1. Introduction
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease involving multiple organ systems, such as the skin, kidneys, blood, joints, and brain [
1]. The disease predominantly affects women of childbearing age, with female-to-male ratio of 9 to 1. The clinical course of SLE is highly variable with recurrent relapses and exacerbations. Despite the advancements in therapeutic options and the improvement in the survival rate for SLE [
2], a high proportion of patients living with SLE have a poor health-related quality of life (HRQoL) compared with healthy individuals as well as patients with other chronic diseases, such as diabetes, hypertension, and even heart failure [
3]. Fatigue, pain, and musculoskeletal distress associated with SLE have been reported to be the main predictors of poor HRQoL [
4]. Older age, poverty, lower educational level, behavioral issues, some clinical manifestations, and comorbidities could also have an impact on HRQoL [
5]. In addition, disease activity status has been suggested to adversely affect HRQoL in patients with SLE [
6,
7,
8,
9,
10,
11].
One of the most commonly used measures for the global disease activity of SLE is the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) introduced in 2002. It is a modification of the original Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) developed by consensus of a group of experienced clinicians in the field of lupus research [
12]. The SLEDAI-2K was validated against SLEDAI in a cohort of 960 patients and a high correlation of 0.97 between the two indices was reported [
13]. More recently, a new 17-item Systemic Lupus Erythematosus Disease Activity Score (SLE-DAS) with improved sensitivity to changes in SLE disease activity as compared with SLEDAI was proposed. In a study of 520 patients with SLE, the SLE-DAS showed a significantly better performance than SLEDAI-2K in identifying clinically meaningful changes in disease activity and in predicting damage accrual [
14]. The scale was subsequently validated in an independent cohort of 227 Latin American patients with Mexican Mestizo ethnicity. Nevertheless, the authors concluded that SLE-DAS did not add an advantage over the existing SLEDAI-2K score, particularly regarding its suboptimal performance in patients with high disease activity [
15]. In addition, the choice of outcome measures for the musculoskeletal component in SLE-DAS has been challenged by a study that reanalyzed the data with SLE-DAS obtained from a longitudinal study of patients with SLE [
16]. Furthermore, another study retrospectively calculated SLE-DAS for 41 patients with lupus nephritis and revealed that the performance of SLE-DAS among patients of high disease activity might not be robust. The authors concluded that there might be no added advantage over the existing SLEDAI-2K score in the current state of SLE-DAS [
17].
Given that measuring SLE disease activity remains a challenging and complex task, it is clear that a broader evaluation of the new SLE-DAS is needed, particularly, in diverse populations across a spectrum of severity and types of clinical manifestations of SLE [
18]. At present, no studies have yet attempted to compare the correlation of these two indices in predicting HRQoL in patients with SLE. Therefore, the aim of this cross-sectional study was to compare the correlation of SLEDAI-2K and SLE-DAS with a disease-specific HRQoL, the Lupus Quality of Life questionnaire (LupusQoL) [
19], in patients with SLE.
3. Results
The demographic and clinical information of the 333 patients with SLE are shown in
Table 1. In brief, 90.4% were female and 40% were between the ages of 20 and 39 years. Approximately 54% of the patients had a normal body mass index, and 50% had an educational level of college or above. About 29% had to change their jobs due to SLE, and 73% rated their own health as average or below. In addition, 64% of the patients had SLE for more than nine years. In addition, 55.3% patients with SLE had low complement levels and 35.1% had increased anti-double strain DNA antibody titer. Clinically, 61.6% patients with SLE had Raynaud’s phenomenon and 51.7% had photosensitivity.
Summary statistics of SLEDAI-2K, SLE-DAS, and individual domains of LupusQoL are also presented in
Table 2. The median SLEDAI-2K and SLE-DAS was 4.00 (interquartile range [IQR] 2.00–7.50) and 2.08 (IQR 1.12–8.24), respectively.
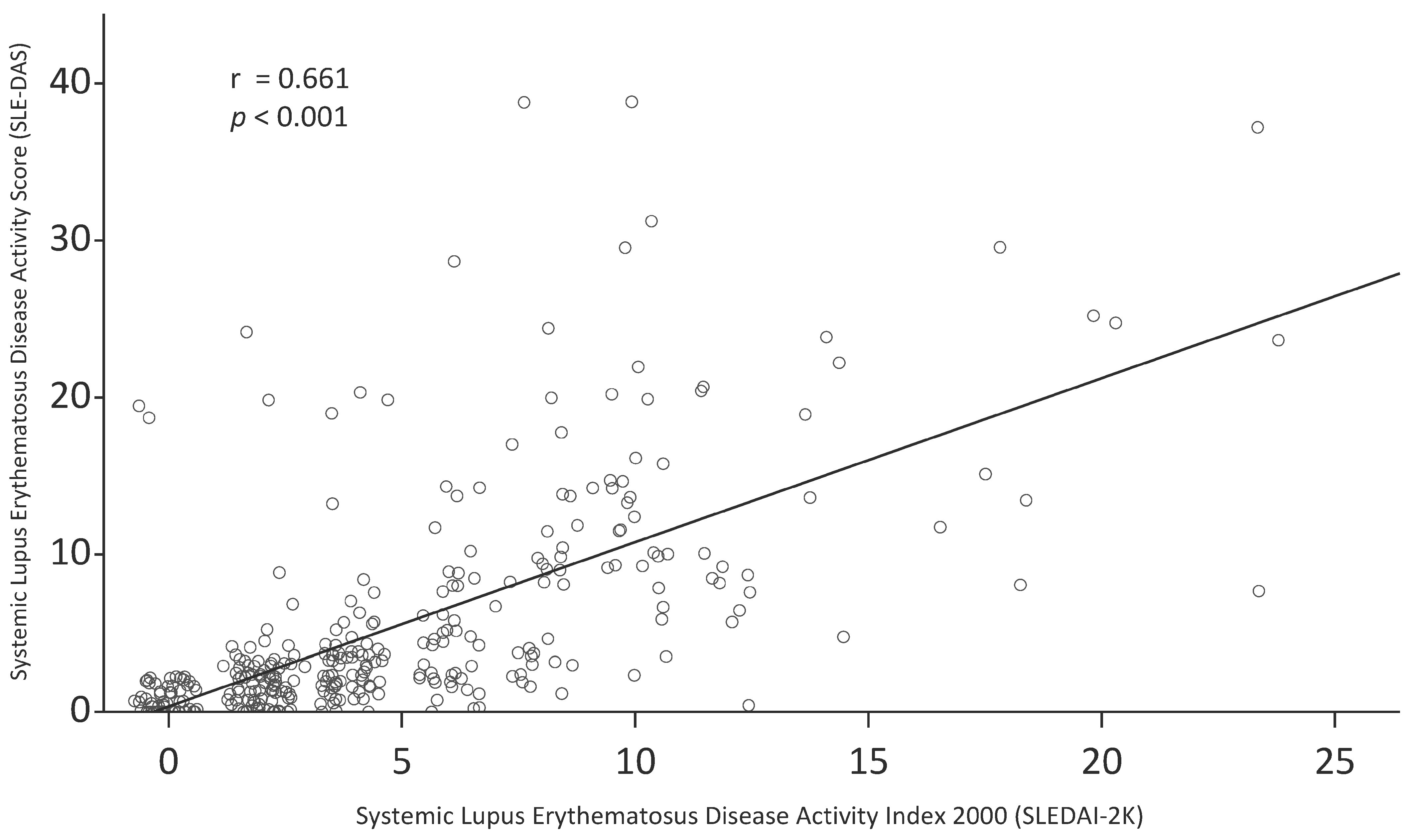
Figure 1 shows a scatter plot of SLEDAI-2K and SLE-DAS. There was a moderate correlation between SLEDAI-2K and SLE-DAS (Pearson’s r = 0.66; 95% CI 0.60, 0.72;
p < 0.001; Spearman’s ρ = 0.78; 95% CI 0.71, 0.83;
p < 0.001).
Table 3 and
Table 4 show the association of the eight domains of LupusQoL with SLEDAI-2K and SLE-DAS, respectively. In
Table 3, SLEDAI-2K was significantly and inversely associated with five domains of LupusQoL, namely, emotional health (
p = 0.036), body image (
p = 0.033), pain (
p = 0.033), fatigue (
p = 0.003), and burden to others (
p < 0.001). When adjusting for sex and age interval, SLEDAI-2K became significantly and inversely associated with all eight domains of LupusQoL. The standardized beta coefficients for the eight domains ranged from the highest at −0.238 in burden to others to the lowest at −0.123 in planning. The three domains with the highest standardized beta coefficients were burden to others (−0.238), followed by pain (−0.196) and physical health (−0.192).
In
Table 4, SLE-DAS was significantly and inversely associated with six domains of LupusQoL, namely, physical health (
p = 0.003), emotional health (
p = 0.007), pain (
p = 0.002), fatigue (
p = 0.001), intimate relationships (
p = 0.022), and burden to others (
p < 0.001). When adjusting for sex and age interval, SLE-DAS also became significantly and inversely associated with all eight domains of LupusQoL. The standardized beta coefficients for the eight domains ranged from the highest at −0.217 in physical health to the two lowest at −0.115 in planning and body image. The three domains with the highest standardized beta coefficients were physical health (−0.217), followed by burden to others (−0.216), and pain (−0.203).
Correlations of SLEDAI-2K and SLE-DAS with LupusQoL were evaluated by comparing five regression model accuracy metrics (
Table 5). The magnitudes of MAE, RMSE, AIC, BIC, and R
2 were comparable between SLEDAI-2K and SLE-DAS. In addition, MAE and RMSE obtained from SLEDAI-2K and SLE-DAS were not significantly different for all eight domains of LupusQoL.
In
Table 6 and
Table 7, correlations of SLEDAI-2K and SLE-DAS with LupusQoL in patients with or without renal involvement were evaluated by comparing five regression model accuracy metrics. The magnitudes of MAE, RMSE, AIC, BIC, and R
2 were comparable between SLEDAI-2K and SLE-DAS. In addition, MAE and RMSE obtained from SLEDAI-2K and SLE-DAS were not significantly different for all eight domains of LupusQoL in patients with SLE with renal involvement or not.
4. Discussion
Measuring disease activity in patients with SLE is important but complex. In this study on 333 patients with SLE, a commonly used SLEDAI-2K was compared with a more recently developed SLE-DAS scoring tool. Overall, we found that the correlations between SLEDAI-2K and SLE-DAS with HRQoL, as measured by LupusQoL, were similar in our patients with SLE. We used five regression model accuracy metrics to assess the performance of the two disease activity measures, and no clear advantages were observed with the newer SLE-DAS over the SLEDAI-2K with respect to their associations with HRQoL. In addition, while there were small differences in the magnitude of the R
2 between the SLEDAI-2K and SLE-DAS, the differences were not in the same direction for the eight domains of LupusQoL. Furthermore, the magnitudes of the R
2 ranged from 0.023 to 0.205 in SLEDAI-2K and 0.021 to 0.216 in SLE-DAS support the view that HRQoL is a different entity from disease activity. Reduced disease activity as a result of treatment may not correlate with improved HRQoL because of the side effects of the medication [
27]. Therefore, both of these entities need to be measured for a more complete clinical picture.
The agreement between SLEDAI-2K and SLE-DAS was evaluated using Spearman’s correlation coefficient. In the original SLE-DAS study, SLE-DAS was shown to be strongly correlated with SLEDAI-2K measured at the last follow-up visit of the external validation cohort, with a ρ = 0.94 [
14]. In our study, a ρ of 0.78 was observed between SLEDAI-2K and SLE-DAS, which is similar to the 0.70 in a study of 41 Indian patients with lupus nephritis [
17]. The low correlation could be attributed to a difference in the distribution of the disease activity between the studies. In a study of 227 Latin American patients with SLE, the authors pointed out that the correlation appeared to depend on the level of the disease activity, with a stronger correlation observed in patients with quiescence or low disease activity [
15].
Regarding the associations with various domains of the LupusQoL, SLEDAI-2K and SLE-DAS were similar. When adjusting for sex and age interval, both SLEDAI-2K and SLE-DAS were significantly and inversely associated with all eight domains of LupusQoL. In terms of the magnitude of the standardized beta coefficients of SLEDAI-2K and SLE-DAS, while their rankings were not identical, they were in general agreement. Burden to others, pain, and physical health were the top three domains, whereas emotional health, body image, and planning were the bottom three domains. Several previous studies on patients with SLE from different cultural and ethnic groups have shown varying degrees of association between disease activity and HRQoL. Some studies showed that all the domains were significantly associated with active disease status, whereas some did not. In a study assessing the psychometric properties of LupusQoL in 208 Chinese patients with SLE, the Chinese version of LupusQoL could discriminate patients with active disease activity, defined as a SLEDAI score >4, in all domains except for body image [
24]. In addition, a study on 132 Turkish patients with SLE found that all domains except planning of the Turkish version of LupusQoL were able to discriminate between active and inactive SLE groups [
28]. Moreover, a study on 78 Iranian patients with SLE showed that active disease, assessed by SLEDAI-2K, was significantly associated with planning, emotional health, and body image domains of the Persian version of the LupusQoL [
29]. Furthermore, a cohort study of 182 French patients with SLE showed that the French version of LupusQoL was significantly lower only for physical health, pain, and intimate relationship in patients with SLEDAI >4 [
30]. Conversely, no significant differences in any domains of an Argentine version of LupusQoL were observed between 147 patients with a SLEDAI score of <4 and ≥4 [
31]. The heterogeneity of the findings from the abovementioned studies might be explained by differences in ethnic composition, cultural setting, and healthcare infrastructure, which could affect the perception of HRQoL in patients with SLE [
32].
Our study has some limitations that deserve mention. First, our patients were enrolled from our outpatient clinic, and therefore, the disease activities were relatively mild. Correlations of SLEDAI-2k and SLE-DAS and LupusQoL in patients with more severe disease activity should be investigated in future studies. Second, we did not measure other variables that might potentially affect HRQoL. Nevertheless, we adjusted the association between the two indexes and HRQoL for age and sex, which are likely to be the two most notable potential confounders of the associations. Despite these limitations, to the best of our knowledge, this is the first study to compare the association of HRQoL between SLEDAI-2k and SLE-DAS. The large sample size is also a strength of this study.
In conclusion, findings from this study showed that there were no clear differences in the use of SLE-DAS over SLEDAI-2K in assessing various domains of HRQoL in patients with SLE. We suggest that, in this aspect, both SLEDAI-2K and SLE-DAS are comparable in their associations with disease activity in patients with SLE.