Effect of Hepatocellular Carcinoma Surveillance Programmes on Overall Survival in a Mixed Cirrhotic UK Population: A Prospective, Longitudinal Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion Criteria
2.3. Outcomes
2.4. Surveillance Adherence
2.5. Lead-Time Bias Estimation
2.5.1. Rate of Transition to Symptomatic Disease
2.5.2. Describing Lead-Time Using Tumour Size
2.5.3. Counterfactual Estimation
2.6. Data Handling and Statistical Analysis
3. Results
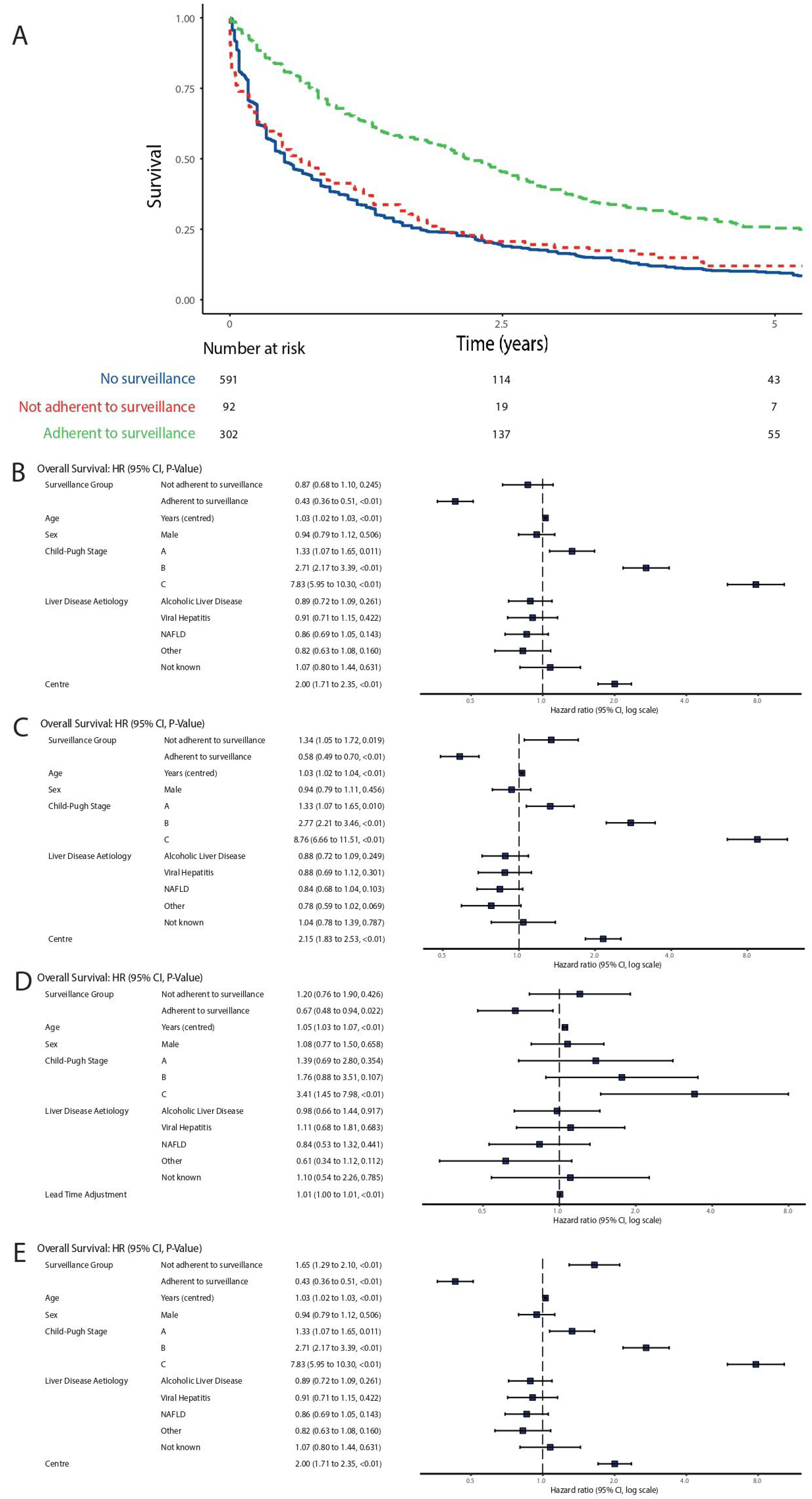
3.1. Enrolment and Adherence to Surveillance
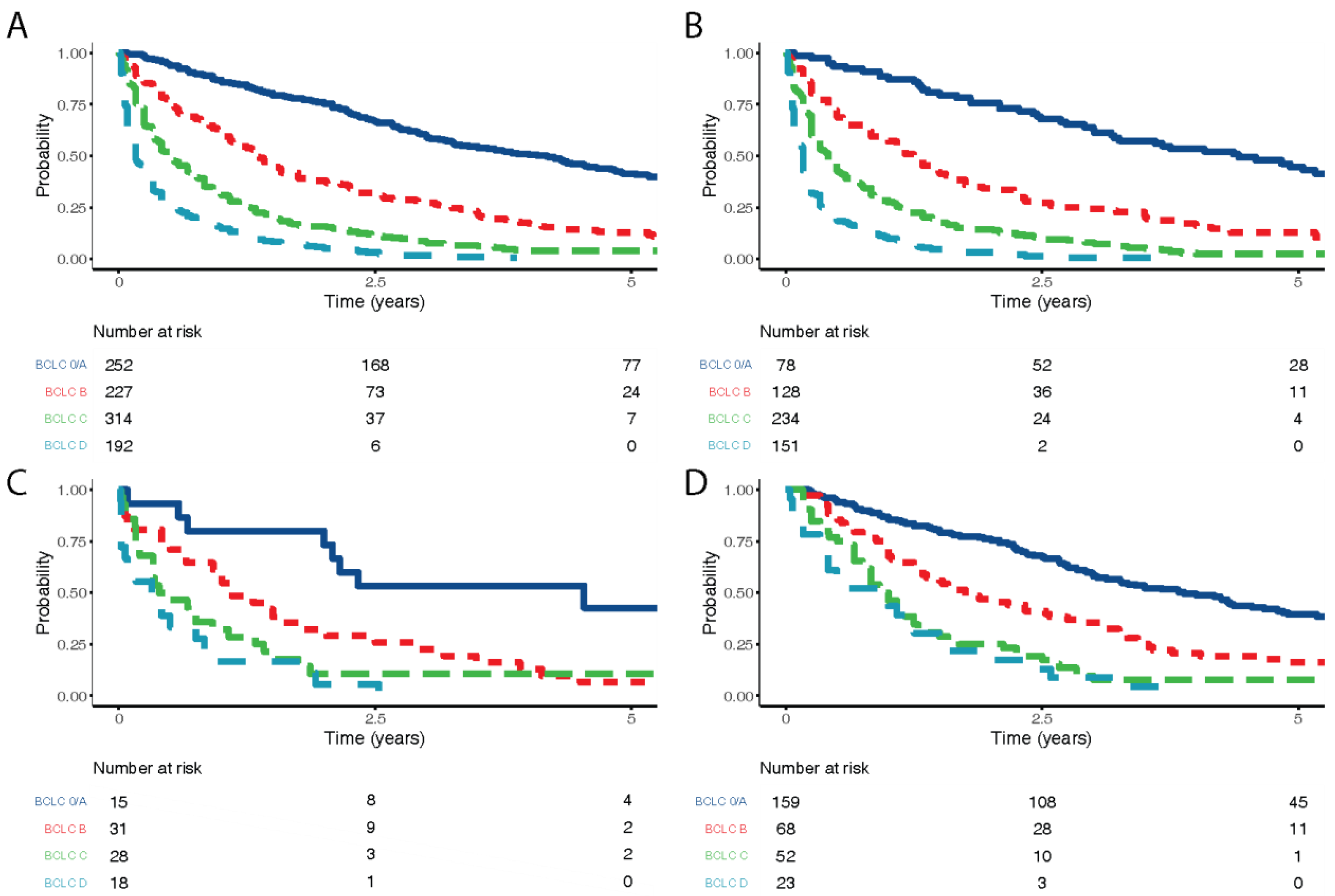
3.2. Stage at Diagnosis
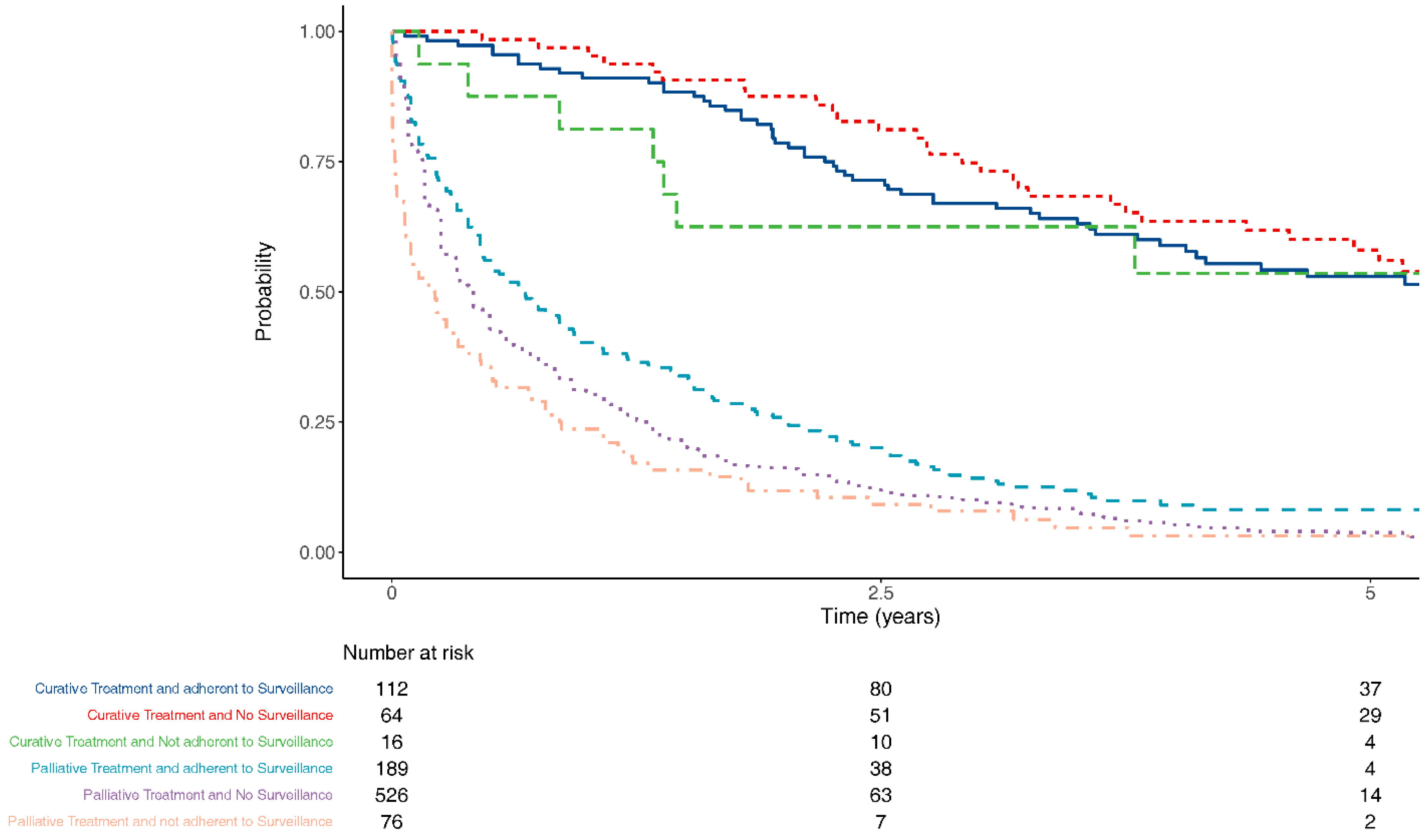
3.3. Treatment Intent
3.4. Survival
3.5. Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2018, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 194–424. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jiang, Y.; Yuan, H.; Fang, Q.; Cai, N.; Suo, C.; Jin, L.; Zhang, T.; Chen, X. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J. Hepatol. 2019, 70, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Available online: www.cancerresearchuk.org/liver-cancer (accessed on 20 October 2018).
- Information Services Division (ISD Scotland). Available online: www.isdscotland.org/Health-Topics/Cancer/Cancer-Statistics/Liver (accessed on 20 October 2018).
- Leon, D.A.; McCambridge, J. Liver cirrhosis mortality rates in Britain from 1950 to 2002: An analysis of routine data. Lancet 2006, 367, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Ioannou, G.N.; Splan, M.F.; Weiss, N.S.; McDonald, G.B.; Beretta, L.; Lee, S.P. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2007, 5, 938–945. [Google Scholar] [CrossRef]
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Ryder, S. Guidelines for the diagnosis and treatment of hepatocellular carcinoma. Gut 2003, 52 (Suppl. S3), iii1–iii8. [Google Scholar] [CrossRef] [Green Version]
- National Institute for Health and Care Excellence. Hepatitis B (Chronic): Diagnosis and management. (NICE Guideline 165). 2013. Available online: https://www.nice.org.uk/guidance/cg165 (accessed on 26 May 2021).
- National Institute for Health and Care Excellence. Cirrhosis in over 16s: Assessment and Management. (NICE Guideline 50). 2016. Available online: https://www.nice.org.uk/guidance/ng50 (accessed on 26 May 2021).
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.; Sirlin, C.B.; Abecassis, M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of HCC. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucchetti, A.; Trevisani, F.; Pecorelli, A.; Erroi, V.; Farinati, F.; Ciccarese, F.; Rapaccini, G.L.; di Marco, M.; Caturelli, E.; Giannini, E.G.; et al. Estimation of lead-time bias and its impact on the outcome of surveillance for the early diagnosis of hepatocellular carcinoma. J. Hepatol. 2014, 61, 333–341. [Google Scholar] [CrossRef]
- Singal, A.G.; Pillai, A.; Tiro, J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: A meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef] [PubMed]
- Pelizzaro, F.; Vitale, A.; Sartori, A.; Vieno, A.; Penzo, B.; Russo, F.P.; Frigo, A.C.; Giannini, E.G.; Piccinnu, M.; Rapaccini, G.L.; et al. Surveillance as determinant of long-term survival in non-transplanted hepatocellular carcinoma patients. Cancers 2021, 13, 897. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, F.T.S. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.A.; Altman, D.G. Basic statistical reporting for articles published in biomedical journals: The “Statistical Analyses and Methods in the Published Literature” or the SAMPL Guidelines. Int. J. Nurs. Stud. 2015, 52, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Duffy, S.W.; Agbaje, O.; Tabar, L.; Vitak, B.; Bjurstam, N.; Björneld, L.; Myles, J.P.; Warwick, J. Overdiagnosis and overtreatment of breast cancer: Estimates of overdiagnosis from two trials of mammographic screening for breast cancer. Breast Cancer Res. 2005, 7, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Bartolomeo, N.; Trerotoli, P.; Serio, G. Progression of liver cirrhosis to HCC: An application of hidden Markov model. BMC Med. Res. Methodol. 2011, 11, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thein, H.-H.; Campitelli, M.A.; Yeung, L.T.; Zaheen, A.; Yoshida, E.M.; Earle, C.C. Improved survival in patients with viral hepatitis-induced hepatocellular carcinoma undergoing recommended abdominal ultrasound surveillance in ontario: A population-based retrospective cohort study. PLoS ONE 2015, 10, e0138907. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Chen, C.J.; Yen, M.F.; Lu, S.-N.; Sun, C.-A.; Huang, G.-T.; Yang, P.-M.; Lee, H.-S.; Duffy, S.W. Ultrasound screening and risk factors for death from hepatocellular carcinoma in a high risk group in Taiwan. Int. J. Cancer 2002, 98, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.H.; Yang, B.H.; Tang, Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef]
- Chen, J.G.; Parkin, D.M.; Chen, Q.G.; Lu, J.-H.; Shen, Q.-J.; Zhang, B.-C.; Zhu, Y.-R. Screening for liver cancer: Results of a randomised controlled trial in Qidong, China. J. Med. Screen 2003, 10, 204–209. [Google Scholar] [CrossRef]
- Von Wagner, C.; Verstraete, W.; Stoffel, S. Psychological aspects of cancer screening. In Oxford Research Encyclopedias; Oxford University Press: Oxford, UK, 2019; Available online: https://oxfordre.com/psychology/view/ (accessed on 26 May 2021).
- Hashimoto, E.; Taniai, M.; Kaneda, H.; Tokushige, K.; Hasegawa, K.; Okuda, H.; Shiratori, K.; Takasaki, K. Comparison of hepatocellular carcinoma patients with alcoholic liver disease and non-alcoholic steatohepatitis. Alcohol. Clin. Exp. Res. 2004, 28, 164S–168S. [Google Scholar] [CrossRef]
- Kansagara, D.; Papak, J.; Pasha, A.S.; O’Neil, M.; Freeman, M.; Relevo, R.; Quiñones, A.; Motu’Apuaka, M.; Jou, J.H. Screening for hepatocellular carcinoma in chronic liver disease: A systematic review. Ann. Intern. Med. 2014, 161, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.H.A.; Yang, J.D.; Giama, N.H.; Choi, J.; Ali, H.M.; Mara, K.C.; Harmsen, W.S.; Wiesner, R.H.; Leise, M.D.; Therneau, T.M.; et al. Factors influencing surveillance for hepatocellular carcinoma in patients with liver cirrhosis. Liver Cancer 2017, 6, 126–136. [Google Scholar] [CrossRef] [PubMed]
- van Meer, S.; de Man, R.A.; Coenraad, M.J.; Sprengers, D.; van Nieuwkerk, K.M.J.; Klümpen, H.J.; Jansen, P.L.M.; Jzermans, j.M.I.; van Oijen, M.G.H.; Siersema, P.D.; et al. Surveillance for hepatocellular carcinoma is associated with increased survival: Results from a large cohort in the Netherlands. J. Hepatol. 2015, 63, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Poustchi, H.; Farrell, G.C.; Strasser, S.I.; Lee, A.U.; McCaughan, G.W.; George, J. Feasibility of conducting a randomized control trial for liver cancer screening: Is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology 2011, 54, 1998–2004. [Google Scholar] [CrossRef]
| Total N | Missing N | Levels | No Surveillance | Not Adherent to Surveillance | Adherent to Surveillance | p | |
|---|---|---|---|---|---|---|---|
| Total N (%) | 591 (60.0) | 92 (9.3) | 302 (30.7) | ||||
| Age at diagnosis | 985 | 0 | Median (25th, 75th centile) | 73.0 (64.5 to 79) | 67.0 (60.8 to 73) | 64.0 (58 to 71) | <0.001 † |
| Sex | 985 | 0 | Female | 106 (17.9) | 22 (23.9) | 68 (22.5) | 0.161 |
| Male | 485 (82.1) | 70 (76.1) | 234 (77.5) | ||||
| Year of diagnosis | 985 | 0 | Median (25th, 75th centile) | 2012.0 (2011 to 2014) | 2012.0 (2011 to 2014) | 2013.0 (2011 to 2014) | 0.007 † |
| Child–Pugh Stage at diagnosis | 985 | 0 | No Cirrhosis | 142 (24.0) | 5 (5.4) | 10 (3.3) | <0.001 |
| A | 169 (28.6) | 32 (34.8) | 150 (49.7) | 0.023 | |||
| B | 209 (35.4) | 46 (50.0) | 114 (37.7) | ||||
| C | 71 (12.0) | 9 (9.8) | 28 (9.3) | ||||
| Alcoholic Liver Disease | 985 | 0 | No | 331 (56.0) | 40 (43.5) | 142 (47.0) | 0.009 |
| Yes | 260 (44.0) | 52 (56.5) | 160 (53.0) | ||||
| Viral hepatitis | 985 | 0 | No | 513 (86.8) | 68 (73.9) | 194 (64.2) | <0.001 |
| Yes | 78 (13.2) | 24 (26.1) | 108 (35.8) | ||||
| Non-alcoholic fatty liver disease | 985 | 0 | No | 447 (75.6) | 69 (75.0) | 236 (78.1) | 0.670 |
| Yes | 144 (24.4) | 23 (25.0) | 66 (21.9) | ||||
| Other | 985 | 0 | No | 387 (65.5) | 69 (75.0) | 250 (82.8) | <0.001 |
| Yes | 204 (34.5) | 23 (25.0) | 52 (17.2) |
| Dependent: Entry to Surveillance | No Surveillance | Entered into Surveillance | OR (Univariable) | OR (Multilevel) | |
|---|---|---|---|---|---|
| Age at diagnosis | Mean (SD) | 70.9 (11.4) | 64.8 (9.7) | 0.95 (0.94–0.96, p < 0.001) | 0.97 (0.95–0.98, p < 0.001) |
| Sex | Female | 106 (54.1) | 90 (45.9) | - | - |
| Male | 485 (61.5) | 304 (38.5) | 0.74 (0.54–1.01, p = 0.059) | 0.60 (0.42–0.85, p = 0.004) | |
| Alcoholic Liver Disease | No | 331 (64.5) | 182 (35.5) | - | - |
| Yes | 260 (55.1) | 212 (44.9) | 1.48 (1.15–1.92, p = 0.003) | 1.75 (1.20–2.55, p = 0.004) | |
| Viral hepatitis | No | 513 (66.2) | 262 (33.8) | - | - |
| Yes | 78 (37.1) | 132 (62.9) | 3.31 (2.42–4.56, p < 0.001) | 2.35 (1.49–3.69, p < 0.001) | |
| Non-alcoholic fatty liver disease | No | 447 (59.4) | 305 (40.6) | - | - |
| Yes | 144 (61.8) | 89 (38.2) | 0.91 (0.67–1.22, p = 0.520) | 1.29 (0.85–1.94, p = 0.231) | |
| Other | No | 387 (54.8) | 319 (45.2) | - | - |
| Yes | 204 (73.1) | 75 (26.9) | 0.45 (0.33–0.60, p < 0.001) | 0.99 (0.62–1.57, p = 0.963) |
| Label | Total N | Missing N | Levels | No Surveillance | Not Adherent to Surveillance | Adherent to Surveillance | p |
|---|---|---|---|---|---|---|---|
| Total N (%) | 591 (60.0) | 92 (9.3) | 302 (30.7) | ||||
| BCLC Stage at diagnosis | 985 | 0 | 0/A | 78 (13.2) | 15 (16.3) | 159 (52.6) | <0.001 |
| B | 128 (21.7) | 31 (33.7) | 68 (22.5) | ||||
| C | 234 (39.6) | 28 (30.4) | 52 (17.2) | ||||
| D | 151 (25.5) | 18 (19.6) | 23 (7.6) | ||||
| AFP level | 941 | 44 | <100 | 299 (50.6) | 61 (66.3) | 241 (79.8) | <0.001 |
| >1000 | 165 (27.9) | 16 (17.4) | 16 (5.3) | ||||
| 100–1000 | 87 (14.7) | 10 (10.9) | 41 (13.6) | ||||
| Not known | 5 (0.8) | 0 (0.0) | 0 (0.0) | ||||
| (Missing) | 35 (5.9) | 5 (5.4) | 4 (1.3) | ||||
| Treatment | 983 | 2 | Liver resection | 36 (6.1) | 4 (4.3) | 24 (7.9) | <0.001 |
| Liver transplant | 8 (1.4) | 3 (3.3) | 45 (14.9) | ||||
| Ablative therapies | 20 (3.4) | 9 (9.8) | 43 (14.2) | ||||
| Sorafenib | 22 (3.7) | 2 (2.2) | 7 (2.3) | ||||
| Supportive care only | 382 (64.6) | 47 (51.1) | 85 (28.1) | ||||
| TACE | 122 (20.6) | 27 (29.3) | 97 (32.1) | ||||
| (Missing) | 1 (0.2) | 0 (0.0) | 1 (0.3) | ||||
| Treatment type | 983 | 2 | Curative therapy | 64 (10.8) | 16 (17.4) | 112 (37.1) | <0.001 |
| Palliative therapy | 144 (24.4) | 29 (31.5) | 104 (34.4) | ||||
| Supportive care only | 382 (64.6) | 47 (51.1) | 85 (28.1) | ||||
| (Missing) | 1 (0.2) | 0 (0.0) | 1 (0.3) | ||||
| Treatment intent | 983 | 2 | Curative | 64 (10.8) | 16 (17.4) | 112 (37.1) | <0.001 |
| Palliative | 526 (89.0) | 76 (82.6) | 189 (62.6) | ||||
| (Missing) | 1 (0.2) | 0 (0.0) | 1 (0.3) |
| Dependent: Treatment Intent | Palliative | Curative | OR (Univariable) | OR (Multilevel) | |
|---|---|---|---|---|---|
| Surveillance group | No surveillance/not compliant | 602 (88.3) | 80 (11.7) | - | - |
| Adherent to surveillance | 189 (62.8) | 112 (37.2) | 0.73 (0.43–1.24, p = 0.249) | 0.67 (0.38–1.20, p = 0.182) | |
| BCLC Stage at diagnosis | 0/A | 98 (39.0) | 153 (61.0) | - | - |
| B | 210 (92.9) | 16 (7.1) | 0.02 (0.01–0.05, p < 0.001) | 0.02 (0.01–0.06, p < 0.001) | |
| C | 296 (94.3) | 18 (5.7) | 0.02 (0.01–0.05, p < 0.001) | 0.02 (0.01–0.05, p < 0.001) | |
| D | 187 (97.4) | 5 (2.6) | 0.00 (0.00–0.01, p < 0.001) | 0.00 (0.00–0.02, p < 0.001) | |
| Age at diagnosis | Median (25th to 75th centile) | 71 (63 to 78) | 71 (63 to 78) | 0.95 (0.93–0.96, p < 0.001) | 0.95 (0.92–0.97, p < 0.001) |
| Sex | Female | 153 (78.1) | 43 (21.9) | - | - |
| Male | 638 (81.1) | 149 (18.9) | 0.83 (0.57–1.23, p = 0.343) | 1.06 (0.62–1.80, p = 0.829) | |
| Alcoholic Liver Disease | No | 406 (79.3) | 106 (20.7) | - | - |
| Yes | 385 (81.7) | 86 (18.3) | 0.86 (0.62–1.17, p = 0.334) | 0.69 (0.40–1.18, p = 0.173) | |
| Child–Pugh Stage | No Cirrhosis | 130 (83.3) | 26 (16.7) | - | - |
| A | 243 (69.4) | 107 (30.6) | 2.20 (1.38–3.61, p = 0.001) | 0.66 (0.31–1.37, p = 0.264) | |
| B | 319 (86.4) | 50 (13.6) | 0.78 (0.47–1.33, p = 0.354) | 0.42 (0.19–0.92, p = 0.029) | |
| C | 99 (91.7) | 9 (8.3) | 0.45 (0.19–0.98, p = 0.054) | 0.78 (0.19–3.31, p = 0.739) | |
| Viral hepatitis | No | 648 (83.8) | 125 (16.2) | - | - |
| Yes | 143 (68.1) | 67 (31.9) | 2.43 (1.71–3.43, p < 0.001) | 0.96 (0.50–1.88, p = 0.913) | |
| Non-alcoholic fatty liver disease | No | 608 (81.1) | 142 (18.9) | - | - |
| Yes | 183 (78.5) | 50 (21.5) | 1.17 (0.81–1.67, p = 0.396) | 1.41 (0.75–2.64, p = 0.288) | |
| Other | No | 556 (78.9) | 149 (21.1) | - | - |
| Yes | 235 (84.5) | 43 (15.5) | 0.68 (0.47–0.98, p = 0.044) | 1.31 (0.64–2.67, p = 0.459) | |
| Adherent to surveillance: BCLC B | Interaction | - | - | 4.50 (1.41–14.81, p = 0.011) | 4.63 (1.41–15.23, p = 0.012) |
| Adherent to surveillance: BCLC C | Interaction | - | - | 4.85 (1.51–14.90, p = 0.006) | 4.46 (1.35–14.72, p = 0.014) |
| Adherent to surveillance: BCLC D | Interaction | - | - | 48.37 (6.26–1006.51, p = 0.001) | 47.55 (4.40–513.48, p = 0.001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, M.I.; Drake, T.M.; Goh, T.L.; Ahmed, A.; Forrest, E.; Barclay, S.; Gillespie, R.; Priest, M.; Evans, J.; Graham, J.; et al. Effect of Hepatocellular Carcinoma Surveillance Programmes on Overall Survival in a Mixed Cirrhotic UK Population: A Prospective, Longitudinal Cohort Study. J. Clin. Med. 2021, 10, 2770. https://doi.org/10.3390/jcm10132770
Haq MI, Drake TM, Goh TL, Ahmed A, Forrest E, Barclay S, Gillespie R, Priest M, Evans J, Graham J, et al. Effect of Hepatocellular Carcinoma Surveillance Programmes on Overall Survival in a Mixed Cirrhotic UK Population: A Prospective, Longitudinal Cohort Study. Journal of Clinical Medicine. 2021; 10(13):2770. https://doi.org/10.3390/jcm10132770
Chicago/Turabian StyleHaq, Mohammad Inamul, Thomas M. Drake, Tee Lin Goh, Asma Ahmed, Ewan Forrest, Stephen Barclay, Ruth Gillespie, Mathew Priest, Jeff Evans, Janet Graham, and et al. 2021. "Effect of Hepatocellular Carcinoma Surveillance Programmes on Overall Survival in a Mixed Cirrhotic UK Population: A Prospective, Longitudinal Cohort Study" Journal of Clinical Medicine 10, no. 13: 2770. https://doi.org/10.3390/jcm10132770
APA StyleHaq, M. I., Drake, T. M., Goh, T. L., Ahmed, A., Forrest, E., Barclay, S., Gillespie, R., Priest, M., Evans, J., Graham, J., Ballantyne, S., McMillan, D. C., Hayes, P. C., Bird, T. G., & Stanley, A. J. (2021). Effect of Hepatocellular Carcinoma Surveillance Programmes on Overall Survival in a Mixed Cirrhotic UK Population: A Prospective, Longitudinal Cohort Study. Journal of Clinical Medicine, 10(13), 2770. https://doi.org/10.3390/jcm10132770






