Prevention of Hypertensive Disorders of Pregnancy—Is There a Place for Metformin?
Abstract
1. Introduction
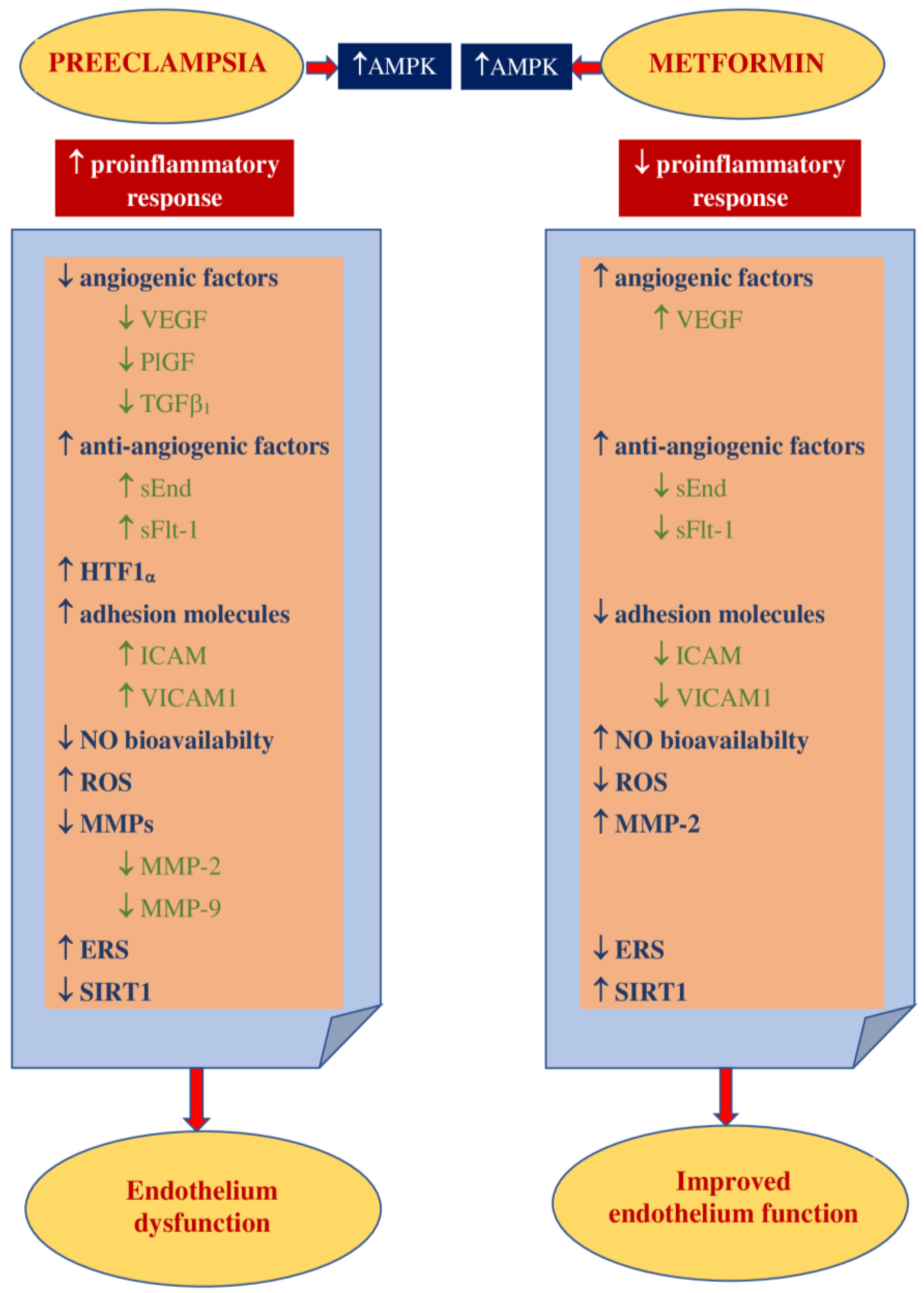
2. Pathophysiology of Preeclampsia
3. Metformin
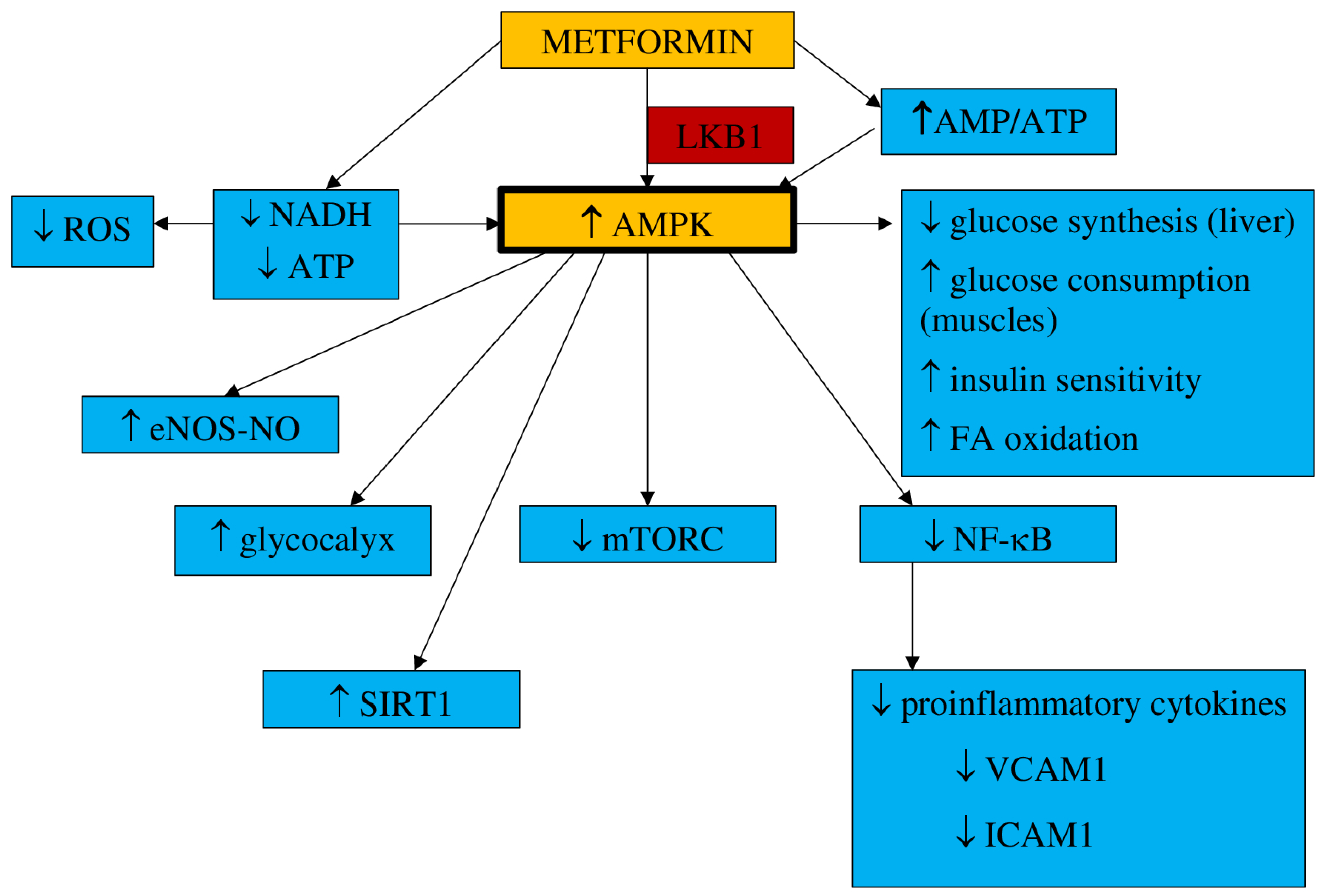
3.1. Pharmacokinetics and Mechanism of Action
- a.
- Inflammation and oxidative stress
- b.
- NO synthesis
- c.
- Endothelial senescence and apoptosis
- d.
- Vascular integrity
3.2. Impact on Preeclampsia Pathophysiology
4. Metformin in Preventing Hypertensive Disorders of Pregnancy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ACOG. Practice Bulletin No. 203: Chronic hypertension in pregnancy. Obstet. Gynecol. 2019, 133, e26–e50. [Google Scholar] [CrossRef] [PubMed]
- ACOG. Practice Bulletin No. 202: Gestational hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, e1–e25. [Google Scholar] [CrossRef]
- Duley, L. The global impact of preeclampsia and eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef]
- Ronsmans, C.; Graham, W.J.; Lancet Maternal Survival Series steering group. Maternal mortality: Who, when, where, and why. Lancet 2006, 368, 1189–1200. [Google Scholar] [CrossRef]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Prejbisz, A.; Dobrowolski, P.; Kosiński, P.; Bomba-Opoń, D.; Adamczak, M.; Bekiesińska-Figatowska, M.; Kądziela, J.; Konopka, A.; Kostka-Jeziorny, K.; Kurnatowska, I.; et al. Management of hypertension in pregnancy: Prevention, diagnosis, treatment and long-term prognosis. Kardiol. Pol. 2019, 77, 757–806. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. International Society for the Study of Hypertension in Pregnancy (ISSHP). The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Pels, A.; Helewa, M.; Rey, E.; von Dadelszen, P.; SOGC Hypertension Guideline Committee. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: Executive summary. J. Obstet. Gynaecol. Can. 2014, 36, 575–576. [Google Scholar] [CrossRef]
- Tong, S.; Kaitu’u-Lino, T.J.; Hastie, R.; Brownfoot, F.; Cluver, C.; Hannan, N. Pravastatin, proton-pump inhibitors, metformin, micronutrients, and biologics: New horizons for the prevention or treatment of preeclampsia. Am. J. Obstet. Gynecol. 2020. [Google Scholar] [CrossRef]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; Davidson, M.B.; Einhorn, D.; Garvey, W.T.; et al. American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm 2013. Endocr. Pract. 2013, 19, 327–336. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 104, 1–52. [Google Scholar] [CrossRef]
- Wang, Y.W.; He, S.J.; Feng, X.; Cheng, J.; Luo, Y.T.; Tian, L.; Huang, Q. Metformin: A review of its potential indications. Drug Des. Devel. Ther. 2017, 11, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef]
- Von Dadelszen, P.; Magee, L.A.; Roberts, J.M. Subclassification of preeclampsia. Hypertens. Pregnancy 2003, 22, 143–148. [Google Scholar] [CrossRef]
- Roberts, J.M. Pathophysiology of ischemic placental disease. Semin. Perinatol. 2014, 38, 139–145. [Google Scholar] [CrossRef]
- Kaufmann, P.; Black, S.; Huppertz, B. Endovascular trophoblast invasion: Implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol. Reprod. 2003, 69, 1–7. [Google Scholar] [CrossRef]
- Perez-Sepulveda, A.; Torres, M.J.; Khoury, M.; Illanes, S.E. Innate immune system and preeclampsia. Front. Immunol. 2014, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.; Sargent, I.L. Immunology of pre-eclampsia. Am. J. Reprod. Immunol. 2010, 63, 534–543. [Google Scholar] [CrossRef]
- Borzychowski, A.M.; Sargent, I.L.; Redman, C.W. Inflammation and pre-eclampsia. Semin. Fetal. Neonatal. Med. 2006, 11, 309–316. [Google Scholar] [CrossRef]
- Luppi, P.; Deloia, J.A. Monocytes of preeclamptic women spontaneously synthesize pro-inflammatory cytokines. Clin. Immunol. 2006, 118, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, A.S.; Rajakumar, A.; Royle, C.M.; Lo, A.; Husain, Z.; Thadhani, R.I.; Sukhatme, V.P.; Karumanchi, S.A.; Kopcow, H.D. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J. Immunol. 2013, 190, 3939–3948. [Google Scholar] [CrossRef]
- Fraser, R.; Whitley, G.S.; Johnstone, A.P.; Host, A.J.; Sebire, N.J.; Thilaganathan, B.; Cartwright, J.E. Impaired decidual natural killer cell regulation of vascular remodelling in early human pregnancies with high uterine artery resistance. J. Pathol. 2012, 228, 322–332. [Google Scholar] [CrossRef]
- Guimond, M.J.; Wang, B.; Croy, B.A. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J. Exp. Med. 1998, 187, 217–223. [Google Scholar] [CrossRef]
- Redman, C.W. Current topic: Pre-eclampsia and the placenta. Placenta 1991, 12, 301–308. [Google Scholar] [CrossRef]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef]
- Brennan, L.J.; Morton, J.S.; Davidge, S.T. Vascular dysfunction in preeclampsia. Microcirculation 2014, 21, 4–14. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, D.Y.; Yang, J.M.; Wang, M.; Zhang, X.T.; Sun, L.; Yun, X.G. Maternal serum uric acid concentration is associated with the expression of tumour necrosis factor-α and intercellular adhesion molecule-1 in patients with preeclampsia. J. Hum. Hypertens. 2016, 30, 456–462. [Google Scholar] [CrossRef]
- Mutter, W.P.; Karumanchi, S.A. Molecular mechanisms of preeclampsia. Microvasc. Res. 2008, 75, 1–8. [Google Scholar] [CrossRef]
- Millauer, B.; Wizigmann-Voos, S.; Schnürch, H.; Martinez, R.; Møller, N.P.; Risau, W.; Ullrich, A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 26, 835–846. [Google Scholar] [CrossRef]
- Mandriota, S.J.; Seghezzi, G.; Vassalli, J.D.; Ferrara, N.; Wasi, S.; Mazzieri, R.; Mignatti, P.; Pepper, M.S. Vascular endothelial growth factor increases urokinase receptor expression in vascular endothelial cells. J. Biol. Chem. 1995, 270, 9709–9716. [Google Scholar] [CrossRef] [PubMed]
- Alon, T.; Hemo, I.; Itin, A.; Pe’er, J.; Stone, J.; Keshet, E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1995, 1, 1024–1028. [Google Scholar] [CrossRef]
- Koroglu, N.; Tola, E.; Temel Yuksel, I.; Aslan Cetin, B.; Turhan, U.; Topcu, G.; Dag, I. Maternal serum AMP-activated protein kinase levels in mild and severe preeclampsia. J. Matern. Fetal. Neonatal. Med. 2019, 32, 2735–2740. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. AMPK in health and disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.A.; Albers, R.E.; Doliboa, S.R.; Hughes, M.; Wyatt, C.N.; Natale, D.R.; Brown, T.L. AMPK knockdown in placental trophoblast cells results in altered morphology and function. Stem. Cells Dev. 2014, 23, 2921–2930. [Google Scholar] [CrossRef]
- Waker, C.A.; Albers, R.E.; Pye, R.L.; Doliboa, S.R.; Wyatt, C.N.; Brown, T.L.; Mayes, D.A. AMPK knockdown in placental labyrinthine progenitor cells results in restriction of critical energy resources and terminal differentiation failure. Stem. Cells Dev. 2017, 26, 808–817. [Google Scholar] [CrossRef]
- Xian, L.; Varshney, R.; Raashdin, N.A.; Shaw, J.H.; Lloyd, P.G. Placenta growth factor and vascular endothelial growth factor-A have differential, cell-type specific atterns of expression in vascular cells. Microcirculation 2014, 21, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Autiero, M.; Luttun, A.; Tjwa, M.; Carmeliet, P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: Novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J. Thromb. Haemost. 2003, 1, 1356–1370. [Google Scholar] [CrossRef] [PubMed]
- Kurz, H.; Wilting, J.; Sandau, K.; Christ, B. Automated evaluation of angiogenic effects mediated by VEGF and PlGF homo- and heterodimers. Microvasc. Res. 1998, 55, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999, 13, 9–22. [Google Scholar] [CrossRef]
- Brownfoot, F.C.; Hastie, R.; Hannan, N.J.; Cannon, P.; Tuohey, L.; Parry, L.J.; Senadheera, S.; Illanes, S.E.; Kaitu’u-Lino, T.J.; Tong, S. Metformin as a prevention and treatment for preeclampsia: Effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am. J. Obstet. Gynecol. 2016, 214, 356.e1–356.e15. [Google Scholar] [CrossRef] [PubMed]
- Luft, F.C. Soluble endoglin (sEng) joins the soluble fms-like tyrosine ki- nase (sFlt) receptor as a pre-eclampsia molecule. Nephrol. Dial. Transplant. 2006, 21, 3052–3054. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.H.; Yuan, H.T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Karumanchi, S.A. Angiogenic factors and preeclampsia. Semin. Nephrol. 2011, 31, 33–46. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Chaemsaithong, P.; Yeo, L.; Romero, R. Pre-eclampsia part 1: Current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014, 10, 466–480. [Google Scholar] [CrossRef]
- Bdolah, Y.; Sukhatme, V.P.; Karumanchi, S.A. Angiogenic imbalance in the pathophysiology of preeclampsia: Newer insights. Semin. Nephrol. 2004, 24, 548–556. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef]
- Govender, L.; Mackraj, I.; Gathiram, P.; Moodley, J. The role of angiogenic, anti-angiogenic and vasoactive factors in pre-eclamptic African women: Early- versus late-onset pre-eclampsia. Cardiovasc. J. Afr. 2012, 23, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Gemmel, M.; Powers, R.W. Nitric oxide signaling in pregnancy and preeclampsia. Nitric. Oxide. 2020, 95, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Nejabati, H.R.; Latifi, Z.; Ghasemnejad, T.; Fattahi, A.; Nouri, M. Placental growth factor (PlGF) as an angiogenic/inflammatory switcher: Lesson from early pregnancy losses. Gynecol. Endocrinol. 2017, 33, 668–674. [Google Scholar] [CrossRef]
- Zafer, E.; Yenisey, C.; Kurek Eken, M.; Ozdemir, E.; Kurt Omurlu, I.; Yuksel, H. Second trimester maternal serum-amniotic fluid nitric oxide and vascular endothelial growth factor levels in relation to uterine artery Doppler indices in pregnancies with normal outcome. J. Obstet. Gynaecol. 2018, 38, 1088–1092. [Google Scholar] [CrossRef]
- Kleinrouweler, C.E.; Wiegerinck, M.M.; Ris-Stalpers, C.; Bossuyt, P.M.; van der Post, J.A.; von Dadelszen, P.; Mol, B.W.; Pajkrt, E. EBM CONNECT Collaboration. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: A systematic review and meta-analysis. BJOG 2012, 119, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Taysi, S.; Tascan, A.S.; Ugur, M.G.; Demir, M. Radicals, oxidative/nitrosative stress and preeclampsia. Mini Rev. Med. Chem. 2019, 19, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Leaños-Miranda, A.; Navarro-Romero, C.S.; Sillas-Pardo, L.J.; Ramírez-Valenzuela, K.L.; Isordia-Salas, I.; Jiménez-Trejo, L.M. Soluble endoglin as a marker for preeclampsia, its severity, and the occurrence of adverse outcomes. Hypertension 2019, 74, 991–997. [Google Scholar] [CrossRef]
- Nevo, O.; Soleymanlou, N.; Wu, Y.; Xu, J.; Kingdom, J.; Many, A.; Zamudio, S.; Caniggia, I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1085–R1093. [Google Scholar] [CrossRef]
- Tal, R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol. Reprod. 2012, 87, 134. [Google Scholar] [CrossRef]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef]
- Burton, G.J.; Yung, H.W.; Cindrova-Davies, T.; Charnock-Jones, D.S. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta 2009, 30, S43–S48. [Google Scholar] [CrossRef]
- Burton, G.J.; Yung, H.W.; Murray, A.J. Mitochondrial-Endoplasmic reticulum interactions in the trophoblast: Stress and senescence. Placenta 2017, 52, 146–155. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, L.; Wang, L.; Zhu, X. Expression of markers of endoplasmic reticulum stress-induced apoptosis in the placenta of women with early and late onset severe pre-eclampsia. Taiwan J. Obstet. Gynecol. 2015, 54, 19–23. [Google Scholar] [CrossRef]
- Isaka, K.; Usuda, S.; Ito, H.; Sagawa, Y.; Nakamura, H.; Nishi, H.; Suzuki, Y.; Li, Y.F.; Takayama, M. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta 2003, 24, 53–64. [Google Scholar] [CrossRef]
- Chen, J.; Khalil, R.A. Matrix metalloproteinases in normal pregnancy and preeclampsia. Prog. Mol. Biol. Transl. Sci. 2017, 148, 87–165. [Google Scholar] [CrossRef]
- Boeldt, D.S.; Bird, I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 2017, 232, R27–R44. [Google Scholar] [CrossRef]
- Bills, V.L.; Salmon, A.H.; Harper, S.J.; Overton, T.G.; Neal, C.R.; Jeffery, B.; Soothill, P.W.; Bates, D.O. Impaired vascular permeability regulation caused by the VEGF₁₆₅b splice variant in preeclampsia. BJOG 2011, 118, 1253–1261. [Google Scholar] [CrossRef]
- Burger, D.; Touyz, R.M. Cellular biomarkers of endothelial health: Microparticles, endothelial progenitor cells, and circulating endothelial cells. J. Am. Soc. Hypertens. 2012, 6, 85–99. [Google Scholar] [CrossRef]
- King, T.F.; Bergin, D.A.; Kent, E.M.; Manning, F.; Reeves, E.P.; Dicker, P.; McElvaney, N.G.; Sreenan, S.; Malone, F.D.; McDermott, J.H. Endothelial progenitor cells in mothers of low-birthweight infants: A link between defective placental vascularization and increased cardiovascular risk? J. Clin. Endocrinol. Metab. 2013, 98, E33–E39. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Luo, Q.; Zheng, Y.; Liu, X.; Hu, Y.; Liu, W.; Luo, M.; Tao, H.; Wu, D.; Zhao, Y.; et al. Notch1 impairs endothelial progenitor cell bioactivity in preeclampsia. Reprod. Sci. 2017, 24, 47–56. [Google Scholar] [CrossRef]
- Sugawara, J.; Mitsui-Saito, M.; Hayashi, C.; Hoshiai, T.; Senoo, M.; Chisaka, H.; Yaegashi, N.; Okamura, K. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 5329–5332. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, X.P.; Zhu, J.H.; Xie, X.D.; Zhang, H.; Wang, X.X.; Chen, J.Z.; Jian, S. Decrease and dysfunction of endothelial progenitor cells in umbilical cord blood with maternal pre-eclampsia. J. Obstet. Gynaecol. Res. 2007, 33, 465–474. [Google Scholar] [CrossRef]
- Matsubara, K.; Abe, E.; Matsubara, Y.; Kameda, K.; Ito, M. Circulating endothelial progenitor cells during normal pregnancy and pre-eclampsia. Am. J. Reprod. Immunol. 2006, 56, 79–85. [Google Scholar] [CrossRef]
- Zhu, J.; Cheng, X.; Wang, Q.; Zhou, Y.; Wang, F.; Zou, L. Transplantation of endothelial progenitor cells for improving placental perfusion in preeclamptic rats. Arch. Gynecol. Obstet. 2014, 291, 1113–1119. [Google Scholar] [CrossRef]
- Rajendrasozhan, S.; Yang, S.R.; Kinnula, V.L.; Rahman, I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 861–870. [Google Scholar] [CrossRef]
- Viana-Mattioli, S.; Nunes, P.; Cavalli, R.; Sandrim, V. Analysis of SIRT1 expression in plasma and in an in vitro model of preeclampsia. Oxid. Med. Cell Longev. 2020, 2020, 4561083. [Google Scholar] [CrossRef]
- Broady, A.J.; Loichinger, M.H.; Ahn, H.J.; Davy, P.M.; Allsopp, R.C.; Bryant-Greenwood, G.D. Protective proteins and telomere length in placentas from patients with pre-eclampsia in the last trimester of gestation. Placenta 2017, 50, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Feng, Y.; Zhao, H.; Zhao, Z.; Yua, H.; Xu, J.; Che, H. SIRT1 inhibits releases of HMGB1 and HSP70 from human umbilical vein endothelial cells caused by IL-6 and the serum from a preeclampsia patient and protects the cells from death. Biomed. Pharmacother. 2017, 88, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Schnettler, W.T.; Powe, C.; Wenger, J.; Salahuddin, S.; Cerdeira, A.S.; Verlohren, S.; Perschel, F.H.; Arany, Z.; Lim, K.H.; et al. Clinical characterization and outcomes of preeclampsia with normal angiogenic profile. Hypertens. Pregnancy 2013, 32, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Velauthar, L.; Plana, M.N.; Kalidindi, M.; Zamora, J.; Thilaganathan, B.; Illanes, S.E.; Khan, K.S.; Aquilina, J.; Thangaratinam, S. First-trimester uterine artery Doppler and adverse pregnancy outcome: A meta-analysis involving 55,974 women. Ultrasound Obstet. Gynecol. 2014, 43, 500–507. [Google Scholar] [CrossRef]
- Poon, L.C.; Nicolaides, K.H. Early prediction of preeclampsia. Obstet. Gynecol. Int. 2014, 2014, 297397. [Google Scholar] [CrossRef]
- Poon, L.C.; Rolnik, D.L.; Tan, M.Y.; Delgado, J.L.; Tsokaki, T.; Akolekar, R.; Singh, M.; Andrade, W.; Efeturk, T.; Jani, J.C.; et al. ASPRE trial: Incidence of preterm pre-eclampsia in patients fulfilling ACOG and NICE criteria according to risk by FMF algorithm. Ultrasound Obstet. Gynecol. 2018, 51, 738–742. [Google Scholar] [CrossRef]
- Rowan, J.A.; Hague, W.M.; Gao, W.; Battin, M.R.; Moore, M.P. MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N. Engl. J. Med. 2008, 358, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wondisford, F.E. Metformin action: Concentrations matter. Cell Metab. 2015, 21, 159–162. [Google Scholar] [CrossRef]
- El-Mir, M.Y.; Nogueira, V.; Fontaine, E.; Avéret, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-Ferraros, C.; Vazquez-Martin, A.; Menendez, J.A. Genome-wide inhibitory impact of the AMPK activator metformin on [kinesins, tubulins, histones, auroras and polo-like kinases] M-phase cell cycle genes in human breast cancer cells. Cell Cycle 2009, 8, 1633–1636. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef]
- Zou, M.H.; Kirkpatrick, S.S.; Davis, B.J.; Nelson, J.S.; Wiles, W.G., IV; Schlattner, U.; Neumann, D.; Brownlee, M.; Freeman, M.B.; Goldman, M.H. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J. Biol. Chem. 2004, 279, 43940–43951. [Google Scholar] [CrossRef]
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546. [Google Scholar] [CrossRef]
- Luo, Z.; Zang, M.; Guo, W. AMPK as a metabolic tumor suppressor: Control of metabolism and cell growth. Future Oncol. 2010, 6, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Sahra, I.B.; Le Marchand-Brustel, Y.; Tanti, J.F. Metformin and cancer therapy. Curr. Opin. Oncol. 2012, 24, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Dowling, R.J.; Zakikhani, M.; Fantus, I.G.; Pollak, M.; Sonenberg, N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007, 67, 10804108012. [Google Scholar] [CrossRef]
- Ben Sahra, I.; Regazzetti, C.; Robert, G.; Laurent, K.; Le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011, 71, 4366–4372. [Google Scholar] [CrossRef]
- Scheen, A.J.; Esser, N.; Paquot, N. Antidiabetic agents: Potential anti-inflammatory activity beyond glucose control. Diabetes Metab. 2015, 41, 183–194. [Google Scholar] [CrossRef]
- Saenz, A.; Fernandez-Esteban, I.; Mataix, A.; Ausejo, M.; Roque, M.; Moher, D. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2005, 3, CD002966. [Google Scholar] [CrossRef]
- Abbasi, F.; Chu, J.W.; McLaughlin, T.; Lamendola, C.; Leary, E.T.; Reaven, G.M. Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus. Metabolism 2004, 53, 159–164. [Google Scholar] [CrossRef] [PubMed]
- De Aguiar, L.G.; Bahia, L.R.; Villela, N.; Laflor, C.; Sicuro, F.; Wiernsperger, N.; Bottino, D.; Bouskela, E. Metformin improves endothelial vascular reactivity in first-degree relatives of type 2 diabetic patients with metabolic syndrome and normal glucose tolerance. Diabetes Care 2006, 29, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 2013, 368, 2004–2013. [Google Scholar] [CrossRef]
- Sena, C.M.; Matafome, P.; Louro, T.; Nunes, E.; Fernandes, R.; Seiça, R.M. Metformin restores endothelial function in aorta of diabetic rats. Br. J. Pharmacol. 2011, 163, 424–437. [Google Scholar] [CrossRef]
- Li, S.N.; Wang, X.; Zeng, Q.T.; Feng, Y.B.; Cheng, X.; Mao, X.B.; Wang, T.H.; Deng, H.P. Metformin inhibits nuclear factor kappaB activation and decreases serum high-sensitivity C-reactive protein level in experimental atherogenesis of rabbits. Heart Vessels. 2009, 24, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 2006, 47, 1183–1888. [Google Scholar] [CrossRef]
- Isoda, K.; Young, J.L.; Zirlik, A.; MacFarlane, L.A.; Tsuboi, N.; Gerdes, N.; Schönbeck, U.; Libby, P. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 611–617. [Google Scholar] [CrossRef]
- Hyun, B.; Shin, S.; Lee, A.; Lee, S.; Song, Y.; Ha, N.J.; Cho, K.H.; Kim, K. Metformin down-regulates TNF-α secretion via suppression of scavenger receptors in macrophages. Immune Netw. 2013, 13, 123–132. [Google Scholar] [CrossRef]
- Gongol, B.; Marin, T.; Peng, I.C.; Woo, B.; Martin, M.; King, S.; Sun, W.; Johnson, D.A.; Chien, S.; Shyy, J.Y. AMPKα2 exerts its anti-inflammatory effects through PARP-1 and Bcl-6. Proc. Natl. Acad. Sci. USA 2013, 110, 3161–3166. [Google Scholar] [CrossRef]
- Kim, S.A.; Choi, H.C. Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2012, 425, 866–872. [Google Scholar] [CrossRef]
- Beisswenger, P.; Ruggiero-Lopez, D. Metformin inhibition of glycation processes. Diabetes Metab. 2003, 29, 6S95–6S103. [Google Scholar] [CrossRef]
- Yan, S.F.; D’Agati, V.; Schmidt, A.M.; Ramasamy, R. Receptor for Advanced Glycation Endproducts (RAGE): A formidable force in the pathogenesis of the cardiovascular complications of diabetes & aging. Curr. Mol. Med. 2007, 7, 699–710. [Google Scholar]
- Di Marco, E.; Gray, S.P.; Jandeleit-Dahm, K. Diabetes alters activation and repression of pro- and anti-inflammatory signaling pathways in the vasculature. Front. Endocrinol. 2013, 4, 68. [Google Scholar] [CrossRef][Green Version]
- Mamputu, J.C.; Wiernsperger, N.F.; Renier, G. Antiatherogenic properties of metformin: The experimental evidence. Diabetes Metab. 2003, 29, 6S71–6S76. [Google Scholar] [CrossRef]
- Saisho, Y. Metformin and inflammation: Its potential beyond glucose-lowering effect. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Ouslimani, N.; Peynet, J.; Bonnefont-Rousselot, D.; Thérond, P.; Legrand, A.; Beaudeux, J.L. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism 2005, 54, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Bakhashab, S.; Ahmed, F.W.; Schulten, H.J.; Bashir, A.; Karim, S.; Al-Malki, A.L.; Gari, M.A.; Abuzenadah, A.M.; Chaudhary, A.G.; Alqahtani, M.H.; et al. Metformin improves the angiogenic potential of human CD34⁺ cells co-incident with downregulating CXCL10 and TIMP1 gene expression and increasing VEGFA under hyperglycemia and hypoxia within a therapeutic window for myocardial infarction. Cardiovasc. Diabetol. 2016, 15, 27. [Google Scholar] [CrossRef]
- Yu, J.W.; Deng, Y.P.; Han, X.; Ren, G.F.; Cai, J.; Jiang, G.J. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc. Diabetol. 2016, 15, 88. [Google Scholar] [CrossRef]
- Arunachalam, G.; Samuel, S.M.; Marei, I.; Ding, H.; Triggle, C.R. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br. J. Pharmacol. 2014, 171, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Kinaan, M.; Ding, H.; Triggle, C.R. Metformin: An old drug for the treatment of diabetes but a new drug for the protection of the endothelium. Med. Princ. Pract. 2015, 24, 401–415. [Google Scholar] [CrossRef]
- Eskens, B.J.; Zuurbier, C.J.; van Haare, J.; Vink, H.; van Teeffelen, J.W. Effects of two weeks of metformin treatment on whole-body glycocalyx barrier properties in db/db mice. Cardiovasc. Diabetol. 2013, 12, 175. [Google Scholar] [CrossRef]
- Vega, M.; Mauro, M.; Williams, Z. Direct toxicity of insulin on the human placenta and protection by metformin. Fertil. Steril. 2019, 111, 489–496.e5. [Google Scholar] [CrossRef]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.B.; de Oliveira, A.C.M.; Goulart, M.O.F. Cross-talk between oxidative stress and inflammation in preeclampsia. Oxid. Med. Cell Longev. 2019, 2019, 8238727. [Google Scholar] [CrossRef]
- Han, C.S.; Herrin, M.A.; Pitruzzello, M.C.; Mulla, M.J.; Werner, E.F.; Pettker, C.M.; Flannery, C.A.; Abrahams, V.M. Glucose and metformin modulate human first trimester trophoblast function: A model and potential therapy for diabetes-associated uteroplacental insufficiency. Am. J. Reprod. Immunol. 2015, 73, 362–371. [Google Scholar] [CrossRef]
- Chiswick, C.; Reynolds, R.M.; Denison, F.; Drake, A.J.; Forbes, S.; Newby, D.E.; Walker, B.R.; Quenby, S.; Wray, S.; Weeks, A.; et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 778–786. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Zhu, B. Protective effect of metformin on a rat model of lipopolysaccharide-induced preeclampsia. Fundam. Clin. Pharmacol. 2019, 33, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Yan, D.; Chen, C.; Mo, Y.; Wu, L.; Gu, J.; Shah, N.K.; He, J.; Dong, S. Metformin exhibits its therapeutic effect in the treatment of pre-eclampsia via modulating the Met/H19/miR-148a-5p/P28 and Met/H19/miR-216-3p/EBI3 signaling pathways. Int. Immunopharmacol. 2019, 74, 105693. [Google Scholar] [CrossRef]
- Correia-Branco, A.; Keating, E.; Martel, F. Involvement of mTOR, JNK and PI3K in the negative effect of ethanol and metformin on the human first-trimester extravillous trophoblast HTR-8/SVneo cell line. Eur. J. Pharmacol. 2018, 833, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Ding, H. Metformin is not just an antihyperglycaemic drug but also has protective effects on the vascular endothelium. Acta Physiol. 2017, 219, 138–151. [Google Scholar] [CrossRef]
- Austgulen, R.; Lien, E.; Vince, G.; Redman, C.W. Increased maternal plasma levels of soluble adhesion molecules (ICAM-1, VCAM-1, E-selectin) in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 71, 53–58. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Romero, R.; Yoshimatsu, J.; Espinoza, J.; Kim, Y.M.; Park, K.; Kalache, K.; Edwin, S.; Bujold, E.; Gomez, R. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J. Matern. Fetal. Neonatal. Med. 2002, 12, 19–27. [Google Scholar] [CrossRef]
- Kaitu’u-Lino, T.J.; Brownfoot, F.C.; Beard, S.; Cannon, P.; Hastie, R.; Nguyen, T.V.; Binder, N.K.; Tong, S.; Hannan, N.J. Combining metformin and esomeprazole is additive in reducing sFlt-1 secretion and decreasing endothelial dysfunction-implications for treating preeclampsia. PLoS ONE 2018, 13, e0188845. [Google Scholar] [CrossRef]
- Soobryan, N.; Murugesan, S.; Pandiyan, A.; Moodley, J.; Mackraj, I. Angiogenic dysregulation in pregnancy-related hypertension-A role for metformin. Reprod. Sci. 2018, 25, 1531–1539. [Google Scholar] [CrossRef]
- Brownfoot, F.C.; Hastie, R.; Hannan, N.J.; Cannon, P.; Nguyen, T.V.; Tuohey, L.; Cluver, C.; Tong, S.; Kaitu’u-Lino, T.J. Combining metformin and sulfasalazine additively reduces the secretion of antiangiogenic factors from the placenta: Implications for the treatment of preeclampsia. Placenta 2020, 95, 78–83. [Google Scholar] [CrossRef]
- Brouillet, S.; Hoffmann, P.; Feige, J.J.; Alfaidy, N. EG-VEGF: A key endocrine factor in placental development. Trends Endocrinol. Metab. 2012, 23, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Jena, M.K.; Sharma, N.R.; Petitt, M.; Maulik, D.; Nayak, N.R. Pathogenesis of preeclampsia and therapeutic approaches targeting the placenta. Biomolecules 2020, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cao, G.; Yi, W.; Li, L.; Cao, X. Effect of metformin on a preeclampsia-like mouse model induced by high-fat diet. Biomed. Res. Int. 2019, 2019, 6547019. [Google Scholar] [CrossRef]
- Liao, Y.F.; Chen, L.L.; Zeng, T.S.; Li, Y.M.; Fan, Y.U.; Hu, L.J.; Ling, Y. Number of circulating endothelial progenitor cells as a marker of vascular endothelial function for type 2 diabetes. Vasc. Med. 2010, 15, 279–285. [Google Scholar] [CrossRef]
- Asadian, S.; Alibabrdel, M.; Daei, N.; Cheraghi, H.; Maedeh Jafari, S.; Noshadirad, E.; Jabarpour, M.; Siavashi, V.; Nassiri, S.M. Improved angiogenic activity of endothelial progenitor cell in diabetic patients treated with insulin plus metformin. J. Cell Biochem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.Z.; Liu, Z.; Sun, L.L.; Zhou, M.; Liu, C.; Li, W.D.; Li, X.Q. Metformin inhibits angiogenesis of endothelial progenitor cells via miR-221-mediated p27 expression and autophagy. Future Med. Chem. 2019, 11, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Li, W.D.; Li, N.P.; Song, D.D.; Rong, J.J.; Qian, A.M.; Li, X.Q. Metformin inhibits endothelial progenitor cell migration by decreasing matrix metalloproteinases, MMP-2 and MMP-9, via the AMPK/mTOR/autophagy pathway. Int. J. Mol. Med. 2017, 39, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Mizuuchi, M.; Baba, T.; Fujibe, Y.; Ino, Y.; Ishioka, S.; Saito, T. The roles of metformin and pravastatin on placental endoplasmic reticulum stress and placental growth factor in human villous-Like trophoblastic BeWo cells. Sapporo Med. J. 2018, 87, 75–84. [Google Scholar] [CrossRef]
- Brink, H.S.; Alkemade, M.; van der Lely, A.J.; van der Linden, J. Metformin in women at high risk of gestational diabetes mellitus. Diabetes Metab. 2018, 44, 300–302. [Google Scholar] [CrossRef]
- Ainuddin, J.; Karim, N.; Hasan, A.A.; Naqvi, S.A. Metformin versus insulin treatment in gestational diabetes in pregnancy in a developing country: A randomized control trial. Diabetes Res. Clin. Pract. 2015, 107, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Tertti, K.; Ekblad, U.; Koskinen, P.; Vahlberg, T.; Rönnemaa, T. Metformin vs. insulin in gestational diabetes. A randomized study characterizing metformin patients needing additional insulin. Diabetes Obes. Metab. 2013, 15, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Niromanesh, S.; Alavi, A.; Sharbaf, F.R.; Amjadi, N.; Moosavi, S.; Akbari, S. Metformin compared with insulin in the management of gestational diabetes mellitus: A randomized clinical trial. Diabetes Res. Clin. Pract. 2012, 98, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.S.; Donovan, L.E.; Zinman, B.; Sanchez, J.J.; Asztalos, E.; Ryan, E.A.; Fantus, I.G.; Hutton, E.; Armson, A.B.; Lipscombe, L.L.; et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): A multicentre, international, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 834–844. [Google Scholar] [CrossRef]
- Ainuddin, J.A.; Karim, N.; Zaheer, S.; Ali, S.S.; Hasan, A.A. Metformin treatment in type 2 diabetes in pregnancy: An active controlled, parallel-group, randomized, open label study in patients with type 2 diabetes in pregnancy. J. Diabetes Res. 2015, 2015, 325851. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, I.B.D.; Sales, W.B.; Dienstmann, G.; Souza, M.L.R.; Fleig, R.; Silva, J.C. Metformin for prevention of cesarean delivery and large-for-gestational-age newborns in non-diabetic obese pregnant women: A randomized clinical trial. Arch. Endocrinol. Metab. 2020, 64, 290–297. [Google Scholar] [CrossRef]
- Syngelaki, A.; Nicolaides, K.H.; Balani, J.; Hyer, S.; Akolekar, R.; Kotecha, R.; Pastides, A.; Shehata, H. Metformin versus placebo in obese pregnant women without diabetes mellitus. N. Engl. J. Med. 2016, 374, 434–443. [Google Scholar] [CrossRef]
- Løvvik, T.S.; Carlsen, S.M.; Salvesen, Ø.; Steffensen, B.; Bixo, M.; Gómez-Real, F.; Lønnebotn, M.; Hestvold, K.V.; Zabielska, R.; Hirschberg, A.L.; et al. Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 256–266. [Google Scholar] [CrossRef]
- Nascimento, I.B.D.; Dienstmann, G.; de Souza, M.L.R.; Fleig, R.; Hoffmann, C.B.P.C.; Silva, J.C. Evaluation of preeclampsia results after use of metformin in gestation: Systematic review and meta-analysis. Rev. Bras. Ginecol. Obstet. 2018, 40, 713–721. [Google Scholar] [CrossRef]
- Kalafat, E.; Sukur, Y.E.; Abdi, A.; Thilaganathan, B.; Khalil, A. Metformin for prevention of hypertensive disorders of pregnancy in women with gestational diabetes or obesity: Systematic review and meta-analysis of randomized trials. Ultrasound Obstet. Gynecol. 2018, 52, 706–714. [Google Scholar] [CrossRef]
- Butalia, S.; Gutierrez, L.; Lodha, A.; Aitken, E.; Zakariasen, A.; Donovan, L. Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: A systematic review and meta-analysis. Diabet. Med. 2017, 34, 27–36. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, H. Metformin-A potentially effective drug for gestational diabetes mellitus: A systematic review and meta-analysis. J. Matern. Fetal. Neonatal. Med. 2017, 30, 1874–1881. [Google Scholar] [CrossRef]
- Li, G.; Zhao, S.; Cui, S.; Li, L.; Xu, Y.; Li, Y. Effect comparison of metformin with insulin treatment for gestational diabetes: A meta-analysis based on RCTs. Arch. Gynecol. Obstet. 2015, 292, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Poolsup, N.; Suksomboon, N.; Amin, M. Efficacy and safety of oral antidiabetic drugs in comparison to insulin in treating gestational diabetes mellitus: A meta-analysis. PLoS ONE 2014, 9, e109985. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, L.; Fan, Y.Y.; Wang, L.; Li, X.G.; Liu, T.; Cao, Y.S.; Zhao, Z.G. Metformin versus insulin in gestational diabetes mellitus: A meta-analysis of randomized clinical trials. Ir. J. Med. Sci. 2016, 185, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Liu, Q.; Feng, L. Metformin vs insulin in the management of gestational diabetes: A meta-analysis. PLoS ONE 2013, 8, e64585. [Google Scholar] [CrossRef]
- Dodd, J.M.; Grivell, R.M.; Deussen, A.R.; Hague, W.M. Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes. Cochrane Database Syst. Rev. 2018, 7, CD010564. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Lin, X.F.; Wan, Z.H.; Hu, D.; Du, Y.K. Efficacy of metformin on pregnancy complications in women with polycystic ovary syndrome: A meta-analysis. Gynecol. Endocrinol. 2015, 31, 833–839. [Google Scholar] [CrossRef]
- Zheng, J.; Shan, P.F.; Gu, W. The efficacy of metformin in pregnant women with polycystic ovary syndrome: A meta-analysis of clinical trials. J. Endocrinol. Investig. 2013, 36, 797–802. [Google Scholar] [CrossRef]
- Stridsklev, S.; Carlsen, S.M.; Salvesen, Ø.; Clemens, I.; Vanky, E. Midpregnancy Doppler ultrasound of the uterine artery in metformin- versus placebo-treated PCOS women: A randomized trial. J. Clin. Endocrinol. Metab. 2014, 99, 972–977. [Google Scholar] [CrossRef]
- Villa, P.M.; Kajantie, E.; Räikkönen, K.; Pesonen, A.K.; Hämäläinen, E.; Vainio, M.; Taipale, P.; Laivuori, H.; PREDO Study group. Aspirin in the prevention of pre-eclampsia in high-risk women: A randomised placebo-controlled PREDO Trial and a meta-analysis of randomised trials. BJOG 2013, 120, 64–74. [Google Scholar] [CrossRef]
- Martis, R.; Crowther, C.A.; Shepherd, E.; Alsweiler, J.; Downie, M.R.; Brown, J. Treatments for women with gestational diabetes mellitus: An overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 2018, 8, CD012327. [Google Scholar] [CrossRef]
| Studied Group | Size of Groups | Metformin Dose | GA at Entry to the Study | PIH and PE | Authors |
|---|---|---|---|---|---|
| GDM high risk | SG: metformin—24 CG:no treatment—25 | 500–1000 mg | 14th week | PE SG: 0% (0) CG: 8.7% (2) p = 0.049 | Brink et al., 2018 [140] |
| GDM | SG: metformin—43 CG:insulin alone—57 | 500–2500 mg | 20th–36th week | PIH SG: 18.6% (8) CG: 24% (18) NS PE SG: 0% (0) CG: 8% (6) p = 0.05 | Ainuddin et al., 2014 [141] |
| GDM | SG: metformin 110 CG insulin 107 | 500–2000 mg | 22nd–34th week | PIH SG: 1.8% (2) CG: 3.7% (4) p = 0.42, RR 0.5 95% CI 0.1–2.7 PE SG: 4.6% (5) CG: 9.4% (10) p = 0.19, RR 0.5 95% CI 0.2–1.4 | Tertti et al., 2013 [142] |
| GDM | SG metformin: 86 CG insulin: 80 | 1000–2500 mg | 20th–34th week | PIH SG: 5% (4) CG 13.8% (11) p = 0.058, RR 0.4 95% CI 0.1–1.1 PE SG: 6.3% (5) CG: 8.8% (7) p = 0.548, RR 0.7 95% CI 0.2–2.22 | Niromanesh et al., 2012 [143] |
| DMt.2 | SG: metformin—233 CG: insulin—240 | 2000 mg | 6th–22th week | PIH SG: 5% (13) CG 6% (15) p = 0.82, RR 0.92 95% CI 0.46–1.8. PE SG: 15% (37) CG 12% (30) p = 0.29, RR 1.27 95% CI 0.82–1.92 Chronic HT ST: 8% (20) CG: 9% (22) p = 0.68, RR 0.89 95% CI 0.51–1.56 | Feig et al., 2020 [144] |
| DMt.2 | SG: metformin alone—16 CG: insulin alone 100 | 500–2500 mg | about 10th week | PIH SG: 6.2% (1) CG: 36% (36) p= 0.020 PE SG: 25% (4) CG: 17% (17) p = 0.084 | Ainuddin et al., 2015 [145] |
| Obesity | SG: metformin—171 CG: placebo—186 | 1000 mg | <20th week | PE SG: 3.5% (6) CG: 4.8% (9) p = 0.01, RR 0.17 95% CI 0.10–1.41 | Nascimento et al., 2020 [146] |
| Obesity (35 kg/m2) | SG: metformin—202 CG: placebo—198 | 1000–3000 mg | 12th–18th week | PIH SG: 6.4% (13) CG: 6.7% (13) p = 0.93, RR 0.96 95% CI 0.43–2.13 PE SG: 3% (6) CG: 11.3% (13) p = 0.001, RR 0.24 95% CI 0.10–0.61 | Syngelaki et al., 2016 [147] |
| Obesity (BMI > 30 kg/m2) | SG: metformin—221 CG: placebo—222 | 500–2500 mg | 12th–16th week | PIH SG: 10% (21) CG 6% (14) p= 0.22, RR 1.56 95% CI 0.77–3.15. PE SG: 3% (7) CG 1% (3) p = 0.21, RR 2.39 95% 0.61–9.36 | Chiswick et al., 2015 [122] |
| PCOS | SG: metformin—238 CG: placebo—240 | 1000–2000 mg | in the 1st trimester | PE SG: 3% (8) CG: 7% (17) p = 0.10, RR 0.46 95% CI 0.17–1.15 | Løvvik et al., 2019 [148] |
| Studied Group | Comparison | Number of Participants | Metformin Impact on PIH/PE | Authors |
|---|---|---|---|---|
| GDM | metformin vs. insulin | 1260 | PIH RR 0.56 95% CI 0.37–0.85 PE RR 0.83 95% CI 0.60–1.14 PE RR 0.74 95% CI 0.09–6.28 | Kalafat et al., 2018 [150] |
| Obesity | metformin vs. placebo | 840 | ||
| GDM | Metformin vs. insulin | 2165 | ↓PIH RR 0.56 95% CI 0.37–0.85 | Butalia et al., 2017 [151] |
| GDM | metformin vs. insulin | 1556 | ↓HDPs RR 0.82 95% CI 0.67–1.0 | Feng et al., 2017 [152] |
| GDM | metformin vs. insulin | 1110 | ↓PIH RR 0.53 95% CI 0.31–0.90 PE RR 0.81 95% CI 0.55–1.17, | Li et al., 2015 [153] |
| 1634 | ||||
| GDM | metformin vs. insulin | 1110 | ↓PIH RR 0.55 95% CI 0.31–0.91 PE RR 0.84 95% CI 0.57–1.23 | Poolsup et al., 2014 [154] |
| 1299 | ||||
| GDM | metformin vs. insulin | 1712 | PE RR = 0.82 95% CI 0.56–1.2 | Zhu et al., 2014 [155] |
| GDM | metformin vs. insulin | 1110 | ↓PIH RR 0.52 95%CI 0.30–0.90 | Gui et al., 2013 [156] |
| Obesity | metformin vs. no-treatment | 840 614 308 | PIH (obesity) RR 1.24 95% CI 0.76–2.02 ↓PE (obesity) RR 0.51 95% CI 0.26–0.98 ↓PIH (PCOS) RR 0.37 95% CI 0.25–0.57 PE (PCOS) RR 1.96 95% CI 0.81–4.77 ↓PIH (GDM) RR 0.53 95% CI 0.31–0.90 PE (GDM) RR 0.70 95% CI 0.45–1.10 | Nascimento et al., 2018 [149] |
| PCOS | ||||
| GDM | metformin vs. insulin | 1120 1120 | ||
| Obesity | metformin vs. no-treatment or placebo | 1034 | PIH RR 1.02 95% CI 0.54–1.94 PE RR 0.74 95% CI 0.09–6.28 | Dodd et al., 2018 [157] |
| PCOS | metformin vs. no-treatment or placebo | 929 | PE RR 0.92 95% CI 0.28–3.00 | Feng et al., 2015 [158] |
| PCOS | metformin vs. no-treatment or placebo | 878 | ↓PE RR 0.53 95% CI 0.30–0.95 | Zheng et al., 2013 [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poniedziałek-Czajkowska, E.; Mierzyński, R.; Dłuski, D.; Leszczyńska-Gorzelak, B. Prevention of Hypertensive Disorders of Pregnancy—Is There a Place for Metformin? J. Clin. Med. 2021, 10, 2805. https://doi.org/10.3390/jcm10132805
Poniedziałek-Czajkowska E, Mierzyński R, Dłuski D, Leszczyńska-Gorzelak B. Prevention of Hypertensive Disorders of Pregnancy—Is There a Place for Metformin? Journal of Clinical Medicine. 2021; 10(13):2805. https://doi.org/10.3390/jcm10132805
Chicago/Turabian StylePoniedziałek-Czajkowska, Elżbieta, Radzisław Mierzyński, Dominik Dłuski, and Bożena Leszczyńska-Gorzelak. 2021. "Prevention of Hypertensive Disorders of Pregnancy—Is There a Place for Metformin?" Journal of Clinical Medicine 10, no. 13: 2805. https://doi.org/10.3390/jcm10132805
APA StylePoniedziałek-Czajkowska, E., Mierzyński, R., Dłuski, D., & Leszczyńska-Gorzelak, B. (2021). Prevention of Hypertensive Disorders of Pregnancy—Is There a Place for Metformin? Journal of Clinical Medicine, 10(13), 2805. https://doi.org/10.3390/jcm10132805






