Fellow-Eye Comparison between Phaco-Microhook Ab-Interno Trabeculotomy and Phaco-iStent Trabecular Micro-Bypass Stent
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Surgical Procedures
2.3. Statistical Analysis
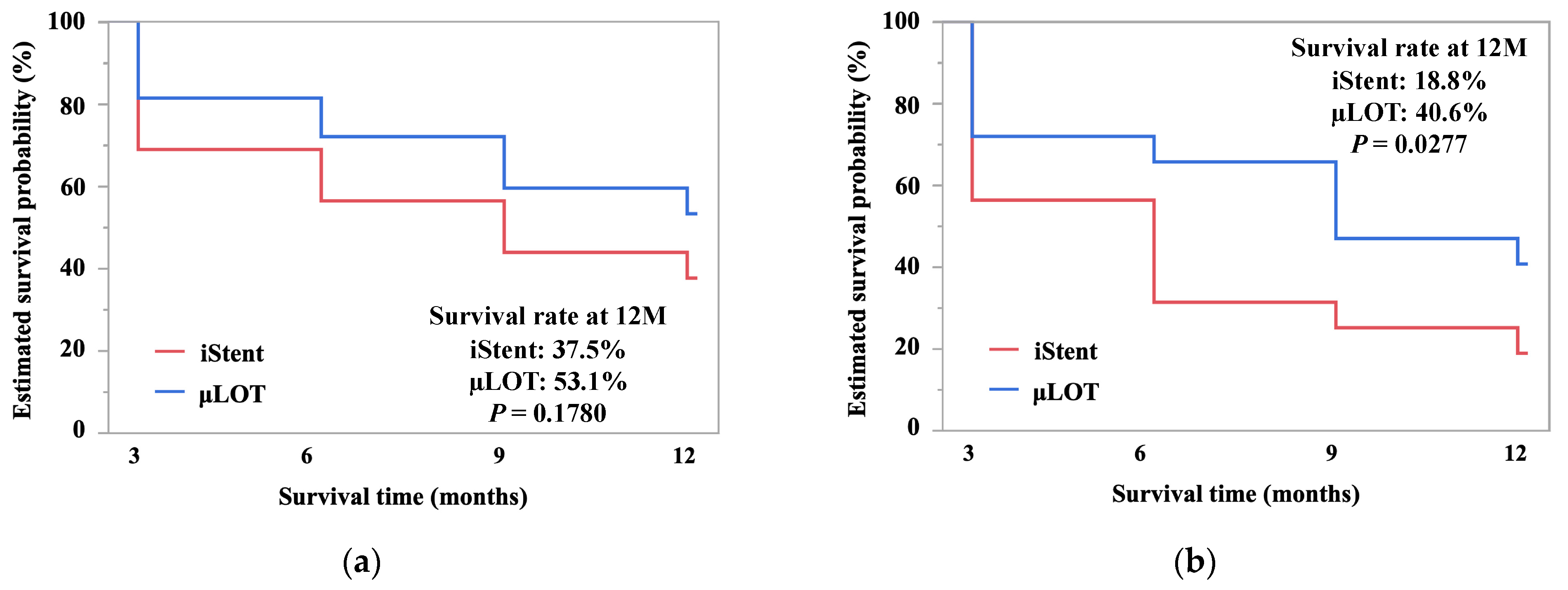
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foster, A.; Resnikoff, S. The impact of Vision 2020 on global blindness. Eye 2005, 19, 1133–1135. [Google Scholar] [CrossRef]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- O’Connor, J.; Soon Ang, G.; Fan Gaskin, J.C.; Nguyen, D.Q.; Crowston, J.G. Wound healing modulation in glaucoma filtration surgery—Conventional practices and new perspectives: Antivascular endothelial growth factor and novel agents (Part II). J. Curr. Glaucoma Pract. 2014, 8, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Konopińska, J.; Deniziak, M.; Saeed, E.; Bartczak, A.; Zalewska, R.; Mariak, Z.; Rękas, M. Prospective randomized study comparing combined phaco-express and phacotrabeculectomy in open angle glaucoma treatment: 12-Month follow-up. J. Ophthalmol. 2015, 2015, 720109. [Google Scholar] [CrossRef] [PubMed]
- Lavia, C.; Dallorto, L.; Maule, M.; Ceccarelli, M.; Fea, A.M. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0183142. [Google Scholar] [CrossRef]
- Agrawal, P.; Bradshaw, S.E. Systematic literature review of clinical and economic outcomes of micro-invasive glaucoma surgery (MIGS) in primary open-angle glaucoma. Ophthalmol. Ther. 2018, 7, 49–73. [Google Scholar] [CrossRef]
- Saheb, H.; Ahmed, I.I. Micro-invasive glaucoma surgery: Current perspectives and future directions. Curr. Opin. Ophthalmol. 2012, 23, 96–104. [Google Scholar] [CrossRef]
- Tanito, M.; Matsuzaki, Y.; Ikeda, Y.; Fujihara, E. Comparison of surgically induced astigmatism following different glaucoma operations. Clin. Ophthalmol. 2017, 11, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Matsuo, M. Ab-interno trabeculotomy-related glaucoma surgeries. Taiwan J. Ophthalmol. 2019, 9, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Sugihara, K.; Tsutsui, A.; Hara, K.; Manabe, K.; Matsuoka, Y. Midterm results of microhook ab interno trabeculotomy in initial 560 eyes with glaucoma. J. Clin. Med. 2021, 10, 814. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Tsutsui, A.; Manabe, K.; Mochiji, M. Comparison of outflow facility before and after the microhook ab interno trabeculotomy. Eye 2021. [Google Scholar] [CrossRef]
- Le, J.T.; Bicket, A.K.; Wang, L.; Li, T. Ab interno trabecular bypass surgery with iStent for open-angle glaucoma. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Popovic, M.; Campos-Moller, X.; Saheb, H.; Ahmed, I.I.K. Efficacy and adverse event profile of the istent and istent inject trabecular micro-bypass for open-angle glaucoma: A meta-analysis. J. Curr. Glaucoma Pract. DVD 2018, 12, 67–84. [Google Scholar] [CrossRef]
- Kozera, M.; Konopińska, J.; Mariak, Z.; Rękas, M. Effectiveness of istent trabecular microbypass system combined with phacoemulsification versus phacoemulsification alone in patients with glaucoma and cataract depending on the initial intraocular pressure. Ophthalmic Res. 2021, 64, 327–336. [Google Scholar] [PubMed]
- Konopińska, J.; Kozera, M.; Kraśnicki, P.; Mariak, Z.; Rękas, M. The effectiveness of first-generation istent microbypass implantation depends on initial intraocular pressure: 24-Month follow-up-prospective clinical trial. J. Ophthalmol. 2020, 2020, 8164703. [Google Scholar] [CrossRef]
- Le, C.; Kazaryan, S.; Hubbell, M.; Zurakowski, D.; Ayyala, R.S. Surgical outcomes of phacoemulsification followed by istent implantation versus goniotomy with the kahook dual blade in patients with mild primary open-angle glaucoma with a minimum of 12-month follow-up. J. Glaucoma 2019, 28, 411–414. [Google Scholar] [CrossRef]
- Iwasaki, K.; Takamura, Y.; Orii, Y.; Arimura, S.; Inatani, M. Performances of glaucoma operations with Kahook Dual Blade or iStent combined with phacoemulsification in Japanese open angle glaucoma patients. Int. J. Ophthalmol. 2020, 13, 941–945. [Google Scholar] [CrossRef]
- Grover, S.; Fishman, G.A.; Anderson, R.J.; Tozatti, M.S.; Heckenlively, J.R.; Weleber, R.G.; Edwards, A.O.; Brown, J., Jr. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology 1999, 106, 1780–1785. [Google Scholar] [CrossRef]
- Elmallah, M.K.; Seibold, L.K.; Kahook, M.Y.; Williamson, B.K.; Singh, I.P.; Dorairaj, S.K. 12-Month retrospective comparison of kahook dual blade excisional goniotomy with istent trabecular bypass device implantation in glaucomatous eyes at the time of cataract surgery. Adv. Ther. 2019, 36, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; King, J.; Thomsen, S.; Hirabayashi, M.; An, J. Comparison of surgical outcomes between excisional goniotomy using the kahook dual blade and istent trabecular micro-bypass stent in combination with phacoemulsification. Clin. Ophthalmol. 2019, 13, 2097–2102. [Google Scholar] [CrossRef]
- Dorairaj, S.K.; Kahook, M.Y.; Williamson, B.K.; Seibold, L.K.; Elmallah, M.K.; Singh, I.P. A multicenter retrospective comparison of goniotomy versus trabecular bypass device implantation in glaucoma patients undergoing cataract extraction. Clin. Ophthalmol. 2018, 12, 791–797. [Google Scholar] [CrossRef]
- Al Yousef, Y.; Strzalkowska, A.; Hillenkamp, J.; Rosentreter, A.; Loewen, N.A. Comparison of a second-generation trabecular bypass (iStent inject) to ab interno trabeculectomy (Trabectome) by exact matching. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2775–2780. [Google Scholar] [CrossRef]
- Gonnermann, J.; Bertelmann, E.; Pahlitzsch, M.; Maier-Wenzel, A.-K.B.; Torun, N.; Klamann, M.K.J. Contralateral eye comparison study in MICS & MIGS: Trabectome® vs. iStent inject®. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 359–365. [Google Scholar]
- Weiner, A.J.; Weiner, Y.; Weiner, A. Intraocular pressure after cataract surgery combined with ab interno trabeculectomy versus trabecular micro-bypass stent: An intrasubject same-surgeon comparison. J. Glaucoma 2020, 29, 773–782. [Google Scholar] [CrossRef]
- Esfandiari, H.; Taubenslag, K.; Shah, P.; Goyal, S.; Weiner, A.J.; Severson, M.L.; Weiner, A.; Grover, D.S.; Bussel, I.I.; Loewen, N.A. Two-year data comparison of ab interno trabeculectomy and trabecular bypass stenting using exact matching. J. Cataract Refract. Surg. 2019, 45, 608–614. [Google Scholar] [CrossRef]
- Aoki, R.; Hirooka, K.; Goda, E.; Yuasa, Y.; Okumichi, H.; Onoe, H.; Kiuchi, Y. Comparison of surgical outcomes between microhook ab interno trabeculotomy and goniotomy with the kahook dual blade in combination with phacoemulsification: A retrospective, comparative case series. Adv. Ther. 2021, 38, 329–336. [Google Scholar] [CrossRef]
- Omoto, T.; Fujishiro, T.; Asano-Shimizu, K.; Sugimoto, K.; Sakata, R.; Murata, H.; Asaoka, R.; Honjo, M.; Aihara, M. Comparison of the short-term effectiveness and safety profile of ab interno combined trabeculotomy using 2 types of trabecular hooks. Jpn. J. Ophthalmol. 2020, 64, 407–413. [Google Scholar] [CrossRef]
- Omoto, T.; Fujishiro, T.; Asano-Shimizu, K.; Sugimoto, K.; Sakata, R.; Murata, H.; Asaoka, R.; Honjo, M.; Aihara, M. Comparison of 12-month surgical outcomes of ab interno trabeculotomy with phacoemulsification between spatula-shaped and dual-blade microhooks. Jpn. J. Ophthalmol. 2021, 65, 402–408. [Google Scholar] [CrossRef]
- Mori, S.; Murai, Y.; Ueda, K.; Sakamoto, M.; Kurimoto, T.; Yamada-Nakanishi, Y.; Nakamura, M. Comparison of efficacy and early surgery-related complications between one-quadrant and two-quadrant microhook ab interno trabeculotomy: A propensity score matched study. Acta Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Tojo, N.; Otsuka, M.; Hayashi, A. Comparison of trabectome and microhook surgical outcomes. Int. Ophthalmol. 2021, 41, 21–26. [Google Scholar] [CrossRef]
- Tanito, M.; Sano, I.; Ikeda, Y.; Fujihara, E. Short-term results of microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery in Japanese eyes: Initial case series. Acta Ophthalmol. 2017, 95, e354–e360. [Google Scholar] [CrossRef]
- Tanito, M.; Ikeda, Y.; Fujihara, E. Effectiveness and safety of combined cataract surgery and microhook ab interno trabeculotomy in Japanese eyes with glaucoma: Report of an initial case series. Jpn. J. Ophthalmol. 2017, 61, 457–464. [Google Scholar] [CrossRef]
- Manning, D. Real-world case series of istent or istent inject trabecular micro-bypass stents combined with cataract surgery. Ophthalmol. Ther. 2019, 8, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M. Delayed-Onset, recurrent hyphema after microhook ab interno trabeculotomy. Case Rep. Ophthalmol. 2021, 12, 57–61. [Google Scholar] [CrossRef]
- Tsutsui, A.; Hamanaka, T.; Manabe, K.; Kaidzu, S.; Kumasaka, T.; Tanito, M. Histologic findings of trabecular meshwork and schlemm’s canal after microhook ab interno trabeculotomy. J. Glaucoma 2021, 30, 203–205. [Google Scholar] [CrossRef]
- Tanito, M.; Manabe, K.; Mochiji, M.; Takai, Y.; Matsuoka, Y. Comparison of anterior chamber flare among different glaucoma surgeries. Clin. Ophthalmol. 2019, 13, 1609–1612. [Google Scholar] [CrossRef]
- Ursell, P.G.; Spalton, D.J.; Whitcup, S.M.; Nussenblatt, R.B. Cystoid macular edema after phacoemulsification: Relationship to blood-aqueous barrier damage and visual acuity. J. Cataract Refract. Surg. 1999, 25, 1492–1497. [Google Scholar] [CrossRef]
- Ersoy, L.; Caramoy, A.; Ristau, T.; Kirchhof, B.; Fauser, S. Aqueous flare is increased in patients with clinically significant cystoid macular oedema after cataract surgery. Br. J. Ophthalmol. 2013, 97, 862–865. [Google Scholar] [CrossRef]
- Chu, L.; Wang, B.; Xu, B.; Dong, N. Aqueous cytokines as predictors of macular edema in non-diabetic patients following uncomplicated phacoemulsification cataract surgery. Mol. Vis. 2013, 19, 2418–2425. [Google Scholar]
- De Maria, M.; Iannetta, D.; Cimino, L.; Coassin, M.; Fontana, L. Measuring anterior chamber inflammation after cataract surgery: A review of the literature focusing on the correlation with cystoid macular edema. Clin. Ophthalmol. 2020, 14, 41–52. [Google Scholar] [CrossRef] [PubMed]
| Parameters | μLOT | iStent | p |
|---|---|---|---|
| No. | 32 | ||
| Age (years) | |||
| Mean ± SD | 75.9 ± 7.6 | ||
| range | 59.5, 88.5 | ||
| Sex | |||
| Men, n (%) | 15 (46.9) | ||
| Women, n (%) | 7 (53.1) | ||
| Laterality | |||
| Left, n (%) | 14 (43.8) | 18 (56.3) | 0.4536 |
| Right, n (%) | 18 (56.3) | 14 (43.8) | |
| Glaucoma types | |||
| POAG, n (%) | 17 (53.1) | 25 (78.1) | 0.0681 |
| EXG, n (%) | 12 (37.5) | 3 (9.4) | |
| Others, n (%) | 3 (9.4) | 4 (12.5) | |
| MD (dB) | |||
| Mean ± SD | −16.3 ± 8.0 | −5.7 ± 6.2 | <0.0001 ** |
| range | −30.8, −3.57 | −27.2, 1.54 | |
| Severity of visual field defects | |||
| Mild (MD > −6 dB), n (%) | 3 (10.3) | 19 (63.3) | <0.0001 ** |
| Moderate (−12 < MD < −6 dB), n (%) | 9 (31.0) | 7 (23.3) | |
| Severe (MD < −12 dB), n (%) | 17 (58.6) | 4 (13.3) | |
| Parameters | IOP (mmHg) | Number of Medications (n) | ||||
|---|---|---|---|---|---|---|
| μLOT | iStent | p | μLOT | iStent | p | |
| Preoperative value | ||||||
| Mean ± SD | 18.8 ± 5.7 | 15.5 ± 3.4 | 0.0001 ** | 3.0 ± 1.2 | 2.7 ± 1.2 | 0.0437 * |
| Range | 12.0, 43.0 | 13.0, 25.0 | 1.0, 5.0 | 1.0, 4.0 | ||
| Two weeks postoperatively | ||||||
| Mean ± SD | 15.3 ± 4.9 | 14.4 ± 3.7 | 0.4857 | 2.0 ± 0.9 | 2.0 ± 0.9 | 1.0000 |
| Range | 7.0, 29.0 | 8.0, 24.0 | 1.0, 3.0 | 1.0, 3.0 | ||
| Three months postoperatively | ||||||
| Mean ± SD | 13.1 ± 4.7 | 12.9 ± 3.3 | 0.6022 | 2.2 ± 0.9 | 2.2 ± 0.9 | 1.0000 |
| Range | 7.0, 33.0 | 8.0, 22.0 | 1.0, 3.0 | 1.0, 3.0 | ||
| Six months postoperatively | ||||||
| Mean ± SD | 12.9 ± 3.3 | 13.3 ± 2.8 | 0.1848 | 2.1 ± 0.9 | 2.2 ± 0.9 | 0.3251 |
| Range | 9.0, 23.0 | 9.0, 21.0 | 0.0, 3.0 | 1.0, 3.0 | ||
| Nine months postoperatively | ||||||
| Mean ± SD | 12.8 ± 3.1 | 13.2 ± 3.2 | 0.3131 | 2.3 ± 0.9 | 2.3 ± 0.9 | 0.3251 |
| range | 6.0, 20.0 | 6.0, 19.0 | 1.0, 4.0 | 1.0, 4.0 | ||
| Twelve months postoperatively | ||||||
| Mean ± SD | 12.6 ± 2.3 | 12.8 ± 2.5 | 0.0934 | 2.3 ± 0.9 | 2.3 ± 0.9 | 0.3251 |
| Range | 7.0, 18.0 | 8.0, 18.0 | 1.0, 4.0 | 1.0, 4.0 | ||
| Parameters | ΔIOP (mmHg) | ΔMedication (n) | ||||
|---|---|---|---|---|---|---|
| μLOT | iStent | p | μLOT | iStent | p | |
| Two weeks postoperatively | ||||||
| Mean ± SD | −3.4 ± 5.1 | −1.1 ± 3.9 | 0.0543 | −0.9 ± 1.2 | −0.7 ± 1.0 | 0.0437 * |
| Range | −20.0, 6.0 | −13.0, 7.0 | −4.0, 1.0 | −3.0, 2.0 | ||
| Three months postoperatively | ||||||
| Mean ± SD | −5.7 ± 6.4 | −2.7 ± 4.3 | 0.0022 ** | −0.8 ± 1.1 | −0.5 ± 1.0 | 0.0437 * |
| Range | −27.0, 7.0 | −10.0, 8.0 | −4.0, 1.0 | −3.0, 2.0 | ||
| Six months postoperatively | ||||||
| Mean ± SD | −5.9 ± 5.5 | −2.4 ± 3.9 | 0.0018 ** | −0.9 ± 1.2 | −0.5 ± 1.0 | 0.0437 * |
| Range | −24.0, 4.0 | −12.0, 4.0 | −4.0, 1.0 | −3.0, 2.0 | ||
| Nine months postoperatively | ||||||
| Mean ± SD | −6.0 ± 6.4 | −2.4 ± 3.7 | <0.0001 ** | −0.7 ± 1.3 | −0.4 ± 1.1 | 0.0437 * |
| Range | −31.0, 1.0 | −11.0, 4.0 | −4.0, 2.0 | −3.0, 2.0 | ||
| Twelve months postoperatively | ||||||
| Mean ± SD | −6.2 ± 5.6 | −2.7 ± 3.2 | 0.0003 ** | −0.7 ± 1.3 | −0.4 ± 1.1 | 0.0437 * |
| Range | −28.0, 2.0 | −12.0, 1.0 | −4.0, 2.0 | −3.0, 2.0 | ||
| Parameters | μLOT | iStent | p |
|---|---|---|---|
| Layered hyphema, n (%) | 8 (25.0) | 0 (0.0) | 0.0048 ** |
| IOP spikes, n (%) | 2 (6.3) | 2 (6.3) | 1.0000 |
| Cystoid macular edema | 3 (9.4) | 4 (12.5) | 1.0000 |
| Additional glaucoma surgery, n (%) | 1 (3.2) | 0 (0.0) | 1.0000 |
| Parameters | BCVA (LogMAR) | ACF (pc/msec) | CECD (Cells/mm²) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| μLOT | iStent | p | μLOT | iStent | p | μLOT | iStent | p | |
| Preoperative value | |||||||||
| Mean ± SD | 0.32 ± 0.51 | 0.23 ± 0.51 | 0.0439 * | 11.0 ± 7.9 | 10.2 ± 6.3 | 0.3337 | 2376.3 ± 408.7 | 2473.5 ± 387.1 | 0.0098 ** |
| Range | −0.08, 2.70 | −0.08, 2.70 | 3.4, 36.2 | 4.3, 26.6 | 785, 2887 | 1203, 3068 | |||
| Two weeks postoperatively | |||||||||
| Mean ± SD | 0.237 ± 0.243 | 0.162 ± 0.486 | 0.0038 ** | 46.9 ± 33.4 | 34.5 ± 29.5 | 0.0026 ** | |||
| Range | −0.079, 0.824 | −0.079, 2.699 | 10.5, 142.9 | 7.9, 152.2 | |||||
| Three months postoperatively | |||||||||
| Mean ± SD | 0.129 ± 0.154 | 0.136 ± 0.469 | 0.0469 ** | 20.4 ± 11.9 | 18.1 ± 7.3 | 0.3217 | 2176.3 ± 366.9 | 2256.0, 415.2 | 0.2269 |
| Range | −0.079, 0.398 | −0.079, 2.602 | 7.2, 55.6 | 7.1, 32.1 | 1182, 3109 | 1284, 2885 | |||
| Six months postoperatively | |||||||||
| Mean ± SD | 0.069 ± 0.133 | 0.119 ± 0.473 | 0.2637 | 15.3 ± 8.4 | 14.2 ± 9.4 | 0.2228 | 2242.8 ± 330.1 | 2265.4 ± 405.2 | 0.3730 |
| Range | −0.079, 0.398 | −0.079, 2.602 | 5.9, 33.9 | 4.8, 39.0 | 1541, 2858 | 1370, 2696 | |||
| Nine months postoperatively | |||||||||
| Mean ± SD | 0.072 ± 0.133 | 0.097 ± 0.472 | 0.0313 * | 12.7 ± 6.4 | 12.3 ± 7.1 | 0.3634 | 2169.4 ±399.7 | 2262.1 ± 414.6 | 0.0158 * |
| Range | −0.079, 0.398 | −0.079, 2.602 | 4.8, 31.3 | 4.8, 36.4 | 801, 2832 | 932, 2852 | |||
| Twelve months postoperatively | |||||||||
| Mean ± SD | 0.071 ± 0.141 | 0.097 ± 0.474 | 0.0072 ** | 12.1 ± 6.4 | 11.8 ± 7.7 | 0.2751 | 2481.1 ± 386.1 | 2296.2 ± 365.8 | 0.2694 |
| Range | −0.079, 0.398 | −0.079, 2.602 | 3.0, 28.0 | 3.0, 33.0 | 1134, 2807 | 1093, 2890 | |||
| Parameters | ΔBCVA (LogMAR) | ΔACF (pc/msec) | ΔCECD (cells/mm²) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| μLOT | iStent | p | μLOT | iStent | p | μLOT | iStent | p | |
| Two weeks postoperatively | |||||||||
| Mean ± SD | −0.081 ± 0.522 | −0.072 ± 0.216 | 0.4292 | 37.1 ± 37.6 | 25.4 ± 29.7 | 0.0156 * | |||
| Range | −2.477, 0.669 | −0.778, 0.176 | 0.0, 158.5 | 0.0, 137.4 | |||||
| Three months postoperatively | |||||||||
| Mean ± SD | −0.189 ± 0.459 | −0.098 ± 0.206 | 0.6604 | 8.7 ± 9.5 | 7.6 ± 6.6 | 0.9916 | −187.6 ± 399.9 | −194.8 ± 300.3 | 0.8963 |
| Range | −2.477, 0.204 | −0.824, 0.255 | −6.7, 34.3 | −8.3, 22.0 | −976, 737 | −918, 552 | |||
| Six months postoperatively | |||||||||
| Mean ± SD | −0.247 ± 0.447 | −0.125 ± 0.220 | 0.1278 | 4.1 ± 7.0 | 3.4 ± 6.2 | 0.6490 | −119.7 ± 400.9 | −183.6 ± 367.1 | 0.7200 |
| Range | −2.398, 0.079 | −0.903, 0.097 | −7.9, 19.2 | −8.3, 20.4 | −983, 1008 | −1116, 521 | |||
| Nine months postoperatively | |||||||||
| Mean ± SD | −0.247 ± 0.439 | −0.137 ± 0.197 | 0.1483 | 2.6 ± 9.8 | 1.8 ± 5.6 | 0.9161 | −200.1 ± 358.3 | −197.2 ± 303.1 | 0.5001 |
| Range | −2.398, 0.0792 | −0.903, 0.079 | −9.7, 42.7 | −11.7, 13.6 | −983, 546 | −1169, 408 | |||
| Twelve months postoperatively | |||||||||
| Mean ± SD | −0.248 ± 0.436 | −0.137 ± 0.195 | 0.1364 | 2.6 ± 9.3 | 1.7 ± 4.9 | 0.5053 | −118.1 ± 349.6 | −178.6 ± 260.4 | 0.1857 |
| Range | −2.398, 0.079 | −0.903, 0.079 | −12.1, 41.7 | −8.2, 14.0 | −819, 722 | −822, 504 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takayanagi, Y.; Ichioka, S.; Ishida, A.; Tsutsui, A.; Tanito, M. Fellow-Eye Comparison between Phaco-Microhook Ab-Interno Trabeculotomy and Phaco-iStent Trabecular Micro-Bypass Stent. J. Clin. Med. 2021, 10, 2129. https://doi.org/10.3390/jcm10102129
Takayanagi Y, Ichioka S, Ishida A, Tsutsui A, Tanito M. Fellow-Eye Comparison between Phaco-Microhook Ab-Interno Trabeculotomy and Phaco-iStent Trabecular Micro-Bypass Stent. Journal of Clinical Medicine. 2021; 10(10):2129. https://doi.org/10.3390/jcm10102129
Chicago/Turabian StyleTakayanagi, Yuji, Sho Ichioka, Akiko Ishida, Aika Tsutsui, and Masaki Tanito. 2021. "Fellow-Eye Comparison between Phaco-Microhook Ab-Interno Trabeculotomy and Phaco-iStent Trabecular Micro-Bypass Stent" Journal of Clinical Medicine 10, no. 10: 2129. https://doi.org/10.3390/jcm10102129
APA StyleTakayanagi, Y., Ichioka, S., Ishida, A., Tsutsui, A., & Tanito, M. (2021). Fellow-Eye Comparison between Phaco-Microhook Ab-Interno Trabeculotomy and Phaco-iStent Trabecular Micro-Bypass Stent. Journal of Clinical Medicine, 10(10), 2129. https://doi.org/10.3390/jcm10102129






