Smaller Intervertebral Disc Volume and More Disc Degeneration after Spinal Distraction in Scoliotic Children
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Intervertebral Disc Volume, Height and Depth
3.2. Intervertebral Disc Degeneration
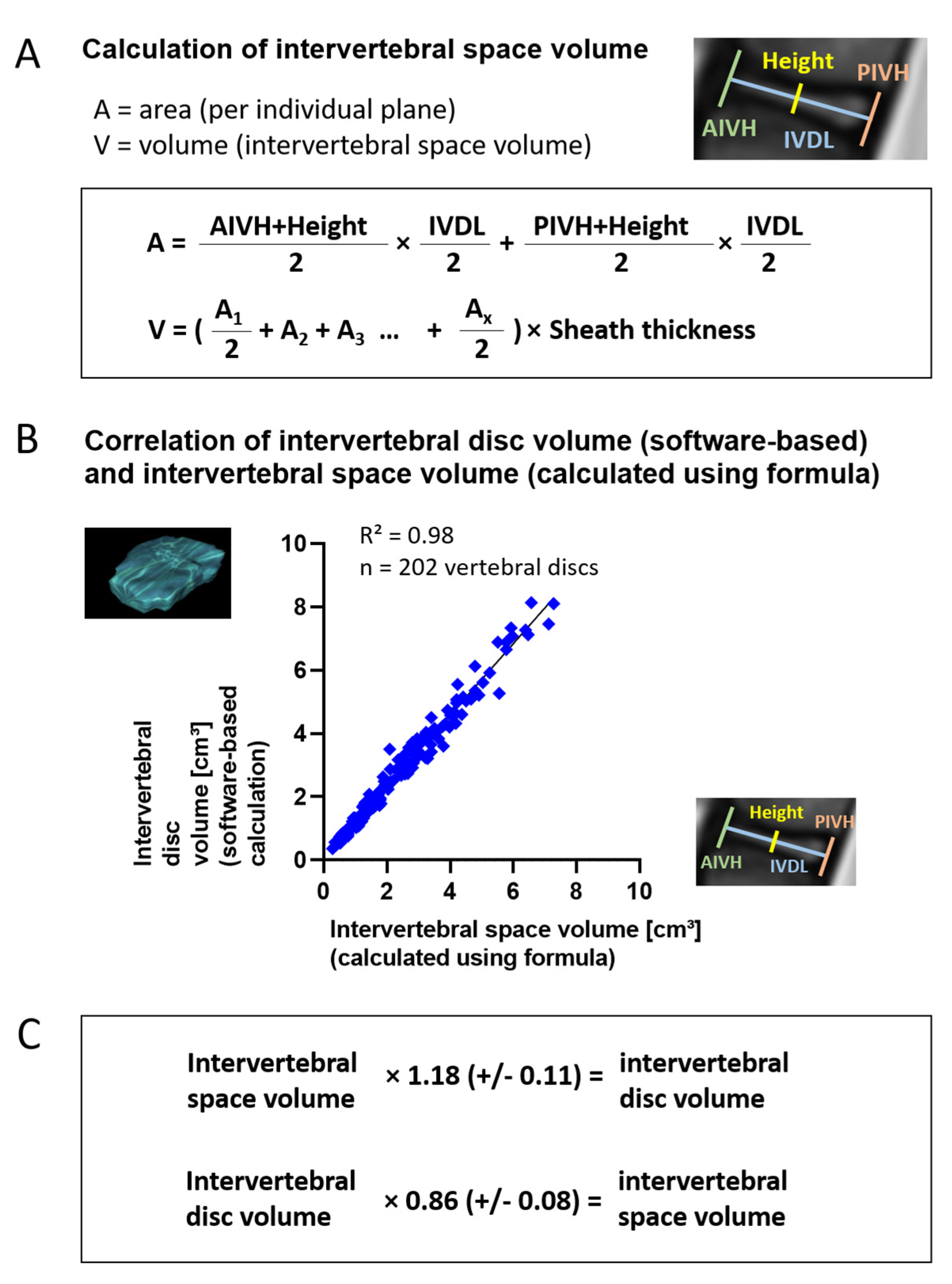
3.3. Volume Calculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bekmez, S.; Afandiyev, A.; Dede, O.; Karaismailoğlu, E.; Demirkiran, H.G.; Yazici, M. Is Magnetically Controlled Growing Rod the Game Changer in Early-onset Scoliosis? A Preliminary Report. J. Pediatr. Orthop. 2019, 39, e195–e200. [Google Scholar] [CrossRef]
- Carbone, M.; Vittoria, F.; Del Sal, A. Treatment of early-onset scoliosis with growing rods in patients with neurofibromatosis-1. J. Pediatr. Orthop. B 2019, 28, 278–287. [Google Scholar] [CrossRef]
- Cahill, P.J.; Marvil, S.; Cuddihy, L.; Schutt, C.; Idema, J.; Clements, D.H.; Antonacci, M.D.; Asghar, J.; Samdani, A.F.; Betz, R.R. Autofusion in the Immature Spine Treated With Growing Rods. Spine 2010, 35, E1199–E1203. [Google Scholar] [CrossRef]
- Groenefeld, B.; Hell, A.K. Ossifications after Vertical Expandable Prosthetic Titanium Rib Treatment in Children with Thoracic Insufficiency Syndrome and Scoliosis. Spine 2013, 38, E819–E823. [Google Scholar] [CrossRef]
- Bouthors, C.; Izatt, M.T.; Adam, C.J.; Pearcy, M.J.; Labrom, R.D.; Askin, G.N. Minimizing Spine Autofusion With the Use of Semiconstrained Growing Rods for Early Onset Scoliosis in Children. J. Pediatr. Orthop. 2018, 38, e562–e571. [Google Scholar] [CrossRef]
- Hasler, C.C.; Studer, D.; Büchler, P. Metamorphosis of human lumbar vertebrae induced by VEPTR growth modulation and stress shielding. J. Child. Orthop. 2015, 9, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Sankar, W.N.; Skaggs, D.L.; Yazici, M.; Johnston, C.E.; Shah, S.A.; Javidan, P.; Kadakia, R.V.; Day, T.F.; Akbarnia, B.A. Lengthening of Dual Growing Rods and the Law of Diminishing Returns. Spine 2011, 36, 806–809. [Google Scholar] [CrossRef]
- Lippross, S.; Grages, A.; Lueders, K.A.; Braunschweig, L.; Austein, F.; Tsaknakis, K.; Lorenz, H.M.; Hell, A.K. Vertebral body changes after continuous spinal distraction in scoliotic children. Eur. Spine J. 2021, 1–7. [Google Scholar] [CrossRef]
- Rong, T.; Shen, J.; Kwan, K.; Zhang, J.; Wang, Y.; Li, S.; Li, Z.; Chen, C.; Lin, Y.; Tan, H. Vertebral Growth Around Distal Instrumented Vertebra in Patients With Early-Onset Scoliosis Who Underwent Traditional Dual Growing Rod Treatment. Spine 2019, 44, 855–865. [Google Scholar] [CrossRef]
- Wijngaarde, C.A.; Brink, R.C.; De Kort, F.A.; Stam, M.; Otto, L.A.M.; Asselman, F.-L.; Bartels, B.; Van Eijk, R.P.; Sombroek, J.; Cuppen, I.; et al. Natural course of scoliosis and lifetime risk of scoliosis surgery in spinal muscular atrophy. Neurology 2019, 93, e149–e158. [Google Scholar] [CrossRef]
- Hell, A.K.; Braunschweig, L.; Tsaknakis, K.; Von Deimling, U.; Lüders, K.A.; Hecker, M.; Lorenz, H.M. Children With Spinal Muscular Atrophy With Prior Growth-Friendly Spinal Implants Have Better Results After Definite Spinal Fusion in Comparison to Untreated Patients. Neurosurgery 2020, 87, 910–917. [Google Scholar] [CrossRef]
- Tunset, A.; Kjaer, P.; Chreiteh, S.S.; Jensen, T.S. A method for quantitative measurement of lumbar intervertebral disc structures: An intra- and inter-rater agreement and reliability study. Chiropr. Man. Ther. 2013, 21, 26. [Google Scholar] [CrossRef] [Green Version]
- Pfirrmann, C.W.A.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic Resonance Classification of Lumbar Intervertebral Disc Degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef]
- Campbell, M.; Matsumoto, H.; Hilaire, T.S.; Roye, B.D.; Roye, D.P.; Vitale, M.G. Burden of care in families of patients with early onset scoliosis. J. Pediatr. Orthop. B 2020, 29, 567–571. [Google Scholar] [CrossRef]
- Roye, B.D.; Children’s Spine Study Group; Simhon, M.E.; Matsumoto, H.; Garg, S.; Redding, G.; Samdani, A.; Smith, J.T.; Sponseller, P.; Vitale, M.G.; et al. Bigger is better: Larger thoracic height is associated with increased health related quality of life at skeletal maturity. Spine Deform. 2020, 8, 771–779. [Google Scholar] [CrossRef]
- Ridderbusch, K.; Rupprecht, M.; Kunkel, P.; Hagemann, C.; Stücker, R. Preliminary Results of Magnetically Controlled Growing Rods for Early Onset Scoliosis. J. Pediatr. Orthop. 2017, 37, e575–e580. [Google Scholar] [CrossRef]
- Campbell, R.M.; Hell-Vocke, A.K. GROWTH OF THE THORACIC SPINE IN CONGENITAL SCOLIOSIS AFTER EXPANSION THORACOPLASTY. J. Bone Jt. Surg. Am. Vol. 2003, 85, 409–420. [Google Scholar] [CrossRef]
- Lattig, F.; Taurman, R.; Hell, A.K. Treatment of Early-Onset Spinal Deformity (EOSD) With VEPTR. Clin. Spine Surg. 2016, 29, E246–E251. [Google Scholar] [CrossRef]
- Lebon, J.; Batailler, C.; Wargny, M.; Choufani, E.; Violas, P.; Fron, D.; Kieffer, J.; Accadbled, F.; Cunin, V.; De Gauzy, J.S. Magnetically controlled growing rod in early onset scoliosis: A 30-case multicenter study. Eur. Spine J. 2017, 26, 1567–1576. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, T.; Ahmad, A.; Mardare, D.M.; Kieser, D.C.; Mayers, D.; Nnadi, C. A six-year observational study of 31 children with early-onset scoliosis treated using magnetically controlled growing rods with a minimum follow-up of two years. Bone Jt. J. 2018, 100, 1187–1200. [Google Scholar] [CrossRef]
- Lorenz, H.M.; Braunschweig, L.; Eberhardt, I.M.; Tsaknakis, K.; Hell, A. Operative „No-touch“-Techniken zur Korrektur kindlicher Skoliosen. Oper. Orthopädie Traumatol. 2019, 31, 321–334. [Google Scholar] [CrossRef]
- Hell, A.K.; Groenefeld, K.; Tsaknakis, K.; Braunschweig, L.; Lorenz, H.M. Combining Bilateral Magnetically Controlled Implants Inserted Parallel to the Spine With Rib to Pelvis Fixation. Clin. Spine Surg. 2018, 31, 239–246. [Google Scholar] [CrossRef]
- Szabó, L.; Gergely, A.; Jakus, R.; Fogarasi, A.; Grosz, Z.; Molnár, M.J.; Andor, I.; Schulcz, O.; Goschler, Á.; Medveczky, E.; et al. Efficacy of nusinersen in type 1, 2 and 3 spinal muscular atrophy: Real world data from Hungarian patients. Eur. J. Paediatr. Neurol. 2020, 27, 37–42. [Google Scholar] [CrossRef]
- Urrutia, J.; Besa, P.; Campos, M.; Cikutovic, P.; Cabezon, M.; Molina, M.; Cruz, J.P. The Pfirrmann classification of lumbar intervertebral disc degeneration: An independent inter- and intra-observer agreement assessment. Eur. Spine J. 2016, 25, 2728–2733. [Google Scholar] [CrossRef]
- Sitte, I.; Kathrein, A.; Pfaller, K.; Pedross, F.; Klosterhuber, M.; Lindtner, R.A.; Zenner, J.; Ferraris, L.; Meier, O.; Koller, H. Morphological Differences in Adolescent Idiopathic Scoliosis. Spine 2013, 38, 1672–1680. [Google Scholar] [CrossRef]
- Demirkiran, G.; Yilgor, C.; Ayvaz, M.; Kosemehmetoglu, K.; Daglioglu, K.; Yazici, M. Effects of the Fusionless Instrumentation on the Disks and Facet Joints of the Unfused Segments. J. Pediatr. Orthop. 2014, 34, 185–193. [Google Scholar] [CrossRef]
- Huber, M.; Gilbert, G.; Roy, J.; Parent, S.; Labelle, H.; Périé, D. Sensitivity of MRI parameters within intervertebral discs to the severity of adolescent idiopathic scoliosis. J. Magn. Reson. Imaging 2016, 44, 1123–1131. [Google Scholar] [CrossRef]
- Veres, S.P.; Robertson, P.A.; Broom, N.D. ISSLS Prize Winner: How Loading Rate Influences Disc Failure Mechanics. Spine 2010, 35, 1897–1908. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Vidyadhara, S.; Subbiah, M.; Kamath, V.; Karunanithi, R.; Shetty, A.P.; Venkateswaran, K.; Babu, M.; Meenakshi, J. ISSLS Prize Winner: A Study of Effects of In Vivo Mechanical Forces on Human Lumbar Discs With Scoliotic Disc as a Biological Model. Spine 2010, 35, 1930–1943. [Google Scholar] [CrossRef]
- Gervais, J.; Périé, D.; Parent, S.; Labelle, H.; Aubin, C.-E. MRI signal distribution within the intervertebral disc as a biomarker of adolescent idiopathic scoliosis and spondylolisthesis. BMC Musculoskelet. Disord. 2012, 13, 239. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.S.; Modic, M.T. Current assessment of spinal degenerative disease with magnetic resonance imaging. Clin. Orthop. Relat. Res. 1992, 279, 68–81. [Google Scholar]
- Louzada, L.L.; Morato, T.N.; Almeida, R.E.F.; Camargos, E.F.; Nóbrega, O.T.; Farage, L. OsiriX™ as a feasible tool for in office manual hippocampal volumetry in the elderly: A technical note. Geriatr. Gerontol. Aging 2016, 10, 34–40. [Google Scholar] [CrossRef]
- Gullbrand, S.E.; Peterson, J.; Ahlborn, J.; Mastropolo, R.; Fricker, A.; Roberts, T.T.; Abousayed, M.; Lawrence, J.P.; Glennon, J.C.; Ledet, E.H. ISSLS Prize Winner. Spine 2015, 40, 1158–1164. [Google Scholar] [CrossRef] [Green Version]
- Gullbrand, S.E.; Kim, D.H.; Ashinsky, B.G.; Bonnevie, E.D.; Smith, H.E.; Mauck, R.L. Restoration of physiologic loading modulates engineered intervertebral disc structure and function in an in vivo model. JOR Spine 2020, 3, e1086. [Google Scholar] [CrossRef]






| Age/Gender (f = Female/ m = Male) | Height/Weight/BMI | Spinal Deformity (Cobb Angle in °) | |
|---|---|---|---|
| Cohort I 1. MRI (n = 16) (before GFSI) | 8.1 (+/−1.4) years(6f/10 m) | 70.1 (+/−22.6) before initial surgery 34.0 (+/−15.4) after initial surgery | |
| 2. MRI (n = 17) (after GFSI) | 13.4 (+/−1.7) years (7f/10 m) | height 146 (+/−8) cm weight 39.1 (+/−9.5) kg BMI 18.4 (+/−3.9) | 65.8 (+/−20.2) after GFSI treatment prior to spinal fusion |
| MCGR index surgery age 8.3 (+/−1.4) years | |||
| Cohort II (n = 16) No surgical treatment | 13.7 (+/−2.6) years (11f/5 m) | height 147 (+/−11) cm weight 42.6 (+/−14.0) kg BMI 19.6 (+/−6.2) | 89.6 (+/−34.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippross, S.; Girmond, P.; Lüders, K.A.; Austein, F.; Braunschweig, L.; Lüders, S.; Tsaknakis, K.; Lorenz, H.M.; Hell, A.K. Smaller Intervertebral Disc Volume and More Disc Degeneration after Spinal Distraction in Scoliotic Children. J. Clin. Med. 2021, 10, 2124. https://doi.org/10.3390/jcm10102124
Lippross S, Girmond P, Lüders KA, Austein F, Braunschweig L, Lüders S, Tsaknakis K, Lorenz HM, Hell AK. Smaller Intervertebral Disc Volume and More Disc Degeneration after Spinal Distraction in Scoliotic Children. Journal of Clinical Medicine. 2021; 10(10):2124. https://doi.org/10.3390/jcm10102124
Chicago/Turabian StyleLippross, Sebastian, Paul Girmond, Katja A. Lüders, Friederike Austein, Lena Braunschweig, Stefan Lüders, Konstantinos Tsaknakis, Heiko M. Lorenz, and Anna K. Hell. 2021. "Smaller Intervertebral Disc Volume and More Disc Degeneration after Spinal Distraction in Scoliotic Children" Journal of Clinical Medicine 10, no. 10: 2124. https://doi.org/10.3390/jcm10102124
APA StyleLippross, S., Girmond, P., Lüders, K. A., Austein, F., Braunschweig, L., Lüders, S., Tsaknakis, K., Lorenz, H. M., & Hell, A. K. (2021). Smaller Intervertebral Disc Volume and More Disc Degeneration after Spinal Distraction in Scoliotic Children. Journal of Clinical Medicine, 10(10), 2124. https://doi.org/10.3390/jcm10102124






