Assessment of the Association of COPD and Asthma with In-Hospital Mortality in Patients with COVID-19. A Systematic Review, Meta-Analysis, and Meta-Regression Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Data Extraction and Outcomes
2.3. Risk of Bias Assessment
2.4. Statistical Analysis
3. Results
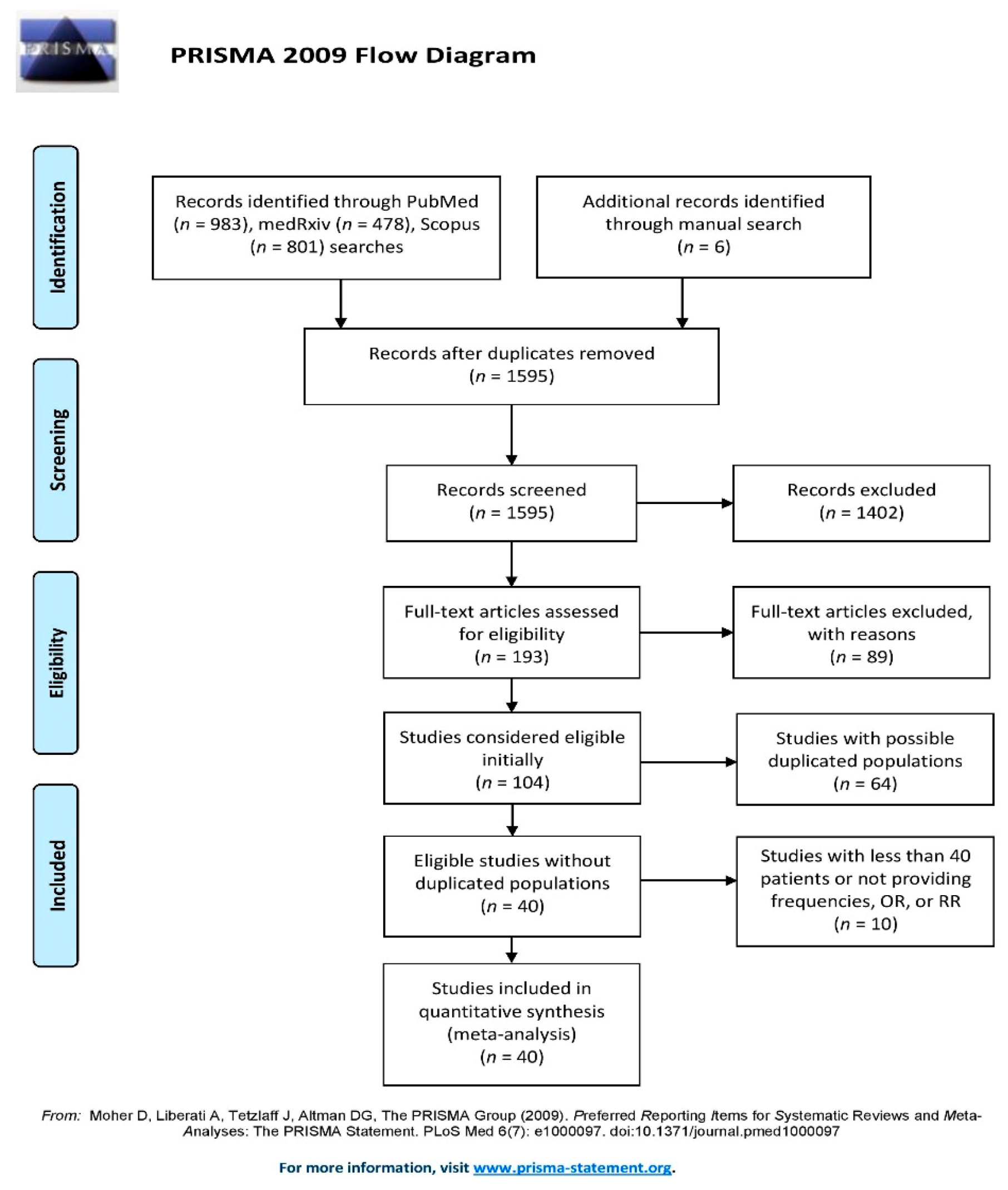
3.1. Search Results
3.2. Patient Characteristics and Primary Events
3.3. COPD vs. Non-COPD
3.4. Asthma vs. Non-Asthma
3.5. Quality Assessment and Heterogeneity
4. Discussion
4.1. COPD and Mortality in Patients with COVID-19
4.2. Asthma and Mortality in Patients with COVID-19
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Exclusion Reasons | n |
|---|---|
| Studies that enrolled special populations (e.g., cancer patients) | 12 |
| Studies that did not report separate data on the outcomes of interest | 28 |
| Studies that did not specifically report data on COPD and/or asthma | 49 |
References
- WHO. Chronic Respiratory Diseases—Burden of COPD Cited 2020 August. Available online: https://www.who.int/respiratory/copd/burden/en/ (accessed on 18 February 2021).
- Prevention CDC. US Centers for Disease Control and Prevention People Who are at Higher Risk for Severe Illness: Center for Disease Control. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html (accessed on 18 February 2021).
- Villarroel, M.A.; Blackwell, D.L.; Jen, A. Adults: 2018 National Health Interview Survey. National Center for Health Statistics. Available online: http://www.cdc.gov/nchs/nhis/SHS/tables.htm (accessed on 18 February 2021).
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Fireman, P. Understanding asthma pathophysiology. Allergy Asthma Proc. 2003, 24, 79–83. [Google Scholar] [PubMed]
- Matsumoto, K.; Inoue, H. Viral infections in asthma and COPD. Respir. Investig. 2014, 52, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.G.; Kent, J.; Ireland, D.C. Respiratory viruses and exacerbations of asthma in adults. BMJ 1993, 307, 982–986. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.-Y.; Xu, Y.-J.; Guan, W.-J.; Lin, L.-F. Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: A literature review. Arch. Virol. 2018, 163, 845–853. [Google Scholar] [CrossRef]
- Kwak, H.J.; Park, D.W.; Kim, J.E.; Koo, G.W.; Moon, J.-Y.; Sohn, J.W.; Shin, D.H.; Park, M.K.; Park, T.S.; Kim, T.H.; et al. Prevalence and Risk Factors of Respiratory Viral Infections in Exacerbations of Chronic Obstructive Pulmonary Disease. Tohoku J. Exp. Med. 2016, 240, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Althani, A.; Bushra, S.; Shaath, N.; Sattar, H.A. Characterisation of winter respiratory viral infections in patients with asthma and COPD in Qatar. Arch. Virol. 2012, 158, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Yormaz, B.; Findik, D.; Süerdem, M. Differences of viral panel positive versus negative by real-time PCR in COPD exacerbated patients. Tuberk. Toraks 2019, 67, 124–130. [Google Scholar] [CrossRef]
- Zwaans, W.; Mallia, P.; van Winden, M.; Rohde, G. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease—A systematic review. J. Clin. Virol. 2014, 61, 181–188. [Google Scholar] [CrossRef]
- Liao, H.; Yang, Z.; Yang, C.; Tang, Y.; Liu, S.; Guan, W.G.; Chen, R.C. Impact of viral infection on acute exacerbation of asthma in out-patient clinics: A prospective study. J. Thorac. Dis. 2016, 8, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Atmar, R.L.; Guy, E.; Guntupalli, K.K.; Zimmerman, J.L.; Bandi, V.D.; Baxter, B.D.; Greenberg, S.B. Respiratory tract viral infections in inner-city asthmatic adults. Arch. Intern. Med. 1998, 158, 2453–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kistler, A.; Avila, P.C.; Rouskin, S.; Wang, D.; Ward, T.; Yagi, S.; Schnurr, D.; Ganem, D.; DeRisi, J.L.; Boushey, H.A. Pan-Viral Screening of Respiratory Tract Infections in Adults With and Without Asthma Reveals Unexpected Human Coronavirus and Human Rhinovirus Diversity. J. Infect. Dis. 2007, 196, 817–825. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Christodoulou, I.; Rohde, G.; Agache, I.; Almqvist, C.; Bruno, A.; Bonini, S.; De Bont, L.G.M.; Bossios, A.; Bousquet, J.; et al. Viruses and bacteria in acute asthma exacerbations—A GA2LEN-DARE* systematic review. Allergy 2010, 66, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Matano, L.; Monroy-Muñoz, I.E.; Angeles-Martínez, J.; Sarquiz-Martinez, B.; Palomec-Nava, I.D.; Pardavé-Alejandre, H.D.; Coy-Arechavaleta, A.S.; Santacruz-Tinoco, C.E.; González-Ibarra, J.; González-Bonilla, C.R.; et al. Prevalence of non-influenza respiratory viruses in acute respiratory infection cases in Mexico. PLoS ONE 2017, 12, e0176298. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 18 February 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Bullen, C.; Howe, C.; Laugesen, M.; McRobbie, H.; Parag, V.; Williman, J.; Walker, N. Electronic cigarettes for smoking cessation: A randomised controlled trial. Lancet 2013, 382, 1629–1637. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Covino, M.; De Matteis, G.; Santoro, M.; Sabia, L.; Simeoni, B.; Candelli, M.; Ojetti, V.; Franceschi, F. Clinical characteristics and prognostic factors in COVID-19 patients aged ≥ 80 years. Geriatr. Gerontol. Int. 2020, 20, 704–708. [Google Scholar] [CrossRef]
- De La Rica, R.; Borges, M.; Aranda, M.; Del Castillo, A.; Socias, A.; Payeras, A.; Rialp, G.; Socias, L.; Masmiquel, L.; Gonzalez-Freire, M. Low Albumin Levels Are Associated with Poorer Outcomes in a Case Series of COVID-19 Patients in Spain: A Retrospective Cohort Study. Microorganisms 2020, 8, 1106. [Google Scholar] [CrossRef]
- Di Bella, S.; Cesareo, R.; De Cristofaro, P.; Palermo, A.; Sanson, G.; Roman-Pognuz, E.; Zerbato, V.; Manfrini, S.; Giacomazzi, D.; Bo, E.D.; et al. Neck circumference as reliable predictor of mechanical ventilation support in adult inpatients with COVID-19: A multicentric prospective evaluation. Diabetes/Metab. Res. Rev. 2021, 37, e3354. [Google Scholar] [CrossRef]
- Tambe, M.P.; Parande, M.; Tapare, V.S.; Borle, P.S.; Lakde, R.N.; Shelke, S.C.; BJMC COVID Epidemiology Group. An epidemiological study of laboratory confirmed COVID-19 cases admitted in a tertiary care hospital of Pune, Maharashtra. Indian J. Public Health 2020, 64, 183–S187. [Google Scholar] [CrossRef]
- Shahriarirad, R.; Khodamoradi, Z.; Erfani, A.; Hosseinpour, H.; Ranjbar, K.; Emami, Y.; Mirahmadizadeh, A.; Lotfi, M.; Yeganeh, B.S.; Nejad, A.D.; et al. Epidemiological and clinical features of 2019 novel coronavirus diseases (COVID-19) in the South of Iran. BMC Infect. Dis. 2020, 20, 427. [Google Scholar] [CrossRef] [PubMed]
- Regina, J.; Papadimitriou-Olivgeris, M.; Burger, R.; Le Pogam, M.-A.; Niemi, T.; Filippidis, P.; Tschopp, J.; Desgranges, F.; Viala, B.; Kampouri, E.; et al. Epidemiology, risk factors and clinical course of SARS-CoV-2 infected patients in a Swiss university hospital: An observational retrospective study. PLoS ONE 2020, 15, e0240781. [Google Scholar] [CrossRef] [PubMed]
- Satici, C.; Demirkol, M.A.; Altunok, E.S.; Gursoy, B.; Alkan, M.; Kamat, S.; Demirok, B.; Surmeli, C.D.; Calik, M.; Cavus, Z.; et al. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int. J. Infect. Dis. 2020, 98, 84–89. [Google Scholar] [CrossRef]
- Lee, L.Y.W.; Cazier, J.-B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef]
- Argenziano, M.G.; Bruce, S.L.; Slater, C.L.; Tiao, J.R.; Baldwin, M.R.; Barr, R.G.; Chang, B.P.; Chau, K.H.; Choi, J.J.; Gavin, N.; et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ 2020, 369, m1996. [Google Scholar] [CrossRef] [PubMed]
- Imam, Z.; Odish, F.; Gill, I.; O’Connor, D.; Armstrong, J.; Vanood, A.; Ibironke, O.; Hanna, A.; Ranski, A.; Halalau, A. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J. Intern. Med. 2020, 288, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.; Szymański, P.; Pańkowski, I.; Szarowska, A.; Życińska, K.; Rogowski, W.; Gil, R.; Furmanek, M.; Tatur, J.; Zaczyński, A.; et al. Clinical characteristics and short-term outcomes of coronavirus disease 2019: Retrospective, single-center experience of designated hospital in Poland. Pol. Arch. Intern. Med. 2020, 130, 407–411. [Google Scholar] [CrossRef]
- Okoh, A.K.; Sossou, C.; Dangayach, N.S.; Meledathu, S.; Phillips, O.; Raczek, C.; Patti, M.; Kang, N.; Hirji, S.A.; Cathcart, C.; et al. Coronavirus disease 19 in minority populations of Newark, New Jersey. Int. J. Equity Health 2020, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Palaiodimos, L.; Kokkinidis, D.G.; Li, W.; Karamanis, D.; Ognibene, J.; Arora, S.; Southern, W.N.; Mantzoros, C.S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabilism 2020, 108, 154262. [Google Scholar] [CrossRef]
- Rentsch, C.T.; Kidwai-Khan, F.; Tate, J.P.; Park, L.S.; King, J.T., Jr.; Skanderson, M.; Hauser, R.G.; Schultze, A.; Jarvis, C.I.; Ho-lodniy, M.; et al. Covid-19 Testing, Hospital Admission, and Intensive Care among 2,026,227 United States Veterans Aged 54-75 Years. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Russo, V.; Di Maio, M.; Attena, E.; Silverio, A.; Scudiero, F.; Celentani, D.; Lodigiani, C.; Di Micco, P. Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: A multicenter observational study. Pharmacol. Res. 2020, 159, 104965. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tanoira, R.; Pérez-García, F.; Romanyk, J.; Gómez-Herruz, P.; Arroyo, T.; González, R.; García, L.L.; Expósito, C.V.; Moreno, J.S.; Gutiérrez, I.; et al. Prevalence and risk factors for mortality related to COVID-19 in a severely affected area of Madrid, Spain. medRxiv 2020. [Google Scholar] [CrossRef]
- Foy, B.H.; Carlson, J.C.; Reinertsen, E.; Valls, R.P.; Lopez, R.P.; Palanques-Tost, E.; Mow, C.; Westover, M.B.; Aguirre, A.D.; Higgins, J.M. Elevated RDW is Associated with Increased Mortality Risk in COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Gui, S.; Pan, F.; Ye, T.; Liang, B.; Hu, Y.; Zheng, C. Association of clinical and radiographic findings with the outcomes of 93 patients with COVID-19 in Wuhan, China. Theranostics 2020, 10, 6113–6121. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef]
- Javanian, M.; Bayani, M.; Shokri, M.; Sadeghi-Haddad-Zavareh, M.; Babazadeh, A.; Yeganeh, B.; Mohseni, S.; Mehraein, R.; Sepidarkish, M.; Bijani, A.; et al. Clinical and laboratory findings from patients with COVID-19 pneumonia in Babol North of Iran: A retrospective cohort study. Roman. J. Intern. Med. 2020, 58, 161–167. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Gao, C.; Cai, Y.; Zhang, K.; Zhou, L.; Zhang, Y.; Zhang, X.; Li, Q.; Li, W.; Yang, S.; Zhao, X.; et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: A retrospective observational study. Eur. Hear. J. 2020, 41, 2058–2066. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, A.; Kumar, R.; Fang, Y.; Chen, G.; Zhu, Y.; Lin, S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J. Med. Virol. 2020, 92, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Urra, J.; Cabrera, C.; Porras, L.; Ródenas, I. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin. Immunol. 2020, 217, 108486. [Google Scholar] [CrossRef]
- Xu, P.P.; Tian, R.H.; Luo, S.; Zu, Z.Y.; Fan, B.; Wang, X.M.; Xu, K.; Wang, J.T.; Zhu, J.; Shi, J.C.; et al. Risk factors for adverse clinical outcomes with COVID-19 in China: A multicenter, retrospective, observational study. Theranostics 2020, 10, 6372–6383. [Google Scholar] [CrossRef] [PubMed]
- Auld, S.C.; Caridi-Scheible, M.; Blum, J.M.; Robichaux, C.; Kraft, C.; Jacob, J.T.; Jabaley, C.S.; Carpenter, D.; Kaplow, R.; Hernandez-Romieu, A.C.; et al. ICU and Ventilator Mortality Among Critically Ill Adults with Coronavirus Disease. Crit. Care Med. 2020. Publish Ahead. [Google Scholar] [CrossRef] [PubMed]
- Borobia, A.M.; Carcas, A.J.; Arnalich, F.; Álvarez-Sala, R.; Monserrat-Villatoro, J.; Quintana, M.; Figueira, J.C.; Santos-Olmo, R.M.T.; García-Rodríguez, J.; Martín-Vega, A.; et al. A Cohort of Patients with COVID-19 in a Major Teaching Hospital in Europe. J. Clin. Med. 2020, 9, 1733. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Hadjadj, S.; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; The CORONADO Investigators; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 2020, 63, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Zamora, E.; Hernandez-Suarez, C.M. Survival in adult inpatients with COVID-19. Public Health 2021, 190, 1–3. [Google Scholar] [CrossRef]
- Israelsen, S.B.; Kristiansen, K.T.; Hindsberger, B.; Ulrik, C.S.; Andersen, O.; Jensen, M.; Andersen, S.; Rasmussen, C.; Jørgensen, H.L.; Østergaard, C.; et al. Characteristics of patients with COVID-19 pneumonia at Hvidovre Hospital, March–April 2020. Dan. Med. J. 2020, 67, A05200313. [Google Scholar]
- Alqahtani, J.S.; Oyelade, T.; Aldhahir, A.M.; Alghamdi, S.M.; Almehmadi, M.; Alqahtani, A.S.; Quaderi, S.; Mandal, S.; Hurst, J.R. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0233147. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.; Cerfolio, R.J.; Francois, F.; Horwitz, L. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Qian, L.; Hong, M.V.; Wei, M.R.; Nadjafi, R.F.; Fischer, H.; Li, M.Z.; Shaw, D.S.F.; Caparosa, M.S.L.; Nau, C.L.; et al. Obesity and Mortality Among Patients Diagnosed With COVID-19: Results From an Integrated Health Care Organization. Ann. Intern. Med. 2020, 173, 773–781. [Google Scholar] [CrossRef]
- Lücker, L.M.; Kherad, O.; Iten, A.; Wagner, N.; Descombes, M.; Camus, V.; Kaiser, L.; Louis-Simonet, M. Clinical features and outcome of hospitalised adults and children with the 2009 influenza A H1N1 infection at Geneva’s University Hospital. Swiss Med. Wkly. 2011, 141. [Google Scholar] [CrossRef] [PubMed]
- Bouneb, R.; Mellouli, M.; Bensoltane, H.; Baroudi, J.; Chouchene, I.; Boussarsar, M. Characteristics and outcome of ill critical patients with influenza a infection. Pan Afr. Med. J. 2018, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Cox, N.; Anderson, L.J.; Fukuda, K. Mortality Associated with Influenza and Respiratory Syncytial Virus in the United States. JAMA 2003, 289, 179–186. [Google Scholar] [CrossRef]
- Kim, H.-C.; Choi, S.-H.; Huh, J.-W.; Sung, H.; Hong, S.B.; Lim, C.-M.; Koh, Y. Different pattern of viral infections and clinical outcomes in patient with acute exacerbation of chronic obstructive pulmonary disease and chronic obstructive pulmonary disease with pneumonia. J. Med. Virol. 2016, 88, 2092–2099. [Google Scholar] [CrossRef]
- Bramley, A.M.; Dasgupta, S.; Skarbinski, J.; Kamimoto, L.; Fry, A.M.; Finelli, L.; Jain, S. Intensive care unit patients with 2009 pandemic influenza A (H1N1pdm09) virus infection—United States. Influ. Other Respir. Viruses 2012, 6, e134–e142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.C.; Kotsimbos, T.; Reynolds, A.; Bowler, S.D.; Brown, S.G.; Hancox, R.J.; Holmes, M.; Irving, L.; Jenkins, C.; Thompson, P.; et al. Clinical and epidemiological profile of patients with severe H1N1/09 pandemic influenza in Australia and New Zealand: An observational cohort study. BMJ Open 2011, 1, e000100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamaati, H.; Mohajerani, S.A.; Shamaee, M.; Chitsazan, M.; Radmand, G.; Maadani, M.; Mansouri, S.D.; Hashemian, S.M.R.; Tabarsi, P.; Nadji, S.A. Secondary infection and clinical aspects after pandemic swine-origin influenza a (H1N1) admission in an Iranian critical care unite. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Jaber, S.; Conseil, M.; Coisel, Y.; Jung, B.; Chanques, G. Grippe A (H1N1) et SDRA: Caractéristiques des patients admis en réanimation et prise en charge. Revue de la littérature. Ann. Fr. d’Anesth. Réanim. 2010, 29, 117–125. [Google Scholar] [CrossRef]
- Fabbiani, M.; Sali, M.; Di Cristo, V.; Pignataro, G.; Prete, V.; Farina, S.; D’Avino, A.; Manzara, S.; Verme, L.Z.D.; Silveri, N.G.; et al. Prospective evaluation of epidemiological, clinical, and microbiological features of pandemic influenza A (H1N1) virus infection in Italy. J. Med. Virol. 2011, 83, 2057–2065. [Google Scholar] [CrossRef]
- Peralta, P.S.-O.; Cortes-García, M.; Vicente-Herrero, M.; Castrillo-Villamandos, C.; Arias-Bohigas, P.; Amo, I.P.-D.; Sierra-Moros, M.J.; on behalf of the Surveillance Group for New Influenza A(H1N1) Virus Investigation and Control Team in Spain. Risk factors for disease severity among hospitalised patients with 2009 pandemic influenza A (H1N1) in Spain, April–December 2009. Eurosurveillance 2010, 15, 19667. [Google Scholar] [CrossRef] [Green Version]
- Booth, C.M.; Matukas, L.M.; Tomlinson, G.A.; Rachlis, A.R.; Rose, D.B.; Dwosh, H.A.; Walmsley, S.L.; Mazzulli, T.; Avendano, M.; Derkach, P.; et al. Clinical Features and Short-term Outcomes of 144 Patients With SARS in the Greater Toronto Area. JAMA 2003, 289, 2801–2809. [Google Scholar] [CrossRef] [Green Version]
- Leung, G.M.; Hedley, A.J.; Ho, L.-M.; Chau, P.; Wong, I.O.; Thach, T.Q.; Ghani, A.C.; Donnelly, C.A.; Fraser, C.; Riley, S.; et al. The Epidemiology of Severe Acute Respiratory Syndrome in the 2003 Hong Kong Epidemic: An Analysis of All 1755 Patients. Ann. Intern. Med. 2004, 141, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, H.S.; Wang, F.M.; Wang, S.J.; Wu, X.D.; Zhang, N.X.; Kan, Z.C.; Qin, Y.Z.; Xiao, L. Preliminary analysis of treatment in 32 patients with critical severe acute respiratory syndrome. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2003, 15, 492–494. [Google Scholar] [PubMed]
- Rohde, G.; Borg, I.; Arinir, U.; Dorsten, C.; Schultze-Werninghaus, G.; Bauer, T. T Evaluation of a real-time polymerase-chain reaction for severe acute respiratory syndrome (SARS) associated coronavirus in patients with hospitalised exacerbation of COPD. Eur. J. Med. Res. 2004, 9, 505–509. [Google Scholar] [PubMed]
- Kherad, O.; Kaiser, L.; Bridevaux, P.-O.; Sarasin, F.; Thomas, Y.; Janssens, J.-P.; Rutschmann, O.T. Upper-Respiratory Viral Infection, Biomarkers, and COPD Exacerbations. Chest 2010, 138, 896–904. [Google Scholar] [CrossRef]
- Radzikowska, U.; Ding, M.; Tan, G.; Zhakparov, D.; Peng, Y.; Wawrzyniak, P.; Wang, M.; Li, S.; Morita, H.; Altunbulakli, C.; et al. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy 2020, 75, 2829–2845. [Google Scholar] [CrossRef]
- Gu, J.; Gong, E.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Zhan, J.; Wang, S.; Xie, Z.; et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhou, J.; To, K.K.-W.; Chu, H.; Li, C.; Wang, D.; Yang, D.; Zheng, S.; Hao, K.; Bossé, Y.; et al. Identification ofTMPRSS2as a Susceptibility Gene for Severe 2009 Pandemic A(H1N1) Influenza and A(H7N9) Influenza. J. Infect. Dis. 2015, 212, 1214–1221. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [Green Version]
- Brake, S.J.; Barnsley, K.; Lu, W.; McAlinden, K.D.; Eapen, M.S.; Sohal, S.S. Smoking Upregulates Angiotensin-Converting Enzyme-2 Receptor: A Potential Adhesion Site for Novel Coronavirus SARS-CoV-2 (Covid-19). J. Clin. Med. 2020, 9, 841. [Google Scholar] [CrossRef] [Green Version]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.-L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef] [Green Version]
- Rossato, M.; Russo, L.; Mazzocut, S.; Di Vincenzo, A.; Fioretto, P.; Vettor, R. Current smoking is not associated with COVID-19. Eur. Respir. J. 2020, 55, 2001290. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif-Askari, N.S.; Sharif-Askari, F.S.; Alabed, M.; Temsah, M.-H.; Al Heialy, S.; Hamid, Q.; Halwani, R. Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD. Mol. Ther. Methods Clin. Dev. 2020, 18, 1–6. [Google Scholar] [CrossRef]
- Kim, W.J.; Lim, J.H.; Lee, J.S.; Lee, S.-D.; Kim, J.H.; Oh, Y.-M. Comprehensive Analysis of Transcriptome Sequencing Data in the Lung Tissues of COPD Subjects. Int. J. Genom. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, B.G.G.; Oliveira, A.E.R.; Singh, Y.; Jimenez, L.; Gonçalves, A.N.; Ogava, R.L.T.; Creighton, R.; Peron, J.P.S.; Nakaya, H.I. ACE2 Expression Is Increased in the Lungs of Patients with Comorbidities Associated with Severe COVID-19. J. Infect. Dis. 2020, 222, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Sausville, E.L.; Girish, V.; Yuan, M.L.; Vasudevan, A.; John, K.M.; Sheltzer, J.M. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev. Cell 2020, 53, 514–529.e3. [Google Scholar] [CrossRef]
- Matusiak, M.; Schürch, C.M. Expression of SARS-CoV-2 entry receptors in the respiratory tract of healthy individuals, smokers and asthmatics. Respir. Res. 2020, 21, 1–6. [Google Scholar] [CrossRef]
- Song, J.; Zeng, M.; Wang, H.; Qin, C.; Hou, H.; Sun, Z.; Xu, S.; Wang, G.; Guo, C.; Deng, Y.; et al. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19. Allergy 2021, 76, 483–496. [Google Scholar] [CrossRef]
- Drummond, M.B.; Wise, R.A.; Hansel, N.N.; Putcha, N. Comorbidities and Chronic Obstructive Pulmonary Disease: Prevalence, Influence on Outcomes, and Management. Semin. Respir. Crit. Care Med. 2015, 36, 575–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Thomas, J.; Sadatsafavi, M.; FitzGerald, J.M. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Lancet Respir. Med. 2015, 3, 631–639. [Google Scholar] [CrossRef]
- Corne, J.M.; Marshall, C.; Smith, S.; Schreiber, J.; Sanderson, G.; Holgate, S.T.; Johnston, S.L. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: A longitudinal cohort study. Lancet 2002, 359, 831–834. [Google Scholar] [CrossRef]
- Lovinsky-Desir, S.; Deshpande, D.R.; De, A.; Murray, L.; Stingone, J.A.; Chan, A.; Patel, N.; Rai, N.; DiMango, E.; Milner, J.; et al. Asthma among hospitalized patients with COVID-19 and related outcomes. J. Allergy Clin. Immunol. 2020, 146, 1027–1034.e4. [Google Scholar] [CrossRef]
- Chhiba, K.D.; Patel, G.B.; Vu, T.H.T.; Chen, M.M.; Guo, A.; Kudlaty, E.; Mai, Q.; Yeh, C.; Muhammad, L.N.; Harris, K.E.; et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J. Allergy Clin. Immunol. 2020, 146, 307–314.e4. [Google Scholar] [CrossRef]
- Avdeev, S.; Moiseev, S.; Brovko, M.; Yavorovskiy, A.; Umbetova, K.; Akulkina, L.; Tsareva, N.; Merzhoeva, Z.; Gainitdinova, V.; Fomin, V. Low prevalence of bronchial asthma and chronic obstructive lung disease among intensive care unit patients with COVID-19. Allergy 2020, 75, 2703–2704. [Google Scholar] [CrossRef] [PubMed]
- Lieberman-Cribbin, W.; Rapp, J.; Alpert, N.; Tuminello, S.; Taioli, E. The Impact of Asthma on Mortality in Patients with COVID-19. Chest 2020, 158, 2290–2291. [Google Scholar] [CrossRef] [PubMed]
- Klemets, P.; Lyytikäinen, O.; Ruutu, P.; Ollgren, J.; Kaijalainen, T.; Leinonen, M.; Nuorti, J.P. Risk of invasive pneumococcal infections among working age adults with asthma. Thorax 2010, 65, 698–702. [Google Scholar] [CrossRef] [Green Version]
- Talbot, T.R.; Hartert, T.V.; Mitchel, E.; Halasa, N.B.; Arbogast, P.G.; Poehling, K.A.; Schaffner, W.; Craig, A.S.; Griffin, M.R. Asthma as a Risk Factor for Invasive Pneumococcal Disease. N. Engl. J. Med. 2005, 352, 2082–2090. [Google Scholar] [CrossRef]
- Black, P.N.; Blasi, F.; Jenkins, C.R.; Scicchitano, R.; Mills, G.D.; Rubinfeld, A.R.; Ruffin, R.E.; Mullins, P.R.; Dangain, J.; Cooper, B.C.; et al. Trial of Roxithromycin in Subjects with Asthma and Serological Evidence of Infection with Chlamydia pneumoniae. Am. J. Respir. Crit. Care Med. 2001, 164, 536–541. [Google Scholar] [CrossRef]
- Busse, W.W.; Lemanske, R.F.; Gern, J. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010, 376, 826–834. [Google Scholar] [CrossRef]
- Grissell, T.V.; Powell, H.; Shafren, D.R.; Boyle, M.J.; Hensley, M.J.; Jones, P.D.; Whitehead, B.F.; Gibson, P.G. Interleukin-10 Gene Expression in Acute Virus-induced Asthma. Am. J. Respir. Crit. Care Med. 2005, 172, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, S.; Sheen, Y.H.; Ha, E.K.; Choi, S.H.; Yang, M.-S.; Hwang, S.; Kim, S.S.; Choi, J.-H.; Han, M.Y. Seasonal Cycle and Relationship of Seasonal Rhino- and Influenza Virus Epidemics With Episodes of Asthma Exacerbation in Different Age Groups. Allergy Asthma Immunol. Res. 2017, 9, 517–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, P.; Jung, T.H.; Keene, D.; Demmer, R.T.; Perzanowski, M.; Lovasi, G. Temporal and spatial associations between influenza and asthma hospitalisations in New York City from 2002 to 2012: A longitudinal ecological study. BMJ Open 2018, 8, e020362. [Google Scholar] [CrossRef]
- Morales-Suárez-Varela, M.; Llopis-González, A.; Vergara-Hernández, C.; Fernandez-Fabrellas, E.; Sanz, F.; Perez-Lozano, M.J.; Martin, V.; Astray, J.; Castilla, J.; Egurrola, M.; et al. Asthma in older people hospitalized with influenza in Spain: A case-control study. Allergy Asthma Proc. 2017, 38, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Feldman, L.Y.; Zhu, J.; To, T. Estimating age-specific influenza-associated asthma morbidity in Ontario, Canada. Respir. Med. 2019, 155, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Van-Tam, J.S.; Openshaw, P.J.M.; Hashim, A.; Gadd, E.M.; Lim, W.S.; Semple, M.G.; Read, R.C.; Taylor, B.L.; Brett, S.J.; McMenamin, J.; et al. Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-September 2009). Thorax 2010, 65, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rieiro, C.; Carrasco-Garrido, P.; Hernandez-Barrera, V.; De Andres, A.L.; Jimenez-Trujillo, I.; Gil De Miguel, Á.; Jimenez-Garcia, R. Pandemic influenza hospitalization in Spain (2009): Incidence, in-hospital mortality, comorbidities and costs. Hum. Vaccines Immunother. 2012, 8, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.H.; Song, W.J.; Yang, M.S.; Lee, S.H.; Kwon, J.W.; Kim, S.H.; Kang, H.R.; Park, H.W.; Cho, Y.J.; Cho, S.H.; et al. Clinical course of asthma patients with H1N1 influenza infection and oseltamivir. Minerva Med. 2018, 109, 7–14. [Google Scholar] [CrossRef]
- Dhawale, V.S.; Amara, V.R.; Karpe, P.A.; Malek, V.; Patel, D.; Tikoo, K. Activation of angiotensin-converting enzyme 2 (ACE2) attenuates allergic airway inflammation in rat asthma model. Toxicol. Appl. Pharmacol. 2016, 306, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Camiolo, M.; Gauthier, M.; Kaminski, N.; Ray, A.; Wenzel, S.E. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J. Allergy Clin. Immunol. 2020, 146, 315–324.e7. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Busse, W.W.; Bacharier, L.B.; Kattan, M.; O’Connor, G.T.; Wood, R.A.; Visness, C.M.; Durham, S.R.; Larson, D.; Esnault, S.; et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J. Allergy Clin. Immunol. 2020, 146, 203–206.e3. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Francisco, D.; Conway, M.; Martinez, F.D.; Vercelli, D.; Polverino, F.; Billheimer, D.; Kraft, M. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J. Allergy Clin. Immunol. 2020, 146, 80–88.e8. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.S.; Thiruchelvam, D.; Chapman, K.R.; Aaron, S.D.; Stanbrook, M.B.; Bourbeau, J.; Tan, W.; To, T. Health Services Burden of Undiagnosed and Overdiagnosed COPD. Chest 2018, 153, 1336–1346. [Google Scholar] [CrossRef]







| Study | Location | Design | Enrollment Start # | Enrollment End # | COPD | Asthma | Sample Size |
|---|---|---|---|---|---|---|---|
| Auld[47] | USA | Retrospective | 6 March | 17 April | Y | Y | 217 |
| Borobia[48] | Spain | Retrospective | 25 February | 5 April | Y | Y | 2226 |
| Cariou[49] | France | Retrospective | 10 March | 31 March | Y | N | 1317 |
| Covino[22] | Italy | Retrospective | 1 March | 31 March | Y | N | 69 |
| Foy[38] | USA | Retrospective | 4 March | 28 April | Y | N | 1198 |
| Gao[43] | China | Retrospective | 1 March | 13 March | Y | N | 2877 |
| Huang[44] | China | Retrospective | 25 January | 24 March | Y | N | 299 |
| Imam[31] | USA | Retrospective | 1 March | 17 April | Y | Y | 1305 |
| Javanian[41] | Iran | Retrospective | 25 February | 12 March | Y | N | 100 |
| Lee[29] | UK | Prospective | 18 March | 26 April | Y | N | 800 |
| Li[39] | China | Retrospective | 10 January | 22 February | Y | N | 93 |
| Liu[40] | China | Retrospective | 1 January | 29 February | Y | N | 245 |
| Murillo-Zamora[50] | Mexico | Retrospective | 4 March | 5 May | Y | Y | 5393 |
| Nowak[32] | Poland | Retrospective | 16 March | 7 April | Y | N | 169 |
| Okoh[33] | USA | Retrospective | 10 March | 10 April | Y | N | 251 |
| Palaiodimos[34] | USA | Retrospective | 9 March | 22 March | Y | Y | 200 |
| Russo[36] | Italy | Prospective | February | April | Y | N | 192 |
| Satici[28] | Turkey | Retrospective | 2 April | 1 May | Y | Y | 681 |
| Shahriarirad[26] | Iran | Retrospective | 20 February | 20 March | Y | Y | 113 |
| Tambe[25] | India | Cross-sectional | 31 March | 24 April | Y | N | 197 |
| Tanoira[37] | Spain | Retrospective | 3 March | 16 March | Y | N | 311 |
| Xu[46] | China | Retrospective | 10 January | 13 March | Y | N | 703 |
| Zhou[42] | China | Retrospective | 19 December | 31 January | Y | N | 191 |
| Argenziano *[30] | USA | Retrospective | 1 March | 5 April | Y | Y | 850 |
| De la Rica[23] | Spain | Retrospective | 15 March | 31 March | Y | N | 48 |
| Israelsen[51] | Denmark | Retrospective | 10 March | 23 April | Y | Y | 175 |
| Rentsch[35] | USA | Retrospective | 8 February | 30 March | Y | N | 585 |
| Urra[45] | Spain | Retrospective | 1 March | 15 April | Y | N | 172 |
| Di Bella[24] | Italy | Prospective | 25 March | 7 April | Y | N | 132 |
| Regina[27] | Switzerland | Retrospective | 1 March | 25 March | Y | N | 200 |
| Study | Age | Female n (%) | Diabetes n (%) | Hypertension n (%) | CAD n (%) | Heart Failure n (%) | CKD n (%) | CVA n (%) | Smoking n (%) | Malignancy n (%) | COPD n (%) | Asthma n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Auld[47] | 64 (54–73) b | 98 (45.2) | 99 (45.4) | 134 (61.7) | 31 (14.3) | 41 (18.9) | 58 (26.7) | NA | NA | NA | 21 (9.7) | 19 (8.8) |
| Borobia[48] | 61 (46–78) b | 1152 (51.8) | 381 (17.1) | 920 (41.3) | NA | NA | 174 (7.8) | NA | 157 (7.1) | 133 (6.0) | 153 (6.9) | 115 (5.2) |
| Cariou[49] | 69.8 (13) a | 462 (35.1) | NA | 1003 (76.1) | 336 (25.5) | 140 (10.6) | 60 (4.5) | 163 (12.4) | 447 (33.9) | NA | 133 (10.1) | NA |
| Covino[22] | 84 (82–89 ) d | 32 (46.4) | 9 (13.0) | 41 (59.4) | 21 (30.4) | 21 (30.4) | NA | 20 (29.0%) | NA | 3 (4.3) | 7 (10.1) | NA |
| Foy[38] | NA | 534 (44.8) | NA | NA | NA | NA | NA | NA | NA | NA | 273 (22.8) | NA |
| Gao[43] | NA | NA | 387 (13.6) | 850 (29.5) | 295 (10.25) | 23 (0.8) | 29 (1.0) | 52 (1.8) | 190 (6.6) | 49 (1.7) | 31 (1.1) | NA |
| Huang[44] | 53.4 (16.7) a | 139 (46.5) | 35 (11.7) | 74 (24.7) | 18 (6) | NA | NA | 13 (4.3) | 48 (16.1) | 9 (3.0) | 8 (2.6) | NA |
| Imam[31] | 61.0 (16.3) a | 603 (46.2) | 393 (30.1) | 734 (56.2) | 208 (15.9) | 75 (5.7) | 228 (17.5) | 95 (7.3) | 356 (27.3) | 83 (6.4) | 107 (8.2) | 115 (8.8) |
| Javanian[41] | 60.12 (13.87) b | 49 (49) | 34 (34.0) | 31 (31.0) | 22 (22.0) | NA | 11 (11.0) | 3 (3.0) | NA | 4 (4.0) | 12 (12.0) | NA |
| Lee[29] | 69 (59.0–76.0) b | 349 (43.6) | 131 (16.4) | 247 (30.9) | NA | NA | NA | NA | NA | 800 (100) | 61 (7.6) | NA |
| Li[39] | 51 (17.5) a | 52 (55.9) | 11 (11.8) | 5 (5.4) | 4 (4.3) | NA | NA | NA | NA | 4 (4.3) | 8 (8.6) | NA |
| Liu[40] | 53.95 (16.9) a | 131 (53.5) | 23 (9.4) | 52 (21.2) | 18 (7.3) | NA | NA | NA | 10 (4.01) | 9 (3.7%) | 8 (3.3) | NA |
| Murillo-Zamora[50] | NA | 1,961 (36.4) | 1,677 (31.1) | 1,973 (36.6) | NA | NA | 299 (5.5) | NA | NA | NA | 273 (5.1) | 146 (2.7) |
| Nowak[32] | 63.7 (19.6) a | 82 (48.5) | 32 (18.9) | 80 (47.3) | 52 (30.8) | NA | 35 (20.7) | 58 (34.3) | NA | 35 (20.7) | 22 (13.3) | NA |
| Okoh[33] | 62 (49–74) b | 122(48.6) | 115 (45.8) | 175 (69.7) | 49 (19.5) | 50 (19.9) | 46 (18.3) | 28 (11.1) | NA | 22 (8.7) | 23 (9.2) | NA |
| Palaiodimos[34] | 64 (50.0–73.5) b | 102 (51.0) | 79 (39.5) | 152 (76.0) | 33 (16.5) | 34 (17.0) | 41 (20.5) | 22 (11.0) | NA | 11 (5.5) | 28 (14.0) | 27 (13.5) |
| Russo[36] | 67.7 (15.2) a | 77 (40.1) | 42 (21.9) | 111 (57.8) | 26 (13.5) | 20 (10.4) | 12 (6.3) | 16 (8.3) | 16 (8..0) | NA | 26 (13.5) | NA |
| Satici[28] | 56.9 (15.7) a | 334 (49.0) | 191 (28.0) | 234 (34.4) | 62 (9.1) | 19 (2.8) | 24 (3.5) | NA | NA | 9 (1.3) | 28 (4.1) | 43 (6.3) |
| Shahriarirad[26] | 53.75 (16.58) a | 42 (37.2) | 16 (14.2) | 22 (19.5) | 16 (14.2) | NA | 6 (5.3) | NA | NA | 1 (0.9) | 9 (7.9) | 7 (6.2) |
| Tambe[25] | 45.8 (17.3) a | 107 (54.5) | 42 (21.3) | 60 (30.5) | 4 (2.0) | NA | 2 (1.0) | NA | NA | NA | 10 (5.1) | NA |
| Tanoira[37] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Xu[46] | 46.1 (15.2) a | 321(45.6) | 64(9.1) | NA | 35 (5.0) | NA | 10(1.4) | NA | NA | 9(1.3) | 13 (1.8) | NA |
| Zhou[42] | 56·0 (46–67) b | 72 (37.7) | 36 (18.8) | 58 (30.4) | 15 (7.9) | NA | 2 (1.0) | NA | 11 (5.8) | 2 (1.0) | 6 (3.1) | NA |
| Argenziano[30] | 63.0 (50.0–75.0) b | 339 (39.9) | 333 (39.2) | 525 (61.8%) | 115 (13.5) | 91 (10.7) | 125 (14.7) | 72 (8.5) | 198 (23.3) | 63 (7.4) | 56 (6.6) | 88 (10.4) |
| De la Rica[23] | 65.98 (13.9) a | 16 (33.3) | 11 (22.9) | 33 (68.8) | 14 (29.2) | NA | 8 (16.7) | NA | 10 (20.8) | 10 (20.8) | 5 (10.4) | NA |
| Israelsen[51] | 71 (55–81) b | 90 (51,4) | 46 (26.3) | 73 (41.7) | 90 (51.4) | NA | NA | NA | 63 (36.0) | NA | 11 (6.3) | 20 (11.4) |
| Rentsch[35] | 66.1 (60.4–71.0) b | 27 (4.6) | 260 (44.4) | 423 (72.3) | 163 (27.3) | NA | NA | NA | 338 (57.8) | 83 (14.2) | 90 (15.4) | 45 (7.7) |
| Urra[45] | NA | 68 (39.5) | 39 (22.7) | 87 (50.6) | 28(16.3) | NA | NA | NA | NA | NA | 17 (9.9) | NA |
| Di Bella[24] | 66 (55–75.8) b | 42 (31.8) | 33 (25.0) | 55 (41.7) | 24 (18.2) | NA | NA | NA | 12 (9.1) | NA | 10 (7.6) | NA |
| Regina[27] | 70 (55–81) b | 80 (40.0) | 43 (21.5) | 87 (43.5) | 35 (17.5) | NA | 28 (14.0) | 21 (10.5) | NA | NA | 16 (8.0) | 8 (4.0) |
| COPD | Asthma | |||||
|---|---|---|---|---|---|---|
| Variables | Coefficient | Standard Error | p-Value | Coefficient | Standard Error | p-Value |
| Age | −0.009 | 0.006 | 0.146 | 0.040 | 0.175 | 0.835 |
| Female | 0.031 | 0.013 | 0.037 | −0.026 | 0.024 | 0.330 |
| HTN | −0.012 | 0.008 | 0.114 | −0.004 | 0.013 | 0.743 |
| CAD | −0.006 | 0.014 | 0.648 | 0.149 | 0.148 | 0.387 |
| HF | −0.016 | 0.027 | 0.584 | −0.033 | 0.049 | 0.568 |
| CKD | −0.015 | 0.022 | 0.489 | 0.001 | 0.023 | 0.979 |
| Diabetes | −0.026 | 0.011 | 0.023 | 0.002 | 0.019 | 0.924 |
| Malignacy | −0.005 | 0.005 | 0.316 | 0.077 | 0.173 | 0.688 |
| CVA | −0.019 | 0.021 | 0.371 | |||
| Smoking | −0.026 | 0.013 | 0.108 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes, F.M.; Hache-Marliere, M.; Karamanis, D.; Berto, C.G.; Estrada, R.; Langston, M.; Ntaios, G.; Gulani, P.; Shah, C.D.; Palaiodimos, L. Assessment of the Association of COPD and Asthma with In-Hospital Mortality in Patients with COVID-19. A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. J. Clin. Med. 2021, 10, 2087. https://doi.org/10.3390/jcm10102087
Reyes FM, Hache-Marliere M, Karamanis D, Berto CG, Estrada R, Langston M, Ntaios G, Gulani P, Shah CD, Palaiodimos L. Assessment of the Association of COPD and Asthma with In-Hospital Mortality in Patients with COVID-19. A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Journal of Clinical Medicine. 2021; 10(10):2087. https://doi.org/10.3390/jcm10102087
Chicago/Turabian StyleReyes, Felix M., Manuel Hache-Marliere, Dimitris Karamanis, Cesar G. Berto, Rodolfo Estrada, Matthew Langston, George Ntaios, Perminder Gulani, Chirag D. Shah, and Leonidas Palaiodimos. 2021. "Assessment of the Association of COPD and Asthma with In-Hospital Mortality in Patients with COVID-19. A Systematic Review, Meta-Analysis, and Meta-Regression Analysis" Journal of Clinical Medicine 10, no. 10: 2087. https://doi.org/10.3390/jcm10102087
APA StyleReyes, F. M., Hache-Marliere, M., Karamanis, D., Berto, C. G., Estrada, R., Langston, M., Ntaios, G., Gulani, P., Shah, C. D., & Palaiodimos, L. (2021). Assessment of the Association of COPD and Asthma with In-Hospital Mortality in Patients with COVID-19. A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Journal of Clinical Medicine, 10(10), 2087. https://doi.org/10.3390/jcm10102087







