Association between Oral Hygiene and Metabolic Syndrome: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
2.5. Statistical Analyses
3. Results
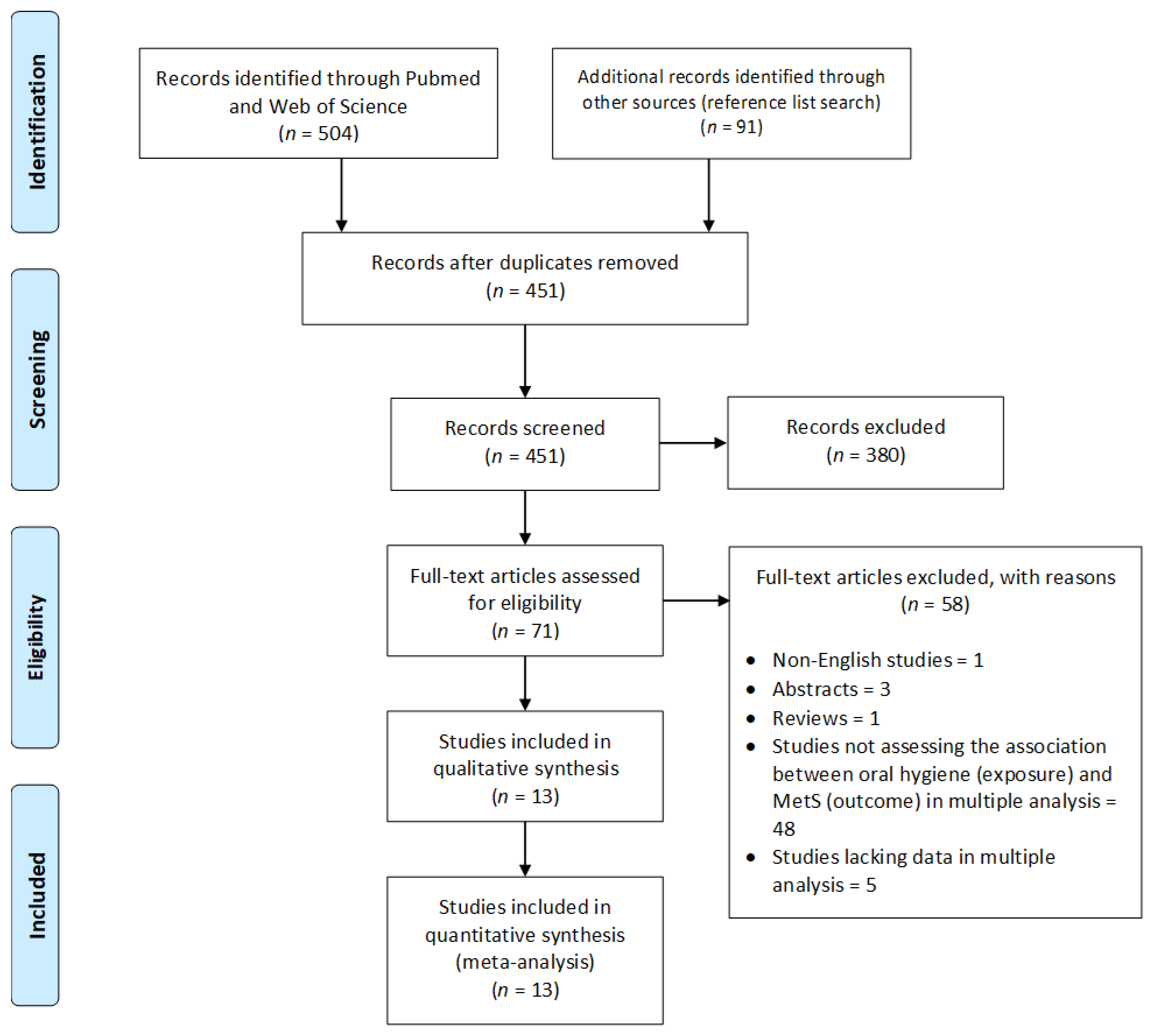
3.1. Literature Search
3.2. Characteristics of Studies
| Author, Publication Year | Country | Study Design | Sample Size (M, F) | Age Range | Type of Oral Hygiene | Diagnostic Criteria for MetS | Number of Cases | Statistical Analysis; Adjustments | Association |
|---|---|---|---|---|---|---|---|---|---|
| Fukui et al., 2012 [45] | Japan | Cross-sectional | 6421 (M: 4944, F: 1477) | 34–77 | Tooth-brushing frequency (times/day) | Modified NCEP ATP III *, except the use of BMI ≥ 25 kg/m2 to define obesity. Treatments for raised TG and reduced HDL were not recorded. | 958 | Logistic regression; age, gender, smoking habit, alcohol consumption, C-reactive protein, number of teeth, periodontal parameter (PD or CAL). | OR (95% CI) Adjusted by PD: ≤1 time daily (reference) 2 times daily = 0.67 (0.57–0.78) ≥3 times daily = 0.50 (0.40–0.64) Adjusted by CAL: ≤1 time daily [reference] 2 times daily = 0.66 (0.57–0.77) ≥3 times daily = 0.50 (0.39–0.63) |
| Kim et al., 2013 [44] | South Korea | Cross-sectional | 18742 (M: 8034, F: 10708) | ≥19 | Tooth-brushing frequency (times/day), use of dental floss (yes or no), use of interdental brush (yes or no) | Modified NCEP ATP III * for Asians. | 5878 | Logistic regression; age, gender, income, education, smoking, alcohol intake, and physical activities. | OR (95% CI) Tooth-brushing frequency: ≥3 times daily (reference) 2 times daily = 1.23 (1.12–1.34) ≤1 time daily = 1.23 (1.04–1.47) Use of dental floss: Yes [reference] No = 1.23 (1.07–1.41) Use of interdental brush: Yes [reference] No = 1.05 (0.92–1.20) |
| Tsutsumi and Kakuma, 2015 [43] | Japan | Cross-sectional | 12548 (M: 7703, F: 4845) | 30–59 | Tooth-brushing frequency (times/day) | Obesity (body mass percentage ≥ 20% in men or ≥30% in women, and/or BMI ≥ 25 kg/m2) and at least one of the following: TG ≥ 150 mg/dL and/or low HDL < 40 mg/dL or drug for hypertriglyceridemia, SBP ≥ 130 mm Hg and/or DBP ≥ 85 mm Hg or drug for hypertension, FPG ≥ 110 mg/dL or drug for diabetes). | 3624 | Logistic regression; Males: age, exercise during holidays, favorite seasoning, eating soup, sugar in coffee, having an interest in losing weight, housekeeping during holidays; Females: age, favorite seasoning, worrying about job, sugar in coffee, pickles and food boiled in soy sauce, exercise during holidays, eating quickly, preparation of dinner, solving problems immediately. | OR (95% CI) Males: None (reference) 1 time daily = 0.57 (0.40–0.81) 2 times daily = 0.50 (0.35–0.71) ≥3 times daily = 0.42 (0.29–0.61) Females: ≤1 time daily (reference) 2 times daily = 0.65 (0.48–0.87) ≥3 times daily = 0.44 (0.32–0.62) |
| Kim et al., 2019 [46] | South Korea | Cross-sectional | 8314 (M: 3860, F: 4454) | 35–79 | Tooth-brushing frequency (times/day) | Three or more of the following five: WC ≥ 90 cm in men or ≥85 cm in women, TG > 150 mg/dL or treatment for raised TG, HDL <40 mg/dL in men or <50 mg/dL in women or treatment for reduced HDL, SBP ≥ 130 mm Hg and DBP ≥ 85 mm Hg or antihypertensive medication, FPG ≥ 100 mg/dL or current use of antidiabetic medication. | 2834 | Logistic regression; age, gender, household income, education, smoking, alcohol intake, physical activity, periodontitis. | OR (95% CI) Frequency of daily tooth-brushing (continuous) = 0.887 (0.84–0.94) |
| Saito et al., 2019 [47] | Japan | Cross-sectional | 2379 (M: 960, F: 1419) | 75 and 80 | Use of secondary oral hygiene products, such as dental floss or interdental brushes (none or sometimes or every day) | JIS ǂ, except the use of BMI ≥ 25 kg/m2 to define obesity and the use of HbA1c levels ≥ 5.6% to additionally define elevated glucose. Treatments for raised TG and reduced HDL were not included. | 563 | Logistic regression; age, gender, smoking, exercise, weight gain, eating speed, cholesterol drug intake, community periodontal index, number of teeth. | OR (95% CI) None (reference) Sometimes = 1.19 (0.92–1.54) Everyday = 0.71 (0.55–0.92) |
| Shearer et al., 2018 [32] | New Zealand | Cross-sectional | 836 | 38 | Modified OHI-S (very low (0–0.5) or low (>0.5–1.0) or moderate (>1.0–1.5) or high (>1.5)) | NCEP ATP III ¤, except the use of HbA1c ≥ 5.7% (≥39 mmol/mol) to define elevated glucose and the use of antihypertensive drugs to additionally define elevated blood pressure. | 152 | Logistic regression; gender, low socioeconomic status, smoking, dysglycemia, inflammatory load. | OR (95% CI) Low (reference) High = 0.95 (0.44, 2.01) |
| Chen et al., 2011 [48] | Taiwan | Cross-sectional | 253 (M:117, F: 136) | >18 | PI | Modified NCEP ATP III * for Asians, except the use of FPG ≥ 110 mg/dL or previously diagnosed T2DM to define elevated glucose. | 145 | Logistic regression; age, gender, education, smoking, high-sensitivity C-reactive protein, and serum albumin. | OR (95% CI) PI score (continuous) = 1.724 (1.135–2.615) |
| Kobayashi et al., 2012 [30] | Japan | Cohort prospective, 3-year follow-up | 685 (M: 513, F: 172) | - | Tooth-brushing frequency (times/day) | JIS ǂ for Asians, except not including treatments for raised TG, reduced HDL, and elevated glucose. | 99 | Logistic regression; age, gender, smoking status, drinking status, breakfast eating, educational level, occupation (desk work or non-desk work), depressive symptoms, physical activity, and total caloric consumption. | OR (95% CI) ≤1 time daily (reference) 2 times daily = 0.80 (0.49–1.31) ≥3 times daily = 0.43 (0.19–0.97) |
| Tanaka et al., 2018 [23] | Japan | Cohort retrospective, 5-year follow-up | 3722 (M: 2897, F: 825) | 35–64 | Tooth-brushing frequency (times/day), dental check-ups (regular or irregular) | JIS ǂ for Asians, except the use of BMI ≥ 25 kg/m2 to define obesity. | 412 | Logistic regression; age, gender, periodontal status, number of present teeth, occupational status, smoking quantity, alcohol consumption, physical activity, dietary behavior, food preference, tooth-brushing frequency, dental check-ups, and number of MetS components at baseline. | OR (95% CI) Tooth-brushing frequency: ≤1 time daily (reference) 2 times daily = 0.83 (0.65–1.05) ≥3 times daily = 0.64 (0.45–0.91) Dental check-ups: Irregular (reference) Regular = 1.10 (0.77–1.55) |
| Pussinen et al., 2020 [31] | Finland | Cohort prospective, 21-, 27-, 31-year follow-up | 586 (M: 270, F: 316) | 27–43 | Presence of visible plaque (yes or no) | JIS ǂ for Europeans. | 153 | Poisson regression; age, gender, childhood BMI, family income, adulthood smoking (ever) and socioeconomic status (education), and interaction terms between caries and periodontal parameters. | RR (95% CI) No (reference) Yes = 1.21 (0.87–1.86) |
| Pham, 2018 [29] | Vietnam | Case–control (case = 206, control = 206) | 412 (M: 114, F: 298) | 50–78 | PI (≤2.5 or 2.51–2.90 or 2.91–3.26 or ≥3.27) | JIS ǂ for Asians. | 206 | Logistic regression; age, gender. | OR (95% CI) ≤2.5 (reference) 2.51–2.90 = 4.81 (1.74–13.27) 2.91–3.26 = 6.12 (2.24–16.70) ≥3.27 = 7.50 (2.80–20.12) |
| Li et al., 2009 [49] | China | Case–control (case = 152, control = 56) | 208 (M: 85, F: 123) | 37–78 | PI (≤1 or >1–1.5 or >1.5–2 or >2) | IDF § | 152 | Logistic regression; age, gender, smoking. | OR (95% CI) ≤1 (reference) >1–1.5 = 4.81 (0.81–28.63) >1.5–2 = 13.06 (2.24–76.18) >2 = 47.4 (6.94–323.68) |
| Li et al., 2020 [50] | China | Case–control (case = 114, control = 49) | 163 (M: 60, F: 103) | 37–78 | PI | IDF § | 114 | Logistic regression (backward); age, gender, smoking habits, bleeding index, PD, biomarkers (serum C-reactive protein, salivary IL-6, IL-1β). | OR (95% CI) PI score (continuous) = 14.69 (5.56–38.84) |
3.3. Quality Aspects of Studies
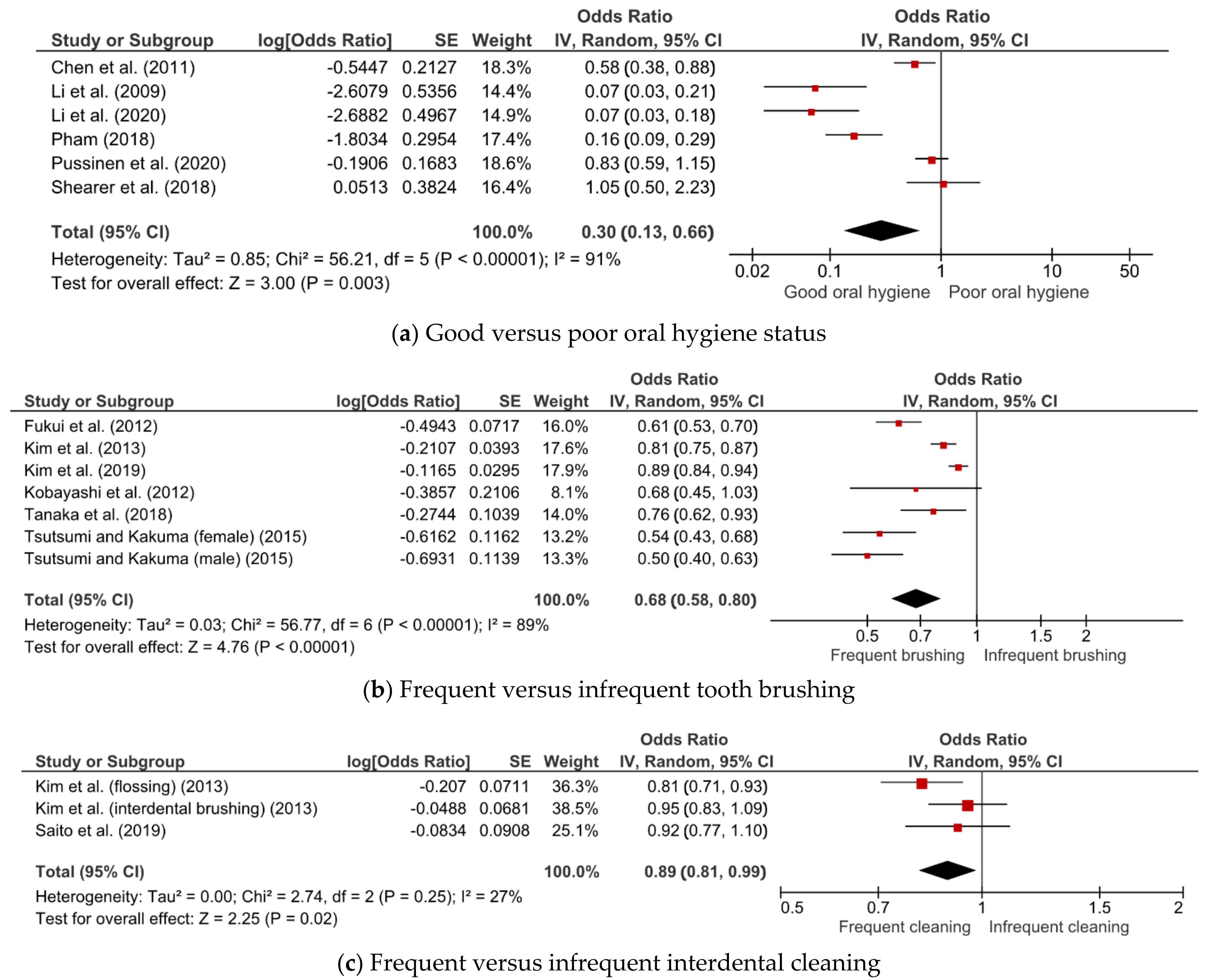
3.4. Association between Oral Hygiene Status, Care, and MetS
3.5. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Lear, S.A.; Gasevic, D. Ethnicity and metabolic syndrome: Implications for assessment, management and prevention. Nutrients 2020, 12, 15. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.H.; Hammill, B.G.; Bethel, M.A.; Anstrom, K.J.; Gottdiener, J.S.; Schulman, K.A. Costs of the metabolic syndrome in elderly individuals: Findings from the Cardiovascular Health Study. Diabetes Care 2007, 30, 2553–2558. [Google Scholar] [CrossRef]
- Blanquet, M.; Legrand, A.; Pélissier, A.; Mourgues, C. Socio-economics status and metabolic syndrome: A meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Liu, J.; Ning, G. Active Smoking and Risk of Metabolic Syndrome: A Meta-Analysis of Prospective Studies. PLoS ONE 2012, 7, e47791. [Google Scholar] [CrossRef]
- Fabiani, R.; Naldini, G.; Chiavarini, M. Dietary patterns and metabolic syndrome in adult subjects: A systematic review and meta-analysis. Nutrients 2019, 11, 2056. [Google Scholar] [CrossRef]
- Joseph, M.S.; Tincopa, M.A.; Walden, P.; Jackson, E.; Conte, M.L.; Rubenfire, M. The impact of structured exercise programs on metabolic syndrome and its components: A systematic review. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2395–2404. [Google Scholar] [CrossRef]
- Gobin, R.; Tian, D.; Liu, Q.; Wang, J. Periodontal Diseases and the Risk of Metabolic Syndrome: An Updated Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 1035–1057. [Google Scholar] [CrossRef]
- Cao, X.; Wang, D.; Zhou, J.; Yuan, H.; Chen, Z. Relationship between dental caries and metabolic syndrome among 13 998 middle-aged urban Chinese. J. Diabetes 2017, 9, 378–385. [Google Scholar] [CrossRef]
- Ojima, M.; Amano, A.; Kurata, S. Relationship between decayed teeth and metabolic syndrome: Data from 4716 middle-aged male Japanese employees. J. Epidemiol. 2015, 25, 204–211. [Google Scholar] [CrossRef]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, K.; Salminen, A.; Salomaa, V.; Pussinen, P.J. Systemic exposure to a common periodontal pathogen and missing teeth are associated with metabolic syndrome. Acta Diabetol. 2015, 52, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Woo, H.G.; Park, J.; Lee, J.S.; Song, T.J. Improved oral hygiene care is associated with decreased risk of occurrence for atrial fibrillation and heart failure: A nationwide population-based cohort study. Eur. J. Prev. Cardiol. 2020, 27, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.S.; Blattner, T.C.; Sant’Ana Filho, M.; Grecca, F.S.; Hugo, F.N.; Fouad, A.F.; Reynolds, M.A. Can apical periodontitis modify systemic levels of inflammatory markers? A systematic review and meta-analysis. J. Endod. 2013, 39, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Cantos, A. Oral inflammation and infection, and chronic medical diseases: Implications for the elderly. Periodontol. 2000 2016, 72, 153–175. [Google Scholar] [CrossRef]
- De Rooij, S.R.; Nijpels, G.; Nilsson, P.M.; Nolan, J.J.; Gabriel, R.; Bobbioni-Harsch, E.; Mingrone, G.; Dekker, J.M. Low-grade chronic inflammation in the relationship between insulin sensitivity and cardiovascular disease (RISC) population: Associations with insulin resistance and cardiometabolic risk profile. Diabetes Care 2009, 32, 1295–1301. [Google Scholar] [CrossRef]
- León-Pedroza, J.I.; González-Tapia, L.A.; del Olmo-Gil, E.; Castellanos-Rodríguez, D.; Escobedo, G.; González-Chávez, A. Low-grade systemic inflammation and the development of metabolic diseases: From the molecular evidence to the clinical practice. Cir. Cir. 2015, 83, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Claydon, N.C. Current concepts in toothbrushing and interdental cleaning. Periodontol. 2000 2008, 48, 10–22. [Google Scholar] [CrossRef]
- Ainamo, J. Prevention of periodontal disease in the dental office. Int. Dent. J. 1984, 34, 56–61. [Google Scholar]
- Lim, L.P.; Davies, W.I.R. Comparison of various modalities of “simple” periodontal therapy on oral cleanliness and bleeding. J. Clin. Periodontol. 1996, 23, 595–600. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.; Watt, R.; Hamer, M. Toothbrushing, inflammation, and risk of cardiovascular disease: Results from Scottish Health Survey. BMJ 2010, 340, 1400. [Google Scholar] [CrossRef]
- Tanaka, A.; Takeuchi, K.; Furuta, M.; Takeshita, T.; Suma, S.; Shinagawa, T.; Shimazaki, Y.; Yamashita, Y. Relationship of toothbrushing to metabolic syndrome in middle-aged adults. J. Clin. Periodontol. 2018, 45, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Nijakowski, K.; Lehmann, A.; Rutkowski, R.; Korybalska, K.; Witowski, J.; Surdacka, A. Poor oral hygiene and high levels of inflammatory cytokines in saliva predict the risk of overweight and obesity. Int. J. Environ. Res. Public Health 2020, 17, 6310. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lee, J.S.; Lee, K.J.; Woo, H.G.; Song, T.J. Improved oral hygiene is associated with decreased risk of new-onset diabetes: A nationwide population-based cohort study. Diabetologia 2020, 63, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Ueno, K.; Hata, A. Lower frequency of daily teeth brushing is related to high prevalence of cardiovascular risk factors. Exp. Biol. Med. 2009, 234, 387–394. [Google Scholar] [CrossRef]
- Choi, H.M.; Han, K.; Park, Y.-G.; Park, J.-B. Associations Among Oral Hygiene Behavior and Hypertension Prevalence and Control: The 2008 to 2010 Korea National Health and Nutrition Examination Survey. J. Periodontol. 2015, 86, 866–873. [Google Scholar] [CrossRef]
- Song, T.J.; Kim, J.W.; Kim, J. Oral health and changes in lipid profile: A nationwide cohort study. J. Clin. Periodontol. 2020, 47, 1437–1445. [Google Scholar] [CrossRef]
- Pham, T. The association between periodontal disease severity and metabolic syndrome in Vietnamese patients. Int. J. Dent. Hyg. 2018, 16, 484–491. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Niu, K.; Guan, L.; Momma, H.; Guo, H.; Cui, Y.; Nagatomi, R. Oral health behavior and metabolic syndrome and its components in adults. J. Dent. Res. 2012, 91, 479–484. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Paju, S.; Viikari, J.; Salminen, A.; Taittonen, L.; Laitinen, T.; Burgner, D.; Kahonen, M.; Lehtimaki, T.; Hutri-Kahonen, N.; et al. Childhood Oral Infections Associate with Adulthood Metabolic Syndrome: A Longitudinal Cohort Study. J. Dent. Res. 2020, 99, 1165–1173. [Google Scholar] [CrossRef]
- Shearer, D.M.; Thomson, W.M.; Cameron, C.M.; Ramrakha, S.; Wilson, G.; Wong, T.Y.; Williams, M.J.A.; McLean, R.; Theodore, R.; Poulton, R. Periodontitis and multiple markers of cardiometabolic risk in the fourth decade: A cohort study. Community Dent. Oral Epidemiol. 2018, 46, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Santoso, C.M.A.; Ketti, F.; Nagy, A. Association between Oral Hygiene and Metabolic Syndrome: A Systematic Review and Meta-analysis. PROSPERO 2021 CRD42021243292. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021243292 (accessed on 17 April 2021).
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 5 February 2021).
- Yuan, T.; Zou, H.; Zhao, J.; Yang, Z.; Li, L.; Cai, W.; Gu, J.; Hao, C.; Li, J.; Hao, Y.; et al. Circumcision to prevent HIV and other sexually transmitted infections in men who have sex with men: A systematic review and meta-analysis of global data. Artic. Lancet Glob. Health 2019, 7, e436–e447. [Google Scholar] [CrossRef]
- Fu, W.; Lv, C.; Zou, L.; Song, F.; Zeng, X.; Wang, C.; Yan, S.; Gan, Y.; Chen, F.; Lu, Z.; et al. Meta-analysis on the association between the frequency of tooth brushing and diabetes mellitus risk. Diabetes Metab. Res. Rev. 2019, 35, e3141. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M. Comparison of methods of extracting information for meta-analysis of observational studies in nutritional epidemiology. Epidemiol. Health 2016, 38, e2016003. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. (Eds.) Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]; The Cochrane Collaboration: Melbourne, Australia, 2011. [Google Scholar]
- Higgins, J.P.T.; Green, S. (Eds.) Addressing reporting biases. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]; The Cochrane Collaboration: Melbourne, Australia, 2011. [Google Scholar]
- Alzahrani, H.; Mackey, M.; Stamatakis, E.; Zadro, J.R.; Shirley, D. The association between physical activity and low back pain: A systematic review and meta-analysis of observational studies. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Review Manager (RevMan) [Computer Program], Version 5.4; The Cochrane Collaboration: Melbourne, Australia, 2020; Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-non-cochrane-reviews (accessed on 16 March 2021).
- Tsutsumi, C.; Kakuma, T. Regular Tooth Brushing is Associated with a Decreased Risk of Metabolic Syndrome According to a Medical Check-Up Database. Kurume Med. J. 2015, 61, 43–52. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, D.-H.; Lim, K.S.; Ko, B.-J.; Han, B.-D.; Nam, G.-E.; Park, Y.-G.; Han, K.D.; Kim, J.-H.; Cho, K.-H. Oral health behaviors and metabolic syndrome: The 2008-2010 Korean National Health and Nutrition Examination Survey. Clin. Oral Investig. 2014, 18, 1517–1524. [Google Scholar] [CrossRef]
- Fukui, N.; Shimazaki, Y.; Shinagawa, T.; Yamashita, Y. Periodontal Status and Metabolic Syndrome in Middle-Aged Japanese. J. Periodontol. 2012, 83, 1363–1371. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, S.Y.; Byon, M.J.; Lee, J.H.; Jeong, S.H.; Kim, J.B. Association between periodontitis and metabolic syndrome in a korean nationally representative sample of adults aged 35–79 years. Int. J. Environ. Res. Public Health 2019, 16, 2930. [Google Scholar] [CrossRef]
- Saito, M.; Shimazaki, Y.; Nonoyama, T.; Tadokoro, Y. Number of teeth, oral self-care, eating speed, and metabolic syndrome in an aged Japanese population. J. Epidemiol. 2019, 29, 26–32. [Google Scholar] [CrossRef]
- Chen, L.-P.; Hsu, S.-P.; Peng, Y.-S.; Chiang, C.-K.; Hung, K.-Y. Periodontal disease is associated with metabolic syndrome in hemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 4068–4073. [Google Scholar] [CrossRef][Green Version]
- Li, P.; He, L.; Sha, Y.Q.; Luan, Q.X. Relationship of Metabolic Syndrome to Chronic Periodontitis. J. Periodontol. 2009, 80, 541–549. [Google Scholar] [CrossRef]
- Li, P.; He, L.; Chen, Z.B.; Luan, Q.X. Biomarkers in Metabolic Syndrome Patients with Chronic Periodontitis. Chin. J. Dent. Res. 2020, 23, 191–197. [Google Scholar] [CrossRef]
- Expert Panel. Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III); NIH Publication: Bethesda, MD, USA, 2002.
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of The International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef]
- Leite, F.R.M.; Nascimento, G.G. The Relationship Between Periodontal Diseases and Chronic Diseases. In Oral Epidemiology—A Textbook on Oral Health Conditions, Research Topics and Methods; Peres, M.A., Antunes, J.L.F., Watt, R.G., Eds.; Springer: Cham, Switzerland, 2021; pp. 379–393. [Google Scholar]
- Iacopino, A.M. Periodontitis and diabetes interrelationships: Role of inflammation. Ann. Periodontol. 2001, 6, 125–137. [Google Scholar] [CrossRef]
- Nishimura, F.; Iwamoto, Y.; Mineshiba, J.; Shimizu, A.; Soga, Y.; Murayama, Y. Periodontal Disease and Diabetes Mellitus: The Role of Tumor Necrosis Factor-α in a 2-Way Relationship. J. Periodontol. 2003, 74, 97–102. [Google Scholar] [CrossRef]
- Wang, C.; Guan, Y.; Yang, J. Cytokines in the progression of pancreatic β-cell dysfunction. Int. J. Endocrinol. 2010, 2010, 515136. [Google Scholar] [CrossRef] [PubMed]
- Cieślak, M.; Wojtczak, A.; Cieślak, M. Role of pro-inflammatory cytokines of pancreatic islets and prospects of elaboration of new methods for the diabetes treatment. Acta Biochim. Pol. 2015, 62, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Grover, H.S.; Luthra, S. Molecular mechanisms involved in the bidirectional relationship between diabetes mellitus and periodontal disease. J. Indian Soc. Periodontol. 2013, 17, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Katagiri, S.; Takahashi, H.; Sasaki, N.; Maekawa, S.; Komazaki, R.; Hatasa, M.; Kitajima, Y.; Maruyama, Y.; Shiba, T.; et al. Porphyromonas gingivalis impairs glucose uptake in skeletal muscle associated with altering gut microbiota. FASEB J. 2020, 35, e21171. [Google Scholar] [CrossRef]
- Lertpimonchai, A.; Rattanasiri, S.; Arj-Ong Vallibhakara, S.; Attia, J.; Thakkinstian, A. The association between oral hygiene and periodontitis: A systematic review and meta-analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.; Zimmermann, N.; Hagenfeld, D.; Veile, A.; Kim, T.S.; Becher, H. Is frequency of tooth brushing a risk factor for periodontitis? A systematic review and meta-analysis. Community Dent. Oral Epidemiol. 2015, 43, 116–127. [Google Scholar] [CrossRef]
- Bourgeois, D.; Bravo, M.; Llodra, J.C.; Inquimbert, C.; Viennot, S.; Dussart, C.; Carrouel, F. Calibrated interdental brushing for the prevention of periodontal pathogens infection in young adults—A randomized controlled clinical trial. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Kim, S.W.; Cho, K.H.; Han, K.D.; Roh, Y.K.; Song, I.S.; Kim, Y.H. Tooth loss and metabolic syndrome in South Korea: The 2012 Korean national health and nutrition examination survey. Medicine 2016, 95. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.A. Gums and heart disease: Healthy gums, healthy heart? BMJ 2010, 341, 113. [Google Scholar] [CrossRef]
- Franchini, R.; Petri, A.; Migliario, M.; Rimondini, L. Poor oral hygiene and gingivitis are associated with obesity and overweight status in paediatric subjects. J. Clin. Periodontol. 2011, 38, 1021–1028. [Google Scholar] [CrossRef]
- Baeza, M.; Morales, A.; Cisterna, C.; Cavalla, F.; Jara, G.; Isamitt, Y.; Pino, P.; Gamonal, J. Effect of periodontal treatment in patients with periodontitis and diabetes: Systematic review and meta-analysis. J. Appl. Oral Sci. 2020, 28. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, A.; Wong, D.T.W.; Lo Muzio, L. Editorial: Saliva and Oral Microbiota: From Physiology to Diagnostic and Therapeutic Implications. Front. Physiol. 2021, 11, 637599. [Google Scholar] [CrossRef]
- Souza, M.L.; Massignan, C.; Peres, K.G.; Peres, M.A. Association between metabolic syndrome and tooth loss: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2019, 150, 1027–1039.e7. [Google Scholar] [CrossRef]
| Subgroup | Number of Studies | OR (95% CI) | I2 (%) | p |
|---|---|---|---|---|
| Cross-sectional | 2 | 0.72 (0.41–1.26) | 46 | 0.17 |
| Case–control | 3 | 0.11 (0.06–0.20) | 39 | 0.19 |
| Cohort | 1 | 0.83 (0.59–1.15) | - | - |
| Subgroup | Number of Studies | OR (95% CI) | I2 (%) | p |
|---|---|---|---|---|
| Study design | ||||
| Cross-sectional | 5 | 0.67 (0.55–0.81) | 93 | <0.001 |
| Cohort | 2 | 0.74 (0.62–0.89) | 0 | 0.64 |
| Country | ||||
| Japan | 5 | 0.61 (0.52–0.70) | 55 | 0.06 |
| Korea | 2 | 0.85 (0.78–0.93) | 73 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoso, C.M.A.; Ketti, F.; Bramantoro, T.; Zsuga, J.; Nagy, A. Association between Oral Hygiene and Metabolic Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2873. https://doi.org/10.3390/jcm10132873
Santoso CMA, Ketti F, Bramantoro T, Zsuga J, Nagy A. Association between Oral Hygiene and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(13):2873. https://doi.org/10.3390/jcm10132873
Chicago/Turabian StyleSantoso, Cornelia Melinda Adi, Fera Ketti, Taufan Bramantoro, Judit Zsuga, and Attila Nagy. 2021. "Association between Oral Hygiene and Metabolic Syndrome: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 13: 2873. https://doi.org/10.3390/jcm10132873
APA StyleSantoso, C. M. A., Ketti, F., Bramantoro, T., Zsuga, J., & Nagy, A. (2021). Association between Oral Hygiene and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(13), 2873. https://doi.org/10.3390/jcm10132873






