Understanding COVID-19 Epidemiology and Implications for Control: The Experience from a Greek Semi-Closed Community
Abstract
:1. Introduction
2. Materials and Methods
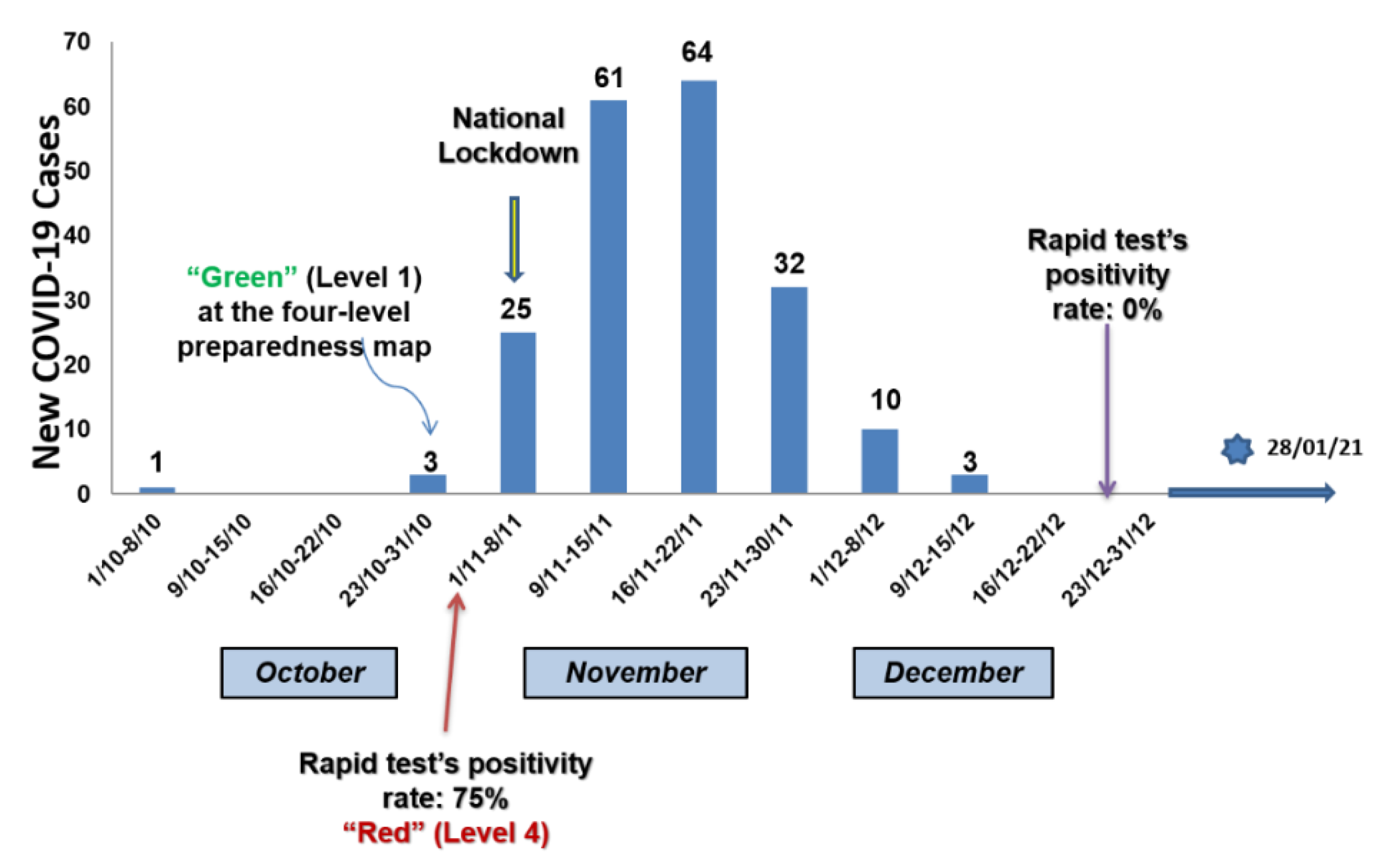
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Novel Coronavirus (2019-nCoV) SITUATION REPORT-1 21 JANUARY 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf (accessed on 1 June 2021).
- National Public Health Organization, (Greek CDC). Current State of Covid-19 Outbreak in Greece and Timeline of Key Con-tainment Events. Available online: https://eody.gov.gr/en/current-state-of-covid-19-outbreak-in-greece-and-timeline-of-key-containment-events/ (accessed on 1 June 2021).
- ECDC. COVID-19 Situation Update Worldwide, as of Week 5, Updated 11 February 2021. Epidemiological Curves. Available online: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (accessed on 1 June 2021).
- Kotsiou, O.; Pantazopoulos, I.; Papagiannis, D.; Fradelos, E.; Kanellopoulos, N.; Siachpazidou, D.; Kirgou, P.; Mouliou, D.; Kyritsis, A.; Kalantzis, G.; et al. Repeated Antigen-Based Rapid Diagnostic Testing for Estimating the Coronavirus Disease 2019 Prevalence from the Perspective of the Workers’ Vulnerability before and during the Lockdown. Int. J. Environ. Res. Public Health 2021, 18, 1638. [Google Scholar] [CrossRef]
- Greek ECDC, daily surveillance report of COVID-19. Available online: https://covid19-surveillance-report.ecdc.europa.eu/archive-COVID19-reports/index.html (accessed on 1 June 2021).
- Wikipedia. COVID-19 pandemic in Greece. Available online: https://en.wikipedia.org/wiki/COVID-19_pandemic_in_Greece#February (accessed on 1 June 2021).
- Chaw, L.; Koh, W.C.; Jamaludin, S.A.; Naing, L.; Alikhan, M.F.; Wong, J. Analysis of SARS-CoV-2 Transmission in Different Settings, Brunei. Emerg. Infect. Dis. 2020, 26, 2598–2606. [Google Scholar] [CrossRef]
- Buss, L.F.; Peter, C.A., Jr.; Abrahim, C.M.M.; Mendrone, A., Jr.; Salomon, T.; de Almeida-Neto, C.; França, R.F.O.; Belotti, M.C.; Carvalho, M.P.S.S.; Costa, A.G.; et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science 2021, 371, 288–292. [Google Scholar] [CrossRef]
- Thomson, S.; Glazebrook, A.J. Infectious diseases in a semiclosed community. J. Hyg. 1941, 41, 570–615. [Google Scholar] [PubMed] [Green Version]
- Polispost. 2020. Available online: https://polispost.com/2021/01/04/istoriko-tou-koronoiou-sti-deskati/?fbclid=IwAR1gETe2HuQjmW37zUk2UYNBykno3V4Vmry5OEIcAzojj76BOa55svto9mg (accessed on 2 June 2021).
- Prognosis Biotech. RAPID TEST AG 2019-NCOV. Available online: https://www.prognosis-biotech.com/products/covid-19/rapid-test-ag-2019-ncov/ (accessed on 2 June 2021).
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 3, CD013705. [Google Scholar] [CrossRef]
- Antigen-Detection in the Diagnosis of SARS-CoV-2, Infection Using Rapid Immunoassays, World Health Organization, Interim Guidance. 11 September 2020. Available online: https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays (accessed on 3 June 2021).
- Applied Biosystems. TaqPath™ COVID-19 CE-IVD RT-PCR Kit Multiplex Real-Time RT-PCR Test Intended for the Qualitative Detection of Nucleic Acid from SARS-CoV-2. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0019215_TaqPathCOVID-19_CE-IVD_RT-PCR%20Kit_IFU.pdf (accessed on 3 June 2021).
- European Commission. COVID-19 In Vitro Diagnostic Devices and Test Methods Database. Available online: https://covid-19-diagnostics.jrc.ec.europa.eu/devices/detail/1499 (accessed on 3 June 2021).
- Abbott. SARS-CoV-2 IgG II Quant Reagent Instructions for Use. December 2020. Available online: https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2 (accessed on 4 March 2021).
- Bryan, A.; Pepper, G.; Wener, M.H.; Fink, S.L.; Morishima, C.; Chaudhary, A.; Jerome, K.R.; Mathias, P.C.; Greninger, A.L. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J. Clin. Microbiol. 2020, 58, e00941-20. [Google Scholar] [CrossRef] [PubMed]
- OECD. Policy Responses to Coronavirus (COVID-19). Testing for COVID-19: How to Best Use the Various Tests? 2020. Available online: http://www.oecd.org/coronavirus/policy-responses/testing-for-covid-19-how-to-best-use-the-various-tests-c76df201/ (accessed on 1 June 2021).
- Pepe, M.S.; Thompson, M.L. Combining diagnostic test results to increase accuracy. Biostatistics 2000, 1, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; Fer-rante Di Ruffano, L.; et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020, 6, CD013652. [Google Scholar] [CrossRef]
- Accorsi, E.K.; Qiu, X.; Rumpler, E.; Kennedy-Shaffer, L.; Kahn, R.; Joshi, K.; Goldstein, E.; Stensrud, M.J.; Niehus, R.; Cevik, M.; et al. How to detect and reduce potential sources of biases in studies of SARS-CoV-2 and COVID-19. Eur. J. Epidemiol. 2021, 36, 179–196. [Google Scholar] [CrossRef]
- Kontou, P.I.; Braliou, G.G.; Dimou, N.L.; Nikolopoulos, G.; Bagos, P.G. Antibody Tests in Detecting SARS-CoV-2 Infection: A Meta-Analysis. Diagnostics 2020, 10, 319. [Google Scholar] [CrossRef]
- Figueiredo-Campos, P.; Blankenhaus, B.; Mota, C.; Gomes, A.; Serrano, M.; Ariotti, S.; Costa, C.; Nunes-Cabaço, H.; Mendes, A.M.; Gaspar, P.; et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur. J. Immunol. 2020, 50, 2025–2040. [Google Scholar] [CrossRef]
- Rostami, A.; Sepidarkish, M.; Leeflang, M.M.; Riahi, S.M.; Shiadeh, M.N.; Esfandyari, S.; Mokdad, A.H.; Hotez, P.J.; Gasser, R.B. SARS-CoV-2 seroprevalence worldwide: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, L.; Lai, A.; Pezzati, L.; Oreni, L.; Bergna, A.; Conti, F.; Meroni, C.; Minisci, D.; Galli, M.; Corbellino, M.; et al. Dynamics of the seroprevalence of SARS-CoV-2 antibodies among healthcare workers at a COVID-19 referral hospital in Milan, Italy. Occup. Environ. Med. 2021, oemed–2020–107060. [Google Scholar] [CrossRef]
- Kolthur-Seeheram, U.; Shah, D.; Shastri, J.; Juneja, S.; Kang, G.; Malani, A.; Mohanan, M.; Lobo, G.A.; Velhal, G.; Gomore, M. Mumbai-Serosurvey Technical Report-NITI. 2021. Available online: http://www.tcs.tifr.res.in/~sandeepj/avail_papers/Mumbai-Serosurvey%20Technical%20report-NITI.pdf?referringSource=articleShare (accessed on 1 June 2021).
- Feikin, D.R.; Widdowson, M.-A.; Mulholland, K. Estimating the Percentage of a Population Infected with SARS-CoV-2 Using the Number of Reported Deaths: A Policy Planning Tool. Pathogens 2020, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Havers, F.P.; Reed, C.; Lim, T.; Montgomery, J.M.; Klena, J.D.; Hall, A.J.; Fry, A.M.; Cannon, D.L.; Chiang, C.-F.; Gibbons, A.; et al. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern. Med. 2020, 180, 1576. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, T.A.; Parker, D.M.; Bartell, S.M.; Vieira, V.M.; Khan, S.; Noymer, A.; Drum, E.; Albala, B.; Zahn, M.; Boden-Albala, B. Estimated seroprevalence of SARS-CoV-2 antibodies among adults in Orange County, California. Sci. Rep. 2021, 11, 3081. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef]
- Han, D.; Li, R.; Han, Y.; Zhang, R.; Li, J. COVID-19: Insight into the asymptomatic SARS-COV-2 infection and transmission. Int. J. Biol. Sci. 2020, 16, 2803–2811. [Google Scholar] [CrossRef]
- Götzinger, F.; Santiago-García, B.; Noguera-Julián, A.; Lanaspa, M.; Lancella, L.; Carducci, F.I.C.; Gabrovska, N.; Velizarova, S.; Prunk, P.; Osterman, V.; et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc. Health 2020, 4, 653–661. [Google Scholar] [CrossRef]
- Steinberg, E.; Wright, E.; Kushner, B. In Young Adults with COVID-19, Obesity Is Associated with Adverse Outcomes. West. J. Emerg. Med. 2020, 21, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, H.R.; Bielecki, M.; Siegrist, D.; Buehrer, T.W.; Züst, R.; Deuel, J.W. Titers of Neutralizing Antibodies against SARS-CoV-2 Are Independent of Symptoms of Non-Severe COVID-19 in Young Adults. Viruses 2021, 13, 284. [Google Scholar] [CrossRef]
- Alshukairi, A.N.; Khalid, I.; Ahmed, W.A.; Dada, A.M.; Bayumi, D.T.; Malic, L.S.; Althawadi, S.; Ignacio, K.; AlSalmi, H.S.; Al-Abdely, H.M.; et al. Antibody Response and Disease Severity in Healthcare Worker MERS Survivors. Emerg. Infect. Dis. 2016, 22, 1113–1115. [Google Scholar] [CrossRef] [Green Version]
- Marklund, E.; Leach, S.; Axelsson, H.; Nyström, K.; Norder, H.; Bemark, M.; Angeletti, D.; Lundgren, A.; Nilsson, S.; Andersson, L.-M.; et al. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PLoS ONE 2020, 15, e0241104. [Google Scholar] [CrossRef]
- Vogelzang, E.H.; Loeff, F.C.; Derksen, N.I.L.; Kruithof, S.; Ooijevaar-de Heer, P.; Van Mierlo, G.; Linty, F.; Mok, J.Y.; Van Esch, W.; De Bruin, S.; et al. Development of a SARS-CoV-2 Total Antibody Assay and the Dynamics of Antibody Response over Time in Hospitalized and Nonhospitalized Patients with COVID-19. J. Immunol. 2020, 205, 3491–3499. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.C.; Deng, K.; Chen, Y.K.; Liao, P.; Qiu, J.F.; Lin, Y.; Cai, X.F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-H.; Zhao, R.; Zhou, J.-B.; Wang, F.; Kong, D.-G.; Sun, J.-B.; Ruan, Q.-F.; Liu, M.-Q. Serologic Response to SARS-CoV-2 in COVID-19 Patients with Different Severity. Virol. Sin. 2020, 35, 752–757. [Google Scholar] [CrossRef]
- Klein, S.L.; Pekosz, A.; Park, H.-S.; Ursin, R.L.; Shapiro, J.R.; Benner, S.E.; Littlefield, K.; Kumar, S.; Naik, H.M.; Betenbaugh, M.J.; et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J. Clin. Investig. 2020, 130, 6141–6150. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front. Mol. Biosci. 2020, 7, 157. [Google Scholar] [CrossRef]
| Variable | Total (n = 388) | Males (n = 180) | Females (n = 208) | |
|---|---|---|---|---|
| Age (years) | 51.5 ± 18.6 | 52.1 ± 19.3 | 51.1 ± 18.1 | |
| BMI (mg/kg2) | 32 ± 6 | 32 ± 5 | 32 ± 6 | |
| Smoking status | ||||
| Ex-smokers n, (%) | 73 (18.8) | 58 (32.2) | 15 (7.2) | |
| Current smokers n, (%) | 74 (19.1) | 45 (25) | 29 (13.9) | |
| Non-smokers n, (%) | 241 (62.1) | 77 (42.8) | 164 (78.8) | |
| Comorbidities (yes, n (%) | 211 (54.4) | 101 (56.1) | 110 (52.9) | |
| Medication (yes), n (%) | 187 (48.1) | 91 (50.6) | 96 (46.2) | |
| Immunosuppression (cancer, autoimmune disease) (yes), n (%) | 7 (1.8) | 3 (1.7) | 4 (1.9) | |
| Respiratory symptoms the last four months (yes), n (%) | 164 (42.3) | 69 (38.3) | 95 (45.7) | |
| Health care visit the last 4 months (yes), n (%) | 81 (20.9) | 40 (22.2) | 41 (19.7) | |
| Travels the last 4 months, (yes), n (%) | 58 (14.9) | 29 (16.1) | 29 (13.9) | |
| Previous exposure to a COVID-19 positive case, n (%) | 20 (5.2) | 10 (5.6) | 10 (4.8) | |
| Past PCR-confirmed COVID-19 infection, (yes), n (%) | 102 (26.3) | 51 (28.3) | 51 (24.5) | |
| Past COVID-19 hospitalizations, (yes), n (%) | 17 (4.4) | 11 (6.1) | 6 (2.9) | |
| Mean duration of hospitalization (days) | 8 ± 5 | 9 ± 5 | 9 ± 7 | |
| Mean duration of home isolation (days) | 21 ± 11 | 20 ± 10 | 21 ± 12 | |
| Previous SARS-CoV-2 antibody testing, (yes), n (%) | 62 (16%) | 29 (16.1) | 33 (15.9) | 0.528 |
| Variable | n of Individuals with Previously Confirmed SARS-CoV-2 infection (n = 102) | Positive Antibody Test (n = 35) | Negative Antibody Test (67) | p-Value |
|---|---|---|---|---|
| Gender Male n, (%) | 51 (0.5) | 20 (57.1) | 31 (46.3) | 0.202 |
| Age (years) | 51.2 ± 18.6 | 61.0 ± 14.6 | 46.0 ± 18.4 | <0.001 |
| BMI (mg/kg2) | 32 ± 6 | 33 ± 5 | 32 ± 6 | 0.385 |
| Smoking status Ex- or non-smokers n, (%) Current smokers n, (%) | 89 (87.3) 13 (12.7) | 34 (97.1) 1 (2.9) | 55 (82.1) 12 (17.9) | 0.025 |
| Comorbidities (yes), n (%) | 64 (62.7) | 25 (71.4) | 39 (71.4) | 0.136 |
| Medication (yes), n (%) | 53 (52.0) | 25 (71.4) | 28 (41.8) | 0.004 |
| Immunosuppression (cancer, autoimmune disease) (yes), n (%) | 4 (3.9) | 3 (8.6) | 1 (1.5) | 0.116 |
| Respiratory symptoms the last four months (yes), n (%) | 74 (72.5) | 26 (74.2) | 48 (71.6) | 0.484 |
| Health care visit the last 4 months (yes), n (%) | 47 (46.1) | 20 (57.1) | 27 (40.3) | 0.079 |
| Past COVID-19 hospitalizations, (yes), n (%) | 17 (16.6) | 11 (31.4) | 6 (8.9) | <0.001 |
| Variables | B | SE | Wald | Sig | Exp (B) | 95% CI for Exp (B) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age a | 0.05 | 0.02 | 6.16 | 0.01 | 1.05 | 1.01 | 1.09 |
| Medication | 0.12 | 0.61 | 0.04 | 0.85 | 1.12 | 0.34 | 3.69 |
| Hospitalization | 1.45 | 0.63 | 5.40 | 0.02 | 4.27 | 1.26 | 14.52 |
| Variable | n of Previously Confirmed SARS-CoV-2 Infection (n = 69) | Previously Known COVID-19 Disease (n = 35) | Previously Unknown COVID-19 Disease (34) | p-Value |
|---|---|---|---|---|
| Gender Male n, (%) | 35 (50.7) | 20 (57.1) | 15 (44.1) | 0.200 |
| Age (years) | 51.2 ± 18.6 | 61.0 ± 14.6 | 47.9 ± 18.2 | 0.002 |
| BMI (mg/kg2) | 32 ± 6 | 33 ± 5 | 30 ± 5 | 0.037 |
| Smoking status Ex- or non-smokers n, (%) Current smokers n, (%) | 60 (87.0) 9 (13.0) | 34 (97.1) 1 (2.9) | 26 (76.5) 8 (23.5) | 0.012 |
| Comorbidities (yes, n (%) | 39 (56.5) | 25 (71.4) | 14 (41.2) | 0.011 |
| Medication (yes), n (%) | 35 (50.7) | 25 (71.4) | 10 (29.4) | 0.001 |
| Immunosuppression (cancer, autoimmune disease) (yes), n (%) | 3 (4.3) | 3 (8.6) | 0 | 0.116 |
| Respiratory symptoms the last four months (yes), n (%) | 40 (58.0) | 26 (74.2) | 14 (41.2) | 0.005 |
| Health care visit the last 4 months (yes), n (%) | 23 (33.3) | 20 (57.1) | 3 (8.8) | <0.001 |
| Past COVID-19 hospitalizations, (yes), n (%) | 11 (15.9) | 11 (31.4) | 0 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsiou, O.S.; Papagiannis, D.; Fradelos, E.C.; Perlepe, G.; Miziou, A.; Siachpazidou, D.S.; Gourgoulianis, K.I. Understanding COVID-19 Epidemiology and Implications for Control: The Experience from a Greek Semi-Closed Community. J. Clin. Med. 2021, 10, 2765. https://doi.org/10.3390/jcm10132765
Kotsiou OS, Papagiannis D, Fradelos EC, Perlepe G, Miziou A, Siachpazidou DS, Gourgoulianis KI. Understanding COVID-19 Epidemiology and Implications for Control: The Experience from a Greek Semi-Closed Community. Journal of Clinical Medicine. 2021; 10(13):2765. https://doi.org/10.3390/jcm10132765
Chicago/Turabian StyleKotsiou, Ourania S., Dimitrios Papagiannis, Evangelos C. Fradelos, Garyfallia Perlepe, Angeliki Miziou, Dimitra S. Siachpazidou, and Konstantinos I. Gourgoulianis. 2021. "Understanding COVID-19 Epidemiology and Implications for Control: The Experience from a Greek Semi-Closed Community" Journal of Clinical Medicine 10, no. 13: 2765. https://doi.org/10.3390/jcm10132765
APA StyleKotsiou, O. S., Papagiannis, D., Fradelos, E. C., Perlepe, G., Miziou, A., Siachpazidou, D. S., & Gourgoulianis, K. I. (2021). Understanding COVID-19 Epidemiology and Implications for Control: The Experience from a Greek Semi-Closed Community. Journal of Clinical Medicine, 10(13), 2765. https://doi.org/10.3390/jcm10132765









