Is There an Association between Herpetic Infections and Giant Cell Arteritis? A Population-Based Study
Abstract
1. Introduction
2. Experimental Section
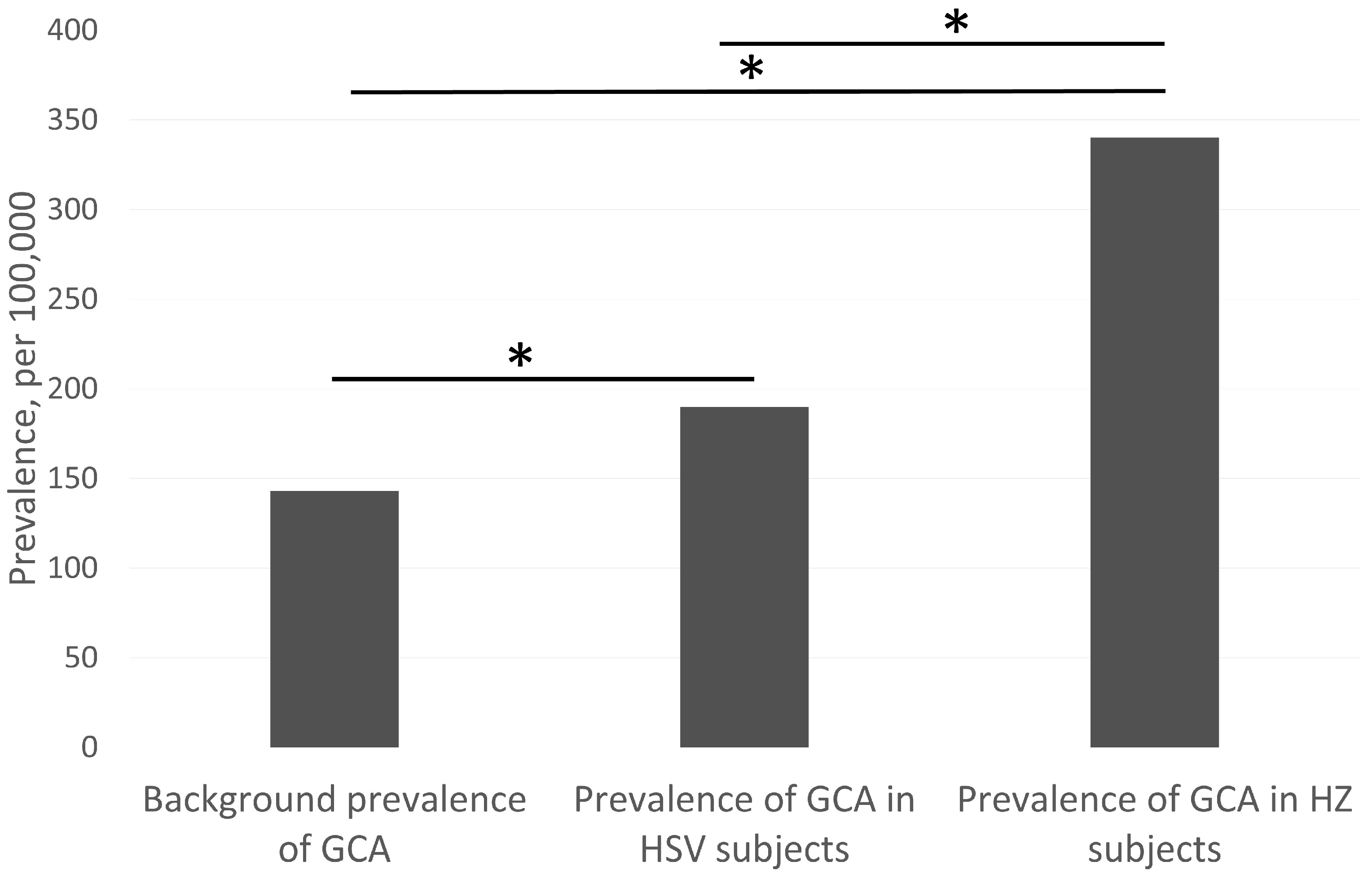
3. Results
4. Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borchers, A.T.; Gershwin, M.E. Giant cell arteritis: A review of classification, pathophysiology, geoepidemiology and treatment. Autoimmun. Rev. 2012, 11, A544–A554. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.; Corbera-Bellalta, M.; Audia, S.; Planas-Rigol, E.; Martin, L.; Cid, M.C.; Bonnotte, B. Recent advances in our understanding of giant cell arteritis pathogenesis. Autoimmun. Rev. 2017, 16, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Ly, K.-H.; Régent, A.; Tamby, M.C.; Mouthon, L. Pathogenesis of giant cell arteritis: More than just an inflammatory condition? Autoimmun. Rev. 2010, 9, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Gilden, D.; Nagel, M.A. Varicella zoster virus triggers the immunopathology of giant cell arteritis. Curr. Opin. Rheumatol. 2016, 28, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Gilden, D.; White, T.; Khmeleva, N.; Heintzman, A.; Choe, A.; Boyer, P.J.; Grose, C.; Carpenter, J.E.; Rempel, A.; Bos, N.; et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology 2015, 84, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Gilden, D.; White, T.; Khmeleva, N.; Boyer, P.J.; Nagel, M.A. VZV in biopsy-positive and -negative giant cell arteritis: Analysis of 100+ temporal arteries. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e216. [Google Scholar] [CrossRef]
- Powers, J.F.; Bedri, S.; Hussein, S.; Salomon, R.N.; Tischler, A.S. High prevalence of herpes simplex virus DNA in temporal arteritis biopsy specimens. Am. J. Clin. Pathol. 2005, 123, 261–264. [Google Scholar] [CrossRef]
- Buckingham, E.M.; Foley, M.A.; Grose, C.; Syed, N.A.; Smith, M.E.; Margolis, T.P.; Thurtell, M.J.; Kardon, R. Identification of Herpes Zoster–Associated Temporal Arteritis Among Cases of Giant Cell Arteritis. Am. J. Ophthalmol. 2018, 187, 51–60. [Google Scholar] [CrossRef]
- Kennedy, P.G.; Grinfeld, E.; Esiri, M.M. Absence of detection of Varicella-Zoster virus DNA in temporal artery biopsies obtained from patients with giant cell arteritis. J. Neurol. Sci. 2003, 215, 27–29. [Google Scholar] [CrossRef]
- Cooper, R.J.; D’Arcy, S.; Kirby, M.; Al-Buhtori, M.; Rahman, M.J.; Proctor, L.; Bonshek, R.E. Infection and temporal arteritis: A PCR-based study to detect pathogens in temporal artery biopsy specimens. J. Med. Virol. 2008, 80, 501–505. [Google Scholar] [CrossRef]
- Alvarez-Lafuente, R.; Fernandez-Gutierrez, B.; Jover, J.A.; Júdez, E.; Loza, E.; Clemente, D.; García-Asenjo, J.A.; Lamas, J.R. Human parvovirus B19, varicella zoster virus, and human herpes virus 6 in temporal artery biopsy specimens of patients with giant cell arteritis: Analysis with quantitative real time polymerase chain reaction. Ann. Rheum. Dis. 2005, 64, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pla, A.; Bosch-Gil, J.A.; Echevarria-Mayo, J.E.; Rossello-Urgell, J.; Solans-Laque, R.; Huguet-Redecilla, P.; Stone, J.H.; Vilardell-Tarres, M. No detection of parvovirus B19 or herpesvirus DNA in giant cell arteritis. J. Clin. Virol. 2004, 31, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sammel, A.M.; Smith, S.; Nguyen, K.; Laurent, R.; Brewer, J.; Hall, N.; Little, C.B. Assessment for varicella zoster virus in patients newly suspected of having giant cell arteritis. Rheumatology 2019, 59, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Rhee, R.L.; Grayson, P.C.; Merkel, P.A.; Tomasson, G. Infections and the risk of incident giant cell arteritis: A population-based, case-control study. Ann. Rheum. Dis. 2017, 76, 1031–1035. [Google Scholar] [CrossRef]
- England, B.R.; Mikuls, T.R.; Xie, F.; Yang, S.; Chen, L.; Curtis, J.R. Herpes Zoster as a Risk Factor for Incident Giant Cell Arteritis. Arthritis Rheumatol. 2017, 69, 2351–2358. [Google Scholar] [CrossRef]
- Lee, J.L.; Naguwa, S.M.; Cheema, G.S.; Gershwin, M.E. The Geo-epidemiology of Temporal (Giant Cell) Arteritis. Clin. Rev. Allergy Immunol. 2008, 35, 88–95. [Google Scholar] [CrossRef]
- British Columbia Ministry of Health, Population Data BC and BC Support Unit. BC DataScoutTM. Version 1; Population Data BC: Victoria, BC, Canada, 2018. [Google Scholar]
- Buttgereit, F.; Dejaco, C.; Matteson, E.L.; Dasgupta, B. Polymyalgia rheumatica and giant cell arteritis: A systematic review. JAMA 2016, 315, 2442–2458. [Google Scholar] [CrossRef]
- Koster, M.J.; Warrington, K.J. Giant cell arteritis: Pathogenic mechanisms and new potential therapeutic targets. BMC Rheumatol. 2017, 1, 2. [Google Scholar] [CrossRef]
- Rondaan, C.; Van Der Geest, K.S.; Eelsing, E.; Boots, A.M.H.; Bos, N.A.; Westra, J.; Brouwer, E. Decreased Immunity to Varicella Zoster Virus in Giant Cell Arteritis. Front. Immunol. 2017, 8, 8. [Google Scholar] [CrossRef]
- Bigler, M.B.; Hirsiger, J.R.; Recher, M.; Mehling, M.; Daikeler, T.; Berger, C.T. Varicella Zoster Virus-Specific T Cell Responses in Untreated Giant Cell Arteritis: Comment on the Article by England et al. Arthritis Rheumatol. 2018, 70, 318–320. [Google Scholar] [CrossRef]
- Ramstead, C.L.; Patel, A.D. Giant cell arteritis in a neuro-ophthalmology clinic in Saskatoon, 1998–2003. Can. J. Ophthalmol. 2007, 42, 295–298. [Google Scholar] [PubMed]
- Statistics Canada. British Columbia and Canada (Table). Census Profile. 2016 Census. Statistics Canada Catalogue no. 98-316-X2016001. 2017. Available online: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E (accessed on 16 December 2019).
- Nordborg, E.; Bengtsson, B.Å. Epidemiology of biopsy-proven giant cell arteritis (GCA). J. Intern. Med. 1990, 227, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Ostberg, G. Temporal arteritis in a large necropsy series. Ann. Rheum. Dis. 1971, 30, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Floris, A.; Ponte, C.; Schmidt, W.A.; Diamantopoulos, A.P.; Pereira, C.; Piper, J.; Luqmani, R. The use of ultrasound to assess giant cell arteritis: Review of the current evidence and practical guide for the rheumatologist. Rheumatology 2018, 57, 227–235. [Google Scholar] [CrossRef]
- Toyama, N.; Shiraki, K. Epidemiology of herpes zoster and its relationship to varicella in Japan: A 10-year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J. Med. Virol. 2009, 81, 2053–2058. [Google Scholar] [CrossRef]
- Lee, T.J.; Hayes, S.; Cummings, D.M.; Cao, Q.; Carpenter, K.; Heim, L.; Edwards, H. Herpes Zoster Knowledge, Prevalence, and Vaccination Rate by Race. J. Am. Board Fam. Med. 2013, 26, 45–51. [Google Scholar] [CrossRef]
- Schmader, K.; George, L.K.; Burchett, B.M.; Pieper, C.F.; Hamilton, J.D. Racial Differences in the Occurrence of Herpes Zoster. J. Infect. Dis. 1995, 171, 701–704. [Google Scholar] [CrossRef]
- Bowsher, D. The lifetime occurrence of Herpes zoster and prevalence of post-herpetic neuralgia: A retrospective survey in an elderly population. Eur. J. Pain 1999, 3, 335–342. [Google Scholar] [CrossRef]
- Malkin, J.-E.; Morand, P.; Malvy, D.; Ly, T.D.; Chanzy, B.; De Labareyre, C.; El Hasnaoui, A.; Hercberg, S. Seroprevalence of HSV-1 and HSV-2 infection in the general French population. Sex. Transm. Infect. 2002, 78, 201–203. [Google Scholar] [CrossRef]
- Fleming, D.T.; McQuillan, G.M.; Johnson, R.E.; Nahmias, A.J.; Aral, S.O.; Lee, F.K.; Louis, M.E.S. Herpes Simplex Virus Type 2 in the United States, 1976 to 1994. N. Engl. J. Med. 1997, 337, 1105–1111. [Google Scholar] [CrossRef]
- Howard, M.; Sellors, J.W.; Jang, D.; Robinson, N.J.; Fearon, M.; Kaczorowski, J.; Chernesky, M. Regional Distribution of Antibodies to Herpes Simplex Virus Type 1 (HSV-1) and HSV-2 in Men and Women in Ontario, Canada. J. Clin. Microbiol. 2003, 41, 84–89. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, G.; Kruszon-Moran, D.; Flagg, E.W.; Paulose-Ram, R. Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons Aged 14–49: United States 2015–2016; CDC: Washington, DC, USA, 2018.
- Looker, K.J.; Magaret, A.S.; Turner, K.M.E.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global Estimates of Prevalent and Incident Herpes Simplex Virus Type 2 Infections in 2012. PLoS ONE 2015, 10, e114989. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Corey, L. Persistence in the Population: Epidemiology, Transmission. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007; ISBN 978-0-521-82714-0. [Google Scholar]
| Age Ranges | Gender | |||||
|---|---|---|---|---|---|---|
| Cohort Group | Total Number | 30–64 | 65+ | Male | Female | Unknown |
| All patients | 3,026,005 | 1,410,370 | 1,615,635 | 1,484,700 | 1,539,970 | 1335 |
| Patients with GCA | 4115 | 0 | 4115 | 1265 | 2850 * | 0 |
| Patients with ION/RAO and TABx | 340 | 0 | 340 | 110 | 225 * | 5 |
| Patients with HSV | 163,170 | 94,220 | 68,950 | 56,450 | 106,715 * | 5 |
| Patients with HZ | 249,900 | 165,010 | 84,865 | 101,000 | 148,870 * | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-h.; Iovieno, A.; Sheldon, C.A. Is There an Association between Herpetic Infections and Giant Cell Arteritis? A Population-Based Study. J. Clin. Med. 2021, 10, 63. https://doi.org/10.3390/jcm10010063
Lee D-h, Iovieno A, Sheldon CA. Is There an Association between Herpetic Infections and Giant Cell Arteritis? A Population-Based Study. Journal of Clinical Medicine. 2021; 10(1):63. https://doi.org/10.3390/jcm10010063
Chicago/Turabian StyleLee, Dong-ho, Alfonso Iovieno, and Claire A. Sheldon. 2021. "Is There an Association between Herpetic Infections and Giant Cell Arteritis? A Population-Based Study" Journal of Clinical Medicine 10, no. 1: 63. https://doi.org/10.3390/jcm10010063
APA StyleLee, D.-h., Iovieno, A., & Sheldon, C. A. (2021). Is There an Association between Herpetic Infections and Giant Cell Arteritis? A Population-Based Study. Journal of Clinical Medicine, 10(1), 63. https://doi.org/10.3390/jcm10010063




