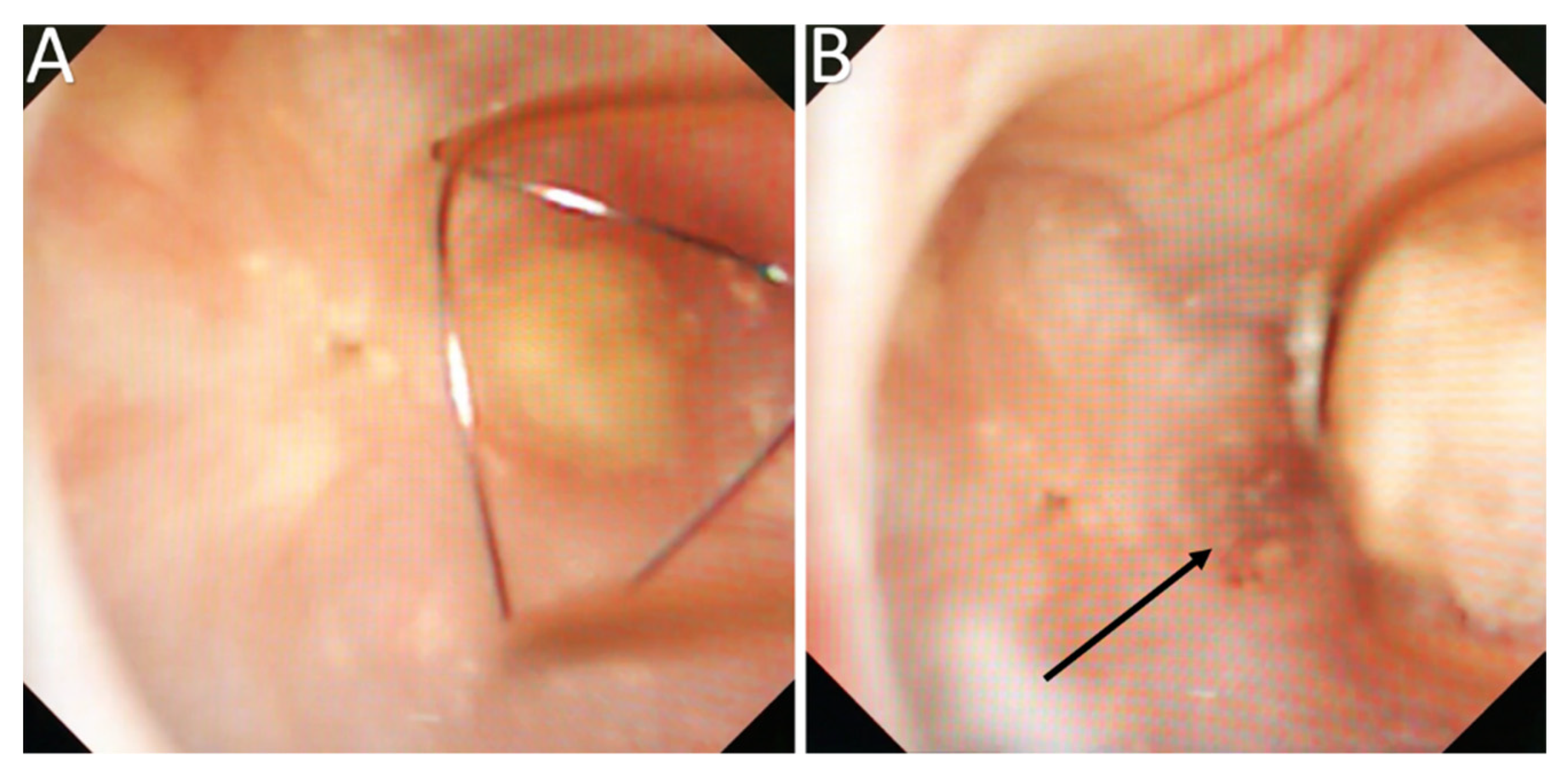
3.3. Endoscopic Papillary Recognition (EPR)
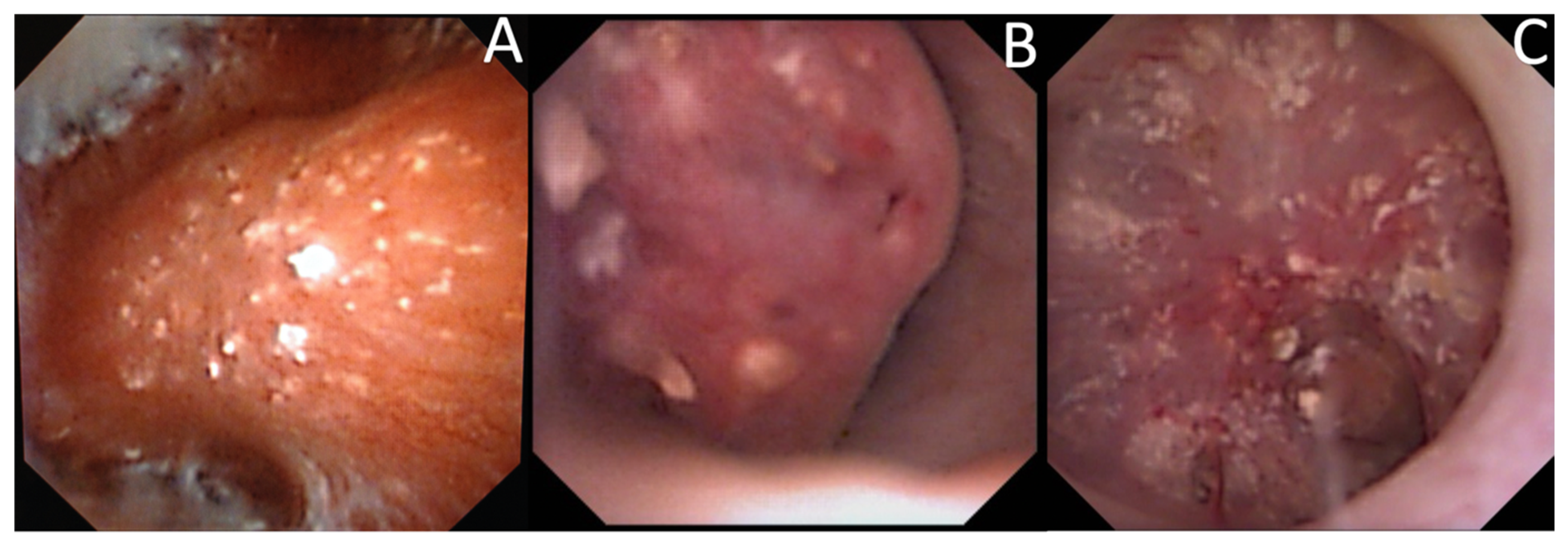
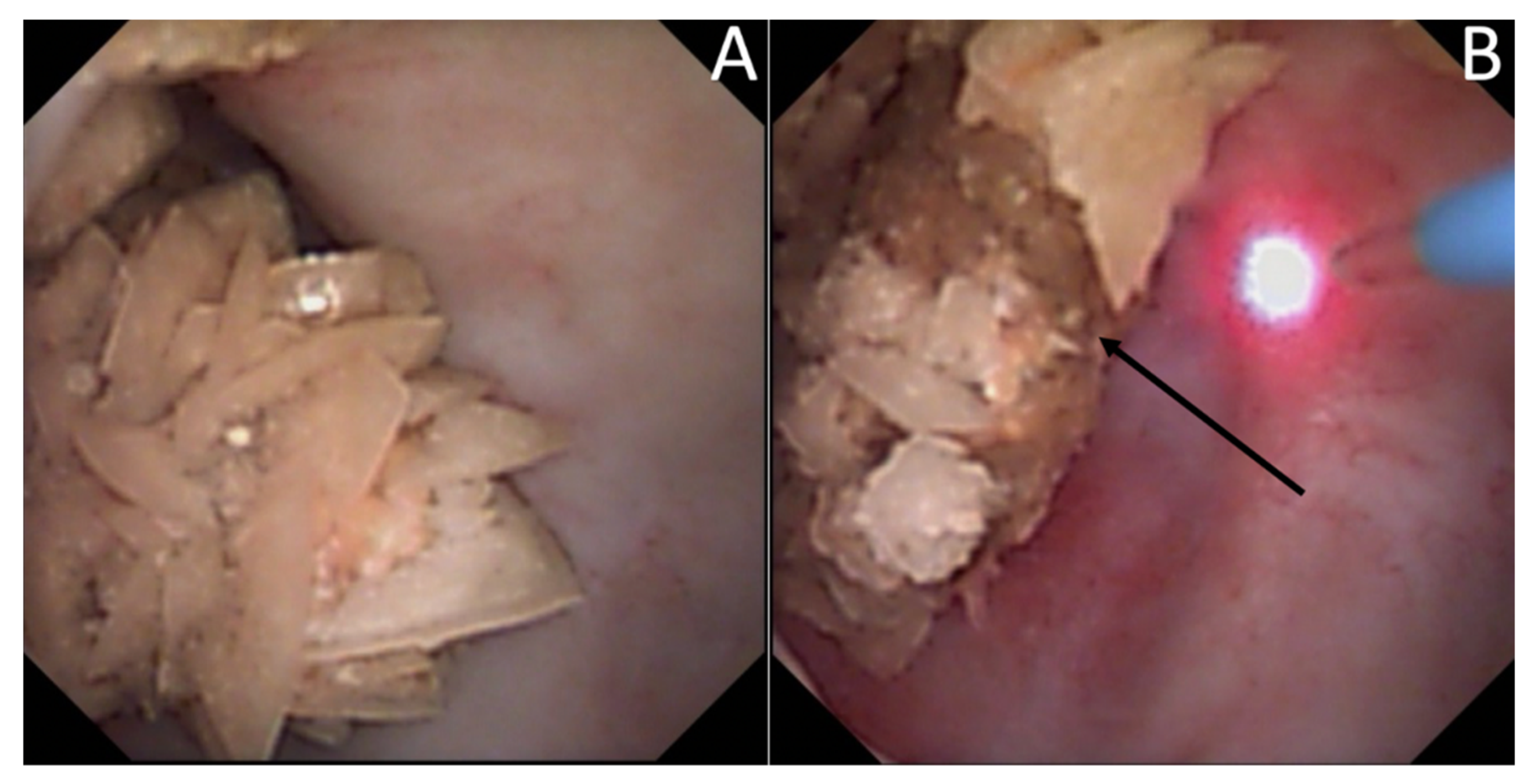
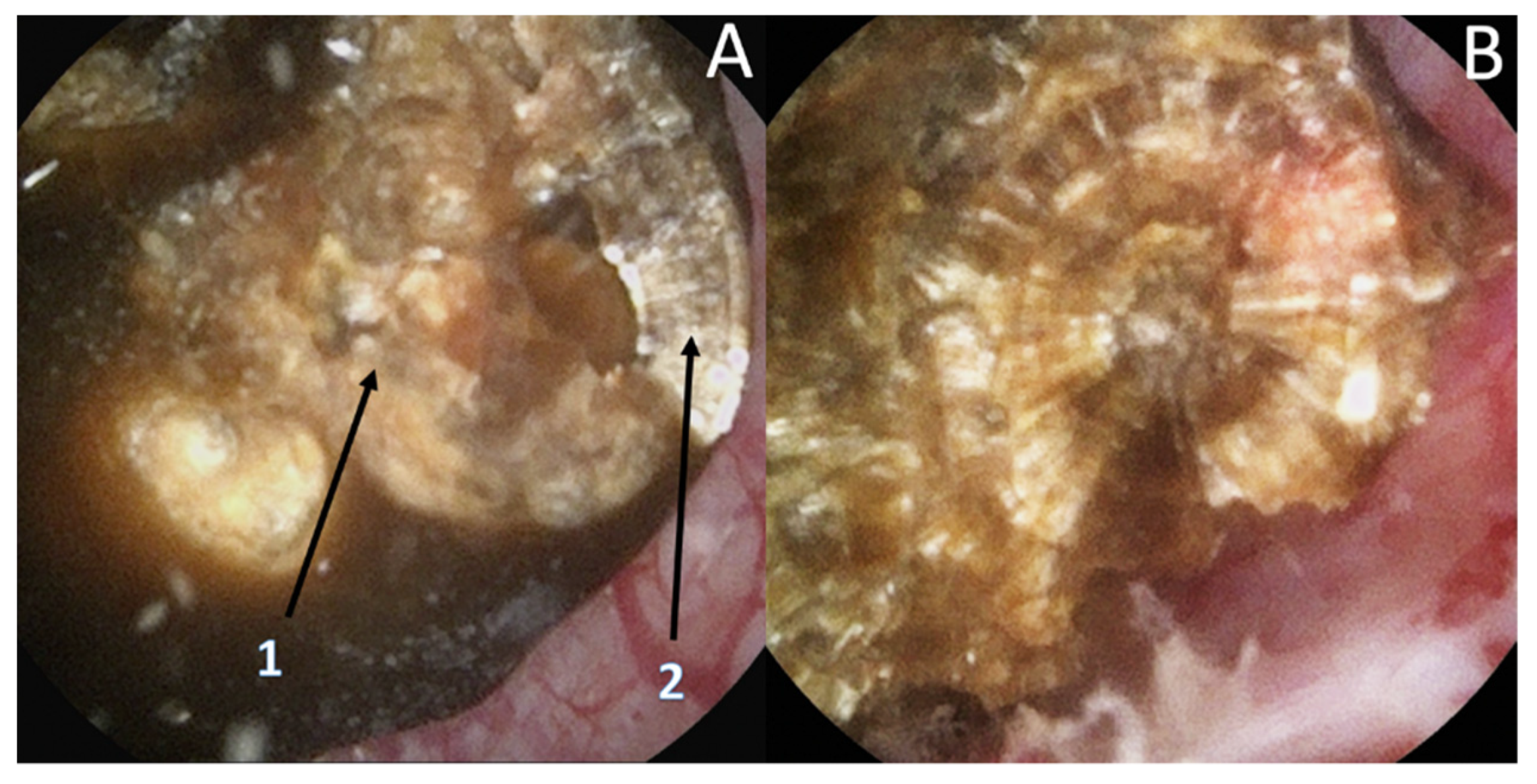
3.4. Endoscopic Stone Recognition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| CA | Carbapatite |
| COD | Calcium Oxalate dihydrate |
| COM | Calcium Oxalate monohydrate |
| CaOx | Calcium Oxalate |
| CP | Calcium Phosphate |
| EPR | Endoscopic Papilla Recognition |
| EPSR | Endoscopic Papilla and Stone Recognition |
| ESR | Endoscopic Stone Recognition |
| RP | Randall’s plaque |
| PICO | Population: Intervention, Comparator, Outcome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| TFL | Thulium Fiber Laser |
| UA | Uric Acid |
References
- Corrales, M.; Doizi, S.; Barghouthy, Y.; Traxer, O.; Daudon, M. Classification of Stones According to Michel Daudon: A Narrative Review. Eur. Urol. Focus 2021, 7, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Jungers, P.; Bazin, D.; Williams, J.C., Jr. Recurrence rates of urinary calculi according to stone composition and morphology. Urolithiasis 2018, 46, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Cloutier, J.; Villa, L.; Traxer, O.; Daudon, M. Kidney stone analysis: “Give me your stone, I will tell you who you are!”. World J. Urol. 2015, 33, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Daudon, M.; Dessombz, A.; Frochot, V.; Letavernier, E.; Haymann, J.-P.; Jungers, P.; Bazin, D. Comprehensive morpho-constitutional analysis of urinary stones improves etiological diagnosis and therapeutic strategy of nephrolithiasis. Comptes Rendus Chim. 2016, 19, 1470–1491. [Google Scholar] [CrossRef]
- Dessombz, A.; Letavernier, E.; Haymann, J.-P.; Bazin, D.; Daudon, M. Calcium Phosphate Stone Morphology Can Reliably Predict Distal Renal Tubular Acidosis. J. Urol. 2015, 193, 1564–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emiliani, E.; Talso, M.; Cho, S.-Y.; Baghdadi, M.; Mahmoud, S.; Pinheiro, H.; Traxer, O. Optimal Settings for the Noncontact Holmium: YAG Stone Fragmentation Popcorn Technique. J. Urol. 2017, 198, 702–706. [Google Scholar] [CrossRef]
- Doizi, S.; Keller, E.X.; De Coninck, V.; Traxer, O. Dusting technique for lithotripsy: What does it mean? Nat. Rev. Urol. 2018, 15, 653–654. [Google Scholar] [CrossRef]
- Keller, E.X.; De Coninck, V.; Audouin, M.; Doizi, S.; Bazin, D.; Daudon, M.; Traxer, O. Fragments and dust after Holmium laser lithotripsy with or without “Moses technology”: How are they different? J. Biophotonics 2018, 12, e201800227. [Google Scholar] [CrossRef]
- Keller, E.X.; De Coninck, V.; Doizi, S.; Daudon, M.; Traxer, O. Thulium fiber laser: Ready to dust all urinary stone composition types? World J. Urol. 2021, 39, 1693–1698. [Google Scholar] [CrossRef]
- Estrade, V.; De Senneville, B.D.; Meria, P.; Almeras, C.; Bladou, F.; Bernhard, J.; Robert, G.; Traxer, O.; Daudon, M. Toward improved endoscopic examination of urinary stones: A concordance study between endoscopic digital pictures vs microscopy. BJU Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Randall, A. The origin and growth of renal calculi. Ann. Surg. 1937, 105, 1009–1027. [Google Scholar] [CrossRef]
- Daudon, M.; Bazin, D.; Letavernier, E. Randall’s plaque as the origin of calcium oxalate kidney stones. Urolithiasis 2014, 43 (Suppl. 1), 5–11. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.C., Jr.; Borofsky, M.S.; Bledsoe, S.B.; Evan, A.P.; Coe, F.L.; Worcester, E.M.; Lingeman, J.E. Papillary ductal plugging is a mechanism for early stone retention in brushite stone disease. J. Urol. 2018, 199, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Coe, F.L.; Evan, A.P.; Worcester, E.M.; Lingeman, J.E. Three pathways for human kidney stone formation. Urol. Res. 2010, 38, 147–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evan, A.; Lingeman, J.; Coe, F.L.; Worcester, E. Randall’s plaque: Pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006, 69, 1313–1318. [Google Scholar] [CrossRef] [Green Version]
- Evan, A.P.; Lingeman, J.E.; Coe, F.L.; Parks, J.H.; Bledsoe, S.B.; Shao, Y.; Sommer, A.J.; Paterson, R.F.; Kuo, R.L.; Grynpas, M. Randall’s plaque of patients with nephrolithiasis begins in basement mem-branes of thin loops of Henle. J. Clin. Investig. 2003, 111, 607–616. [Google Scholar] [CrossRef] [Green Version]
- Evan, A.; Lingeman, J.; Coe, F.; Shao, Y.; Miller, N.; Matlaga, B.; Phillips, C.; Sommer, A.; Worcester, E. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int. 2007, 71, 795–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, R.K.; Stoller, M.L. Endoscopic mapping of renal papillae for Randall’s plaques in patients with urinary stone dis-ease. J. Urol. 1997, 158, 2062–2064. [Google Scholar] [CrossRef]
- Matlaga, B.R.; Williams, J.; Kim, S.C.; Kuo, R.L.; Evan, A.P.; Bledsoe, S.B.; Coe, F.L.; Worcester, E.M.; Munch, L.C.; Lingeman, J.E. Endoscopic Evidence of Calculus Attachment to Randall’s Plaque. J. Urol. 2006, 175, 1720–1724. [Google Scholar] [CrossRef]
- Borofsky, M.S.; Paonessa, J.E.; Evan, A.P.; Williams, J.C.; Coe, F.L.; Worcester, E.M.; Lingeman, J.E. A proposed grading system to standardize the description of renal papil-lary appearance at the time of endoscopy in patients with nephrolithiasis. J. Endourol. 2016, 30, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Almeras, C.; Daudon, M.; Ploussard, G.; Gautier, J.R.; Traxer, O.; Meria, P. Endoscopic description of renal papillary abnormalities in stone disease by flexible ureteroscopy: A proposed classification of severity and type. World J. Urol. 2016, 34, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, C.D.; Rule, A.D.; Mehta, R.A.; Vaughan, L.E.; Vrtiska, T.J.; Holmes, D.R.; McCollough, C.M.; Ziegelmann, M.J.; Hernandez, L.P.H.; Lieske, J.C.; et al. Endoscopic and Pathologic Characterization of Papillary Architecture in Struvite Stone Formers. Urology 2016, 90, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.J.; Borofsky, M.S.; Anderson, B.B.; Dauw, C.A.; Gillen, D.L.; Gerber, G.S.; Worcester, E.M.; Coe, F.L.; Lingeman, J.E. Endoscopic Evidence That Randall’s Plaque is Associated with Surface Erosion of the Renal Papilla. J. Endourol. 2017, 31, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borofsky, M.S.; Williams, J.C., Jr.; Dauw, C.A.; Cohen, A.; Evan, A.C.; Coe, F.L.; Worcester, E.M.; Lingeman, J.E.; Coe, F. Association between Randall’s Plaque Stone Anchors and Renal Papillary Pits. J. Endourol. 2019, 33, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Almeras, C.; Daudon, M.; Estrade, V.; Gautier, J.R.; Traxer, O.; Meria, P. Classification of the renal papillary abnormalities by flexible ureteroscopy: Evaluation of the 2016 version and update. World J. Urol. 2021, 39, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pless, M.S.; Williams, J.C.; Andreassen, K.H.; Jung, H.; Osther, S.S.; Christensen, D.R.; Osther, P.J.S. Endoscopic observations as a tool to define underlying pathology in kidney stone formers. World J. Urol. 2019, 37, 2207–2215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darves-Bornoz, A.L.; Marien, T.; Thomas, J.; Fiscus, G.; Brock, J.; Clayton, D.B.; Miller, N.L. Renal Papillary Mapping and Quantification of Randall’s Plaque in Pediatric Calcium Oxalate Stone Formers. J. Endourol. 2019, 33, 863–867. [Google Scholar] [CrossRef]
- Arroyo, X.S.; Freixedas, F.G.; Quetglas, J.L.B.; Garcia, J.G.; Ayala, E.P. Relationship of endoscopic lesions of the renal papilla with type of renal stone and 24 h urine analysis. BMC Urol. 2020, 20, 1–6. [Google Scholar] [CrossRef]
- Strohmaier, W.L.; Hörmann, M.; Schubert, G. Papillary calcifications: A new prognostic factor in idiopathic calcium oxalate urolithiasis. Urolithiasis 2013, 41, 475–479. [Google Scholar] [CrossRef]
- Kim, S.C.; Coe, F.L.; Tinmouth, W.W.; Kuo, R.L.; Paterson, R.F.; Parks, J.H.; Munch, L.C.; Evan, A.P.; Lingeman, J.E. Stone formation is proportional to papillary surface coverage by randall’s plaque. J. Urol. 2005, 173, 117–119. [Google Scholar] [CrossRef]
- Wang, X.; Krambeck, A.E.; Williams, J.C.; Tang, X.; Rule, A.D.; Zhao, F.; Bergstralh, E.; Haskic, Z.; Edeh, S.; Holmes, D.R.; et al. Distinguishing Characteristics of Idiopathic Calcium Oxalate Kidney Stone Formers with Low Amounts of Randall’s Plaque. Clin. J. Am. Soc. Nephrol. 2014, 9, 1757–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, K.; Korinek, M.; Camp, J.; Lieske, J.; Holmes, D. Automatic detection of calcium phosphate deposit plugs at the terminal ends of kidney tubules. Healthc. Technol. Lett. 2019, 6, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Marien, T.P.; Miller, N.L. Advanced ureteroscopy for stone disease: Characteristics of renal papillae in kidney stone formers. Minerva Urol. Nefrol. 2016, 68, 496–515. [Google Scholar]
- Keller, E.X.; Doizi, S.; Villa, L.; Traxer, O. Which flexible ureteroscope is the best for upper tract urothelial carcinoma treatment? World J. Urol. 2019, 37, 2325–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciudin, A.; Luque, M.P.; Salvador, R.; Diaconu, M.G.; Franco, A.; Constantin, V.; Alvarez-Vijande, R.; Nicolau, C.; Alcaraz, A. Abdominal Computed Tomography—A New Tool for Predicting Recurrent Stone Disease. J. Endourol. 2013, 27, 965–969. [Google Scholar] [CrossRef]
- Kuo, R.L.; Lingeman, J.E.; Evan, A.P.; Paterson, R.F.; Parks, J.H.; Bledsoe, S.B.; Munch, L.C.; Coe, F.L. Urine calcium and volume predict coverage of renal papilla by Randall’s plaque. Kidney Int. 2003, 64, 2150–2154. [Google Scholar] [CrossRef] [Green Version]
- Coe, F.L.; Worcester, F.L.C.E.M.; Evan, A.P. Idiopathic hypercalciuria and formation of calcium renal stones. Nat. Rev. Nephrol. 2016, 12, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Bouderlique, E.; Tang, E.; Perez, J.; Coudert, A.; Bazin, D.; Verpont, M.-C.; Duranton, C.; Rubera, I.; Haymann, J.-P.; Leftheriotis, G.; et al. Vitamin D and Calcium Supplementation Accelerates Randall’s Plaque Formation in a Murine Model. Am. J. Pathol. 2019, 189, 2171–2180. [Google Scholar] [CrossRef]
- Siener, R.; Hesse, A. Fluid intake and epidemiology of urolithiasis. Eur. J. Clin. Nutr. 2003, 57, S47–S51. [Google Scholar] [CrossRef] [Green Version]
- Kittanamongkolchai, W.; Vaughan, L.E.; Enders, F.T.; Dhondup, T.; Mehta, R.A.; Krambeck, A.E.; McCollough, C.H.; Vrtiska, T.J.; Lieske, J.C.; Rule, A.D. The Changing Incidence and Presentation of Urinary Stones Over 3 Decades. Mayo Clin. Proc. 2018, 93, 291–299. [Google Scholar] [CrossRef]
- Robertson, W.; Peacock, M. The Pattern of Urinary Stone Disease in Leeds and in the United Kingdom in Relation to Animal Protein Intake during the Period 1960–1980. Urol. Int. 1982, 37, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Siener, R. The Effect of Different Diets on Urine Composition and the Risk of Calcium Oxalate Crystallisation in Healthy Subjects. Eur. Urol. 2002, 42, 289–296. [Google Scholar] [CrossRef]
- Bergot, C.; Robert, G.; Bernhard, J.C.; Ferrière, J.-M.; Bensadoun, H.; Capon, G.; Estrade, V. The basis of endoscopic stones recognition, a prospective monocentric study [Article in French]. Prog. Urol. 2019, 29, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Black, K.M.; Law, H.; Aldoukhi, A.; Deng, J.; Ghani, K.R. Deep learning computer vision algorithm for detecting kidney stone composition. BJU Int. 2020, 125, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Marchini, G.S.; Batagello, C.A.; Monga, M.; Torricelli, F.C.M.; Vicentini, F.C.; Danilovic, A.; Srougi, M.; Nahas, W.C.; Mazzucchi, E. In Vitro Evaluation of Single-Use Digital Flexible Ureteroscopes: A Practical Comparison for a Patient-Centered Approach. J. Endourol. 2018, 32, 184–191. [Google Scholar] [CrossRef]
- Dragos, L.B.; Somani, B.; Keller, E.X.; De Coninck, V.M.J.; Herrero, M.R.-M.; Kamphuis, G.M.; Bres-Niewada, E.; Sener, E.T.; Doizi, S.; Wiseman, O.J.; et al. Characteristics of current digital single-use flexible ureteroscopes versus their reusable counterparts: An in-vitro comparative analysis. Transl. Androl. Urol. 2019, 8 (Suppl. 4), S359–S370. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Agrawal, S.; Singh, A.; Ganpule, A.; Sabnis, R.; Desai, M. A Single-Center Prospective Comparative Study of Two Single-Use Flexible Ureteroscopes: LithoVue (Boston Scientific, USA) and Uscope PU3022a (Zhuhai Pusen, China). J. Endourol. 2021, 35, 274–278. [Google Scholar] [CrossRef]


| Type | Subject | Number | Year | |
|---|---|---|---|---|
| Low [18] | EPR | RP | 57 | 1997 |
| Darves-Bornoz [27] | EPR | RP in pediatric stone formers | 8 | 2019 |
| Strohmaier [29] | EPR | RP and number of stone episodes | 100 | 2013 |
| Kim [30] | EPR | RP and number of stones | 17 | 2005 |
| Wang [31] | EPR | Low RP and CaOx stone formers | 42 | 2014 |
| Matlaga [19] | EPR | Anchored stone and RP | 23 | 2006 |
| Borofsky [20] | EPR | Grading Score | 342 | 2016 |
| Almeras [21] | EPR | Classification | 164 | 2016 |
| Jaeger [22] | EPR | Struvite | 119 | 2016 |
| Cohen [23] | EPR | Score use and correlation RP/pitting | 76 | 2019 |
| Borofsky [24] | EPR | Anchored stone/pitting | 28 | 2019 |
| Almeras [25] | EPR | Classification use and correlations RP, stones, … | 88 | 2021 |
| Pless [26] | EPR | Score use | 46 | 2019 |
| Sabaté [28] | EPR | Description | 41 | 2020 |
| Fernandez [32] | EPR | CP plugs detection by AI | 200 | 2019 |
| Estrade [10] | ESR | Correlation endoscopy/microscopy | 399 | 2020 |
| Marien [33] | EPR | Review | 13 | 2016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeras, C.; Pradere, B.; Estrade, V.; Meria, P.; on behalf of the Lithiasis Committee of the French Urological Association. Endoscopic Papillary Abnormalities and Stone Recognition (EPSR) during Flexible Ureteroscopy: A Comprehensive Review. J. Clin. Med. 2021, 10, 2888. https://doi.org/10.3390/jcm10132888
Almeras C, Pradere B, Estrade V, Meria P, on behalf of the Lithiasis Committee of the French Urological Association. Endoscopic Papillary Abnormalities and Stone Recognition (EPSR) during Flexible Ureteroscopy: A Comprehensive Review. Journal of Clinical Medicine. 2021; 10(13):2888. https://doi.org/10.3390/jcm10132888
Chicago/Turabian StyleAlmeras, Christophe, Benjamin Pradere, Vincent Estrade, Paul Meria, and on behalf of the Lithiasis Committee of the French Urological Association. 2021. "Endoscopic Papillary Abnormalities and Stone Recognition (EPSR) during Flexible Ureteroscopy: A Comprehensive Review" Journal of Clinical Medicine 10, no. 13: 2888. https://doi.org/10.3390/jcm10132888
APA StyleAlmeras, C., Pradere, B., Estrade, V., Meria, P., & on behalf of the Lithiasis Committee of the French Urological Association. (2021). Endoscopic Papillary Abnormalities and Stone Recognition (EPSR) during Flexible Ureteroscopy: A Comprehensive Review. Journal of Clinical Medicine, 10(13), 2888. https://doi.org/10.3390/jcm10132888







