Fecal Microbial Transplantation versus Mesalamine Enema for Treatment of Active Left-Sided Ulcerative Colitis—Results of a Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Randomization
2.4. Interventions
2.5. Clinical Outcomes
2.6. FMT Enema Preparation
2.7. Assessment and Analysis of the Microbiome
2.8. Statistical Analysis
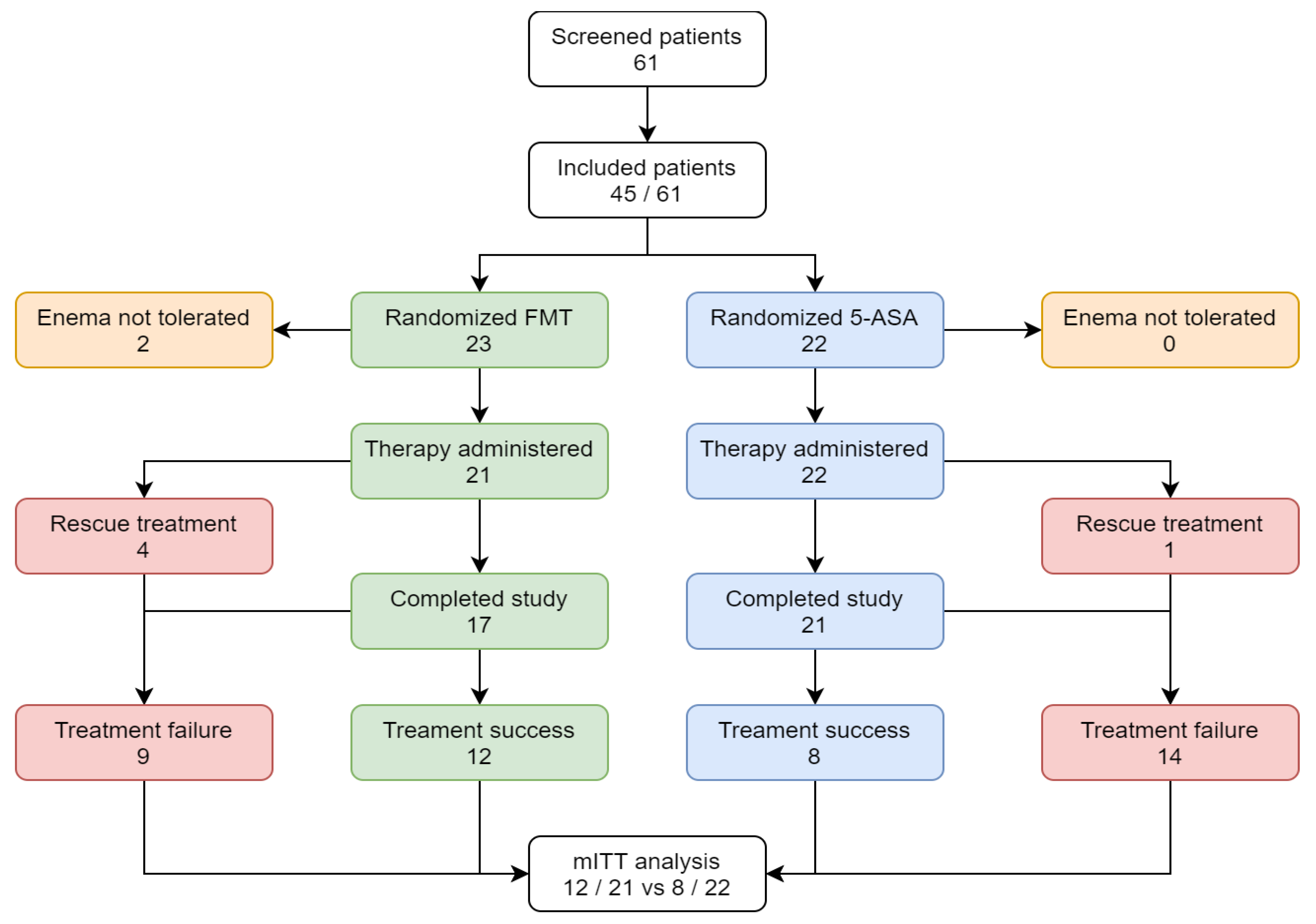
3. Results
3.1. Clinical Outcome
3.2. Safety
3.3. Donor Selection
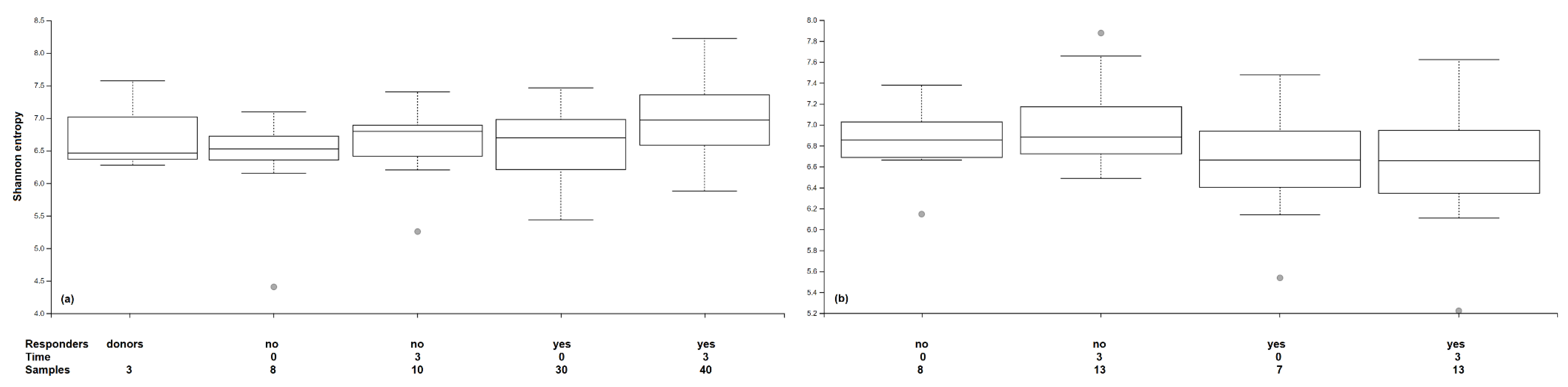
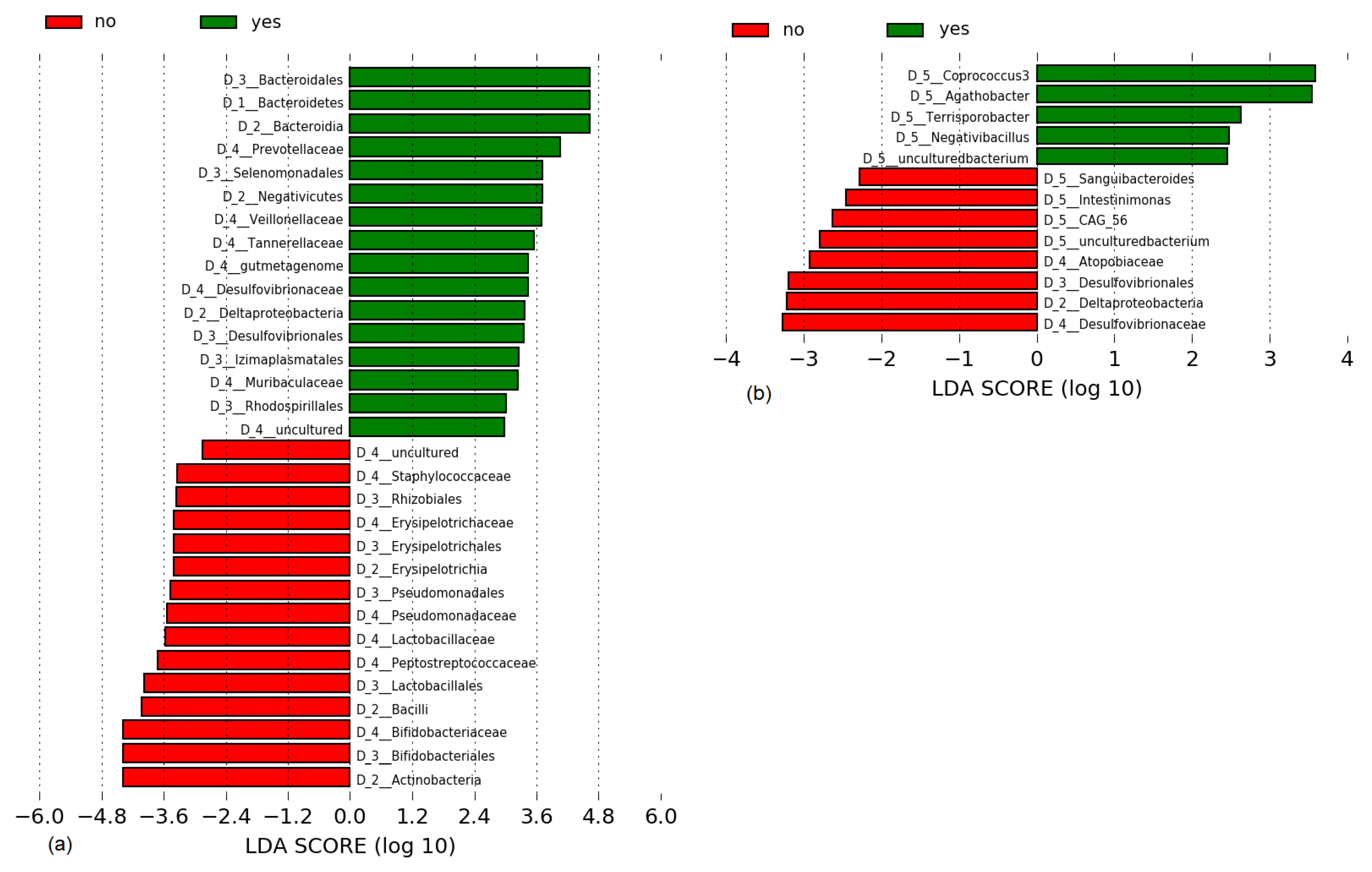
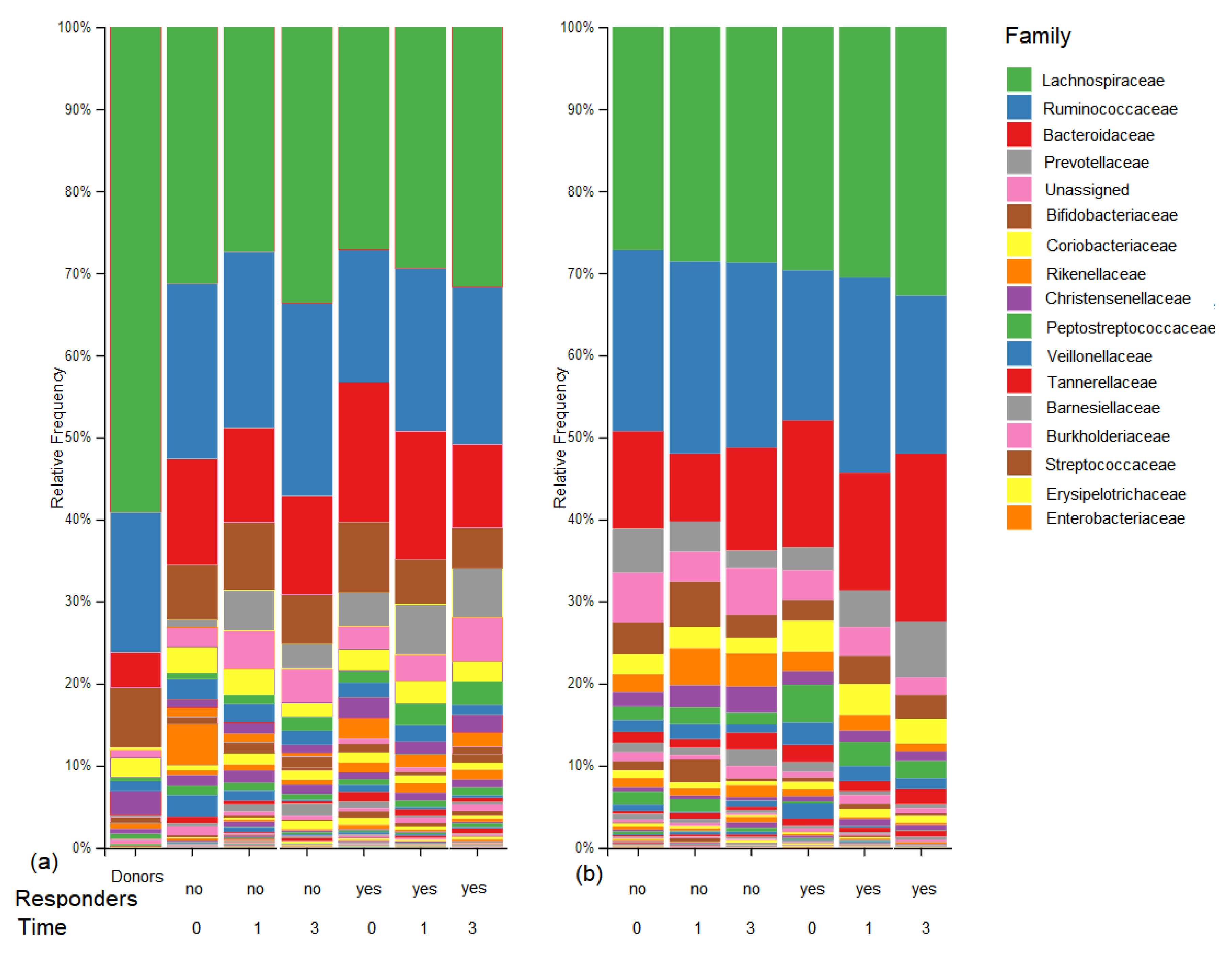
3.4. Microbiome Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, L.; Gordon, M.; Baines, P.A.; Iheozor-Ejiofor, Z.; Sinopoulou, V.; Akobeng, A.K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020, 3, CD005573. [Google Scholar] [CrossRef] [PubMed]
- Laurell, A.; Sjoberg, K. Prebiotics and synbiotics in ulcerative colitis. Scand. J. Gastroenterol. 2017, 52, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Quraishi, M.N.; Widlak, M.; Bhala, N.; Moore, D.; Price, M.; Sharma, N.; Iqbal, T.H. Systematic review with meta-analysis: The efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment. Pharmacol. Ther. 2017, 46, 479–493. [Google Scholar] [CrossRef] [Green Version]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [Green Version]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.; Duflou, A.; Lowenberg, M.; van den Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M.; et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology 2015, 149, 110–118.e4. [Google Scholar] [CrossRef]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; Leong, R.W.L.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.; Mahajan, R.; Singh, A.; Midha, V.; Mehta, V.; Narang, V.; Singh, T.; Singh Pannu, A. Role of Faecal Microbiota Transplantation for Maintenance of Remission in Patients With Ulcerative Colitis: A Pilot Study. J. Crohns Colitis 2019, 13, 1311–1317. [Google Scholar] [CrossRef]
- Da Silva, B.C.; Lyra, A.C.; Rocha, R.; Santana, G.O. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J. Gastroenterol. 2014, 20, 9458–9467. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.D.; Woseth, D.M.; Thisted, R.A.; Hanauer, S.B. A meta-analysis and overview of the literature on treatment options for left-sided ulcerative colitis and ulcerative proctitis. Am. J. Gastroenterol. 2000, 95, 1263–1276. [Google Scholar] [CrossRef]
- Fliegerova, K.; Tapio, I.; Bonin, A.; Mrazek, J.; Callegari, M.L.; Bani, P.; Bayat, A.; Vilkki, J.; Kopecny, J.; Shingfield, K.J.; et al. Effect of DNA extraction and sample preservation method on rumen bacterial population. Anaerobe 2014, 29, 80–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Biancone, L.; Gionchetti, P.; Blanco Gdel, V.; Orlando, A.; Annese, V.; Papi, C.; Sostegni, R.; D’Inca, R.; Petruzziello, C.; Casa, A.; et al. Beclomethasone dipropionate versus mesalazine in distal ulcerative colitis: A multicenter, randomized, double-blind study. Dig. Liver Dis. 2007, 39, 329–337. [Google Scholar] [CrossRef]
- Anderson, J.L.; Edney, R.J.; Whelan, K. Systematic review: Faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment. Pharmacol. Ther. 2012, 36, 503–516. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; Shanahan, F.; Guarner, F.; de Vos, W.M. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm. Bowel Dis. 2013, 19, 481–488. [Google Scholar] [CrossRef]
- Kassam, Z.; Hundal, R.; Marshall, J.K.; Lee, C.H. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch. Intern. Med. 2012, 172, 191–193. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.H.; Zhu, C.X.; Quan, Y.S.; Yang, Z.Y.; Wu, S.; Luo, W.W.; Tan, B.; Wang, X.Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef]
- Xu, J.; Chen, N.; Wu, Z.; Song, Y.; Zhang, Y.; Wu, N.; Zhang, F.; Ren, X.; Liu, Y. 5-Aminosalicylic Acid Alters the Gut Bacterial Microbiota in Patients With Ulcerative Colitis. Front. Microbiol. 2018, 9, 1274. [Google Scholar] [CrossRef]
- Khanna, S.; Raffals, L.E. The Microbiome in Crohn’s Disease: Role in Pathogenesis and Role of Microbiome Replacement Therapies. Gastroenterol. Clin. N. Am. 2017, 46, 481–492. [Google Scholar] [CrossRef]
- Schierova, D.; Brezina, J.; Mrazek, J.; Fliegerova, K.O.; Kvasnova, S.; Bajer, L.; Drastich, P. Gut Microbiome Changes in Patients with Active Left-Sided Ulcerative Colitis after Fecal Microbiome Transplantation and Topical 5-aminosalicylic Acid Therapy. Cells 2020, 9, 2283. [Google Scholar] [CrossRef]
- Chen, H.T.; Huang, H.L.; Xu, H.M.; Luo, Q.L.; He, J.; Li, Y.Q.; Zhou, Y.L.; Nie, Y.Q.; Zhou, Y.J. Fecal microbiota transplantation ameliorates active ulcerative colitis. Exp. Ther. Med. 2020, 19, 2650–2660. [Google Scholar] [CrossRef] [Green Version]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef]
- Olaisen, M.; Spigset, O.; Flatberg, A.; Granlund, A.V.B.; Brede, W.R.; Albrektsen, G.; Royset, E.S.; Gilde, B.; Sandvik, A.K.; Martinsen, T.C.; et al. Mucosal 5-aminosalicylic acid concentration, drug formulation and mucosal microbiome in patients with quiescent ulcerative colitis. Aliment. Pharmacol. Ther. 2019, 49, 1301–1313. [Google Scholar] [CrossRef] [Green Version]
- Jalanka, J.; Hillamaa, A.; Satokari, R.; Mattila, E.; Anttila, V.J.; Arkkila, P. The long-term effects of faecal microbiota transplantation for gastrointestinal symptoms and general health in patients with recurrent Clostridium difficile infection. Aliment. Pharmacol. Ther. 2018, 47, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Goloshchapov, O.V.; Olekhnovich, E.I.; Sidorenko, S.V.; Moiseev, I.S.; Kucher, M.A.; Fedorov, D.E.; Pavlenko, A.V.; Manolov, A.I.; Gostev, V.V.; Veselovsky, V.A.; et al. Long-term impact of fecal transplantation in healthy volunteers. BMC Microbiol. 2019, 19, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Fecal Microbiota Transplantation (n = 23) | 5–ASA Enema (n = 22) | |
|---|---|---|
| Male | 12 (52%) | 11 (50%) |
| Female | 11 (48%) | 11 (50%) |
| Age | 39 (25–63) | 39.5 (27–70) |
| Disease duration (years) | 9 (1–20) | 4.5 (0.6–20) |
| Prior biologic exposure | 0 (0%) | 0 (0%) |
| Concomitant therapy | ||
| Oral 5-aminosalicylate | 19 (83%) | 18 (82%) |
| Oral steroids | 3 (13%) | 2 (9%) |
| Oral immunomodulator | 5 (22%) | 4 (18%) |
| Total Mayo score | 6 (4–10) | 6 (4–10) |
| Endoscopic Mayo = 2 | 19 (83%) | 18 (82%) |
| Mayo = 3 | 4 (17%) | 4 (18%) |
| Fecal calprotectin (µg/g) | 1817.5 (166–6000) | 1220 (80–6000) |
| C-reactive protein (mg/L) | 2.3 (0.3–25) | 2.1 (0.4–32.8) |
| White-cell count (×10⁹ cells/L) | 7.9 (5.4–11.7) | 6.3 (3.9–12.3) |
| Hemoglobin (g/L) | 141 (107–163) | 142 (104–161) |
| Fecal Microbiota Transplantation (n = 21) | 5-ASA Enema (n = 22) | 95% CI for Difference | |
|---|---|---|---|
| Primary outcome | |||
| Clinical remission (week 12) | 12 (57%) | 8 (36%) | (−7.6%, 48.9%) |
| Secondary outcome | p-value | ||
| Clinical response (week 6) | 14 (64%) | 12 (55%) | 0.53 |
| Clinical response (week 12) | 15 (71%) | 12 (55%) | 0.35 |
| Endoscopic remission (week 6) | 3 (14%) | 1 (5%) | 0.34 |
| Endoscopic remission (week 12) | 3 (14%) | 3 (14%) | 1.0 |
| Fecal Microbiota Transplantation (n = 21) | 5-ASA Enema (n = 22) | |
|---|---|---|
| Total adverse events | 22 | 21 |
| Total patients with adverse events | 12 (57%) | 13 (59%) |
| Total patients with serious adverse events | 4 (19%) | 1 (5%) |
| Infection | 1 (5%) | 0 (0%) |
| Worsening of ulcerative colitis | 8 | 9 |
| Abdominal pain | 5 | 8 |
| Bloating | 2 | 1 |
| Rash | 0 | 1 |
| Fever | 2 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Březina, J.; Bajer, L.; Wohl, P.; Ďuricová, D.; Hrabák, P.; Novotný, A.; Koželuhová, J.; Lukáš, M.; Mrázek, J.; Fliegerová, K.O.; et al. Fecal Microbial Transplantation versus Mesalamine Enema for Treatment of Active Left-Sided Ulcerative Colitis—Results of a Randomized Controlled Trial. J. Clin. Med. 2021, 10, 2753. https://doi.org/10.3390/jcm10132753
Březina J, Bajer L, Wohl P, Ďuricová D, Hrabák P, Novotný A, Koželuhová J, Lukáš M, Mrázek J, Fliegerová KO, et al. Fecal Microbial Transplantation versus Mesalamine Enema for Treatment of Active Left-Sided Ulcerative Colitis—Results of a Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(13):2753. https://doi.org/10.3390/jcm10132753
Chicago/Turabian StyleBřezina, Jan, Lukáš Bajer, Pavel Wohl, Dana Ďuricová, Pavel Hrabák, Aleš Novotný, Jana Koželuhová, Milan Lukáš, Jakub Mrázek, Kateřina Olša Fliegerová, and et al. 2021. "Fecal Microbial Transplantation versus Mesalamine Enema for Treatment of Active Left-Sided Ulcerative Colitis—Results of a Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 13: 2753. https://doi.org/10.3390/jcm10132753
APA StyleBřezina, J., Bajer, L., Wohl, P., Ďuricová, D., Hrabák, P., Novotný, A., Koželuhová, J., Lukáš, M., Mrázek, J., Fliegerová, K. O., Kvasnová, S., Chahrazed, M., Mareš, J., Špičák, J., & Drastich, P. (2021). Fecal Microbial Transplantation versus Mesalamine Enema for Treatment of Active Left-Sided Ulcerative Colitis—Results of a Randomized Controlled Trial. Journal of Clinical Medicine, 10(13), 2753. https://doi.org/10.3390/jcm10132753







