Serum Levels of Soluble Triggering Receptor Expressed on Myeloid Cells-1 Associated with the Severity and Outcome of Acute Ischemic Stroke
Abstract
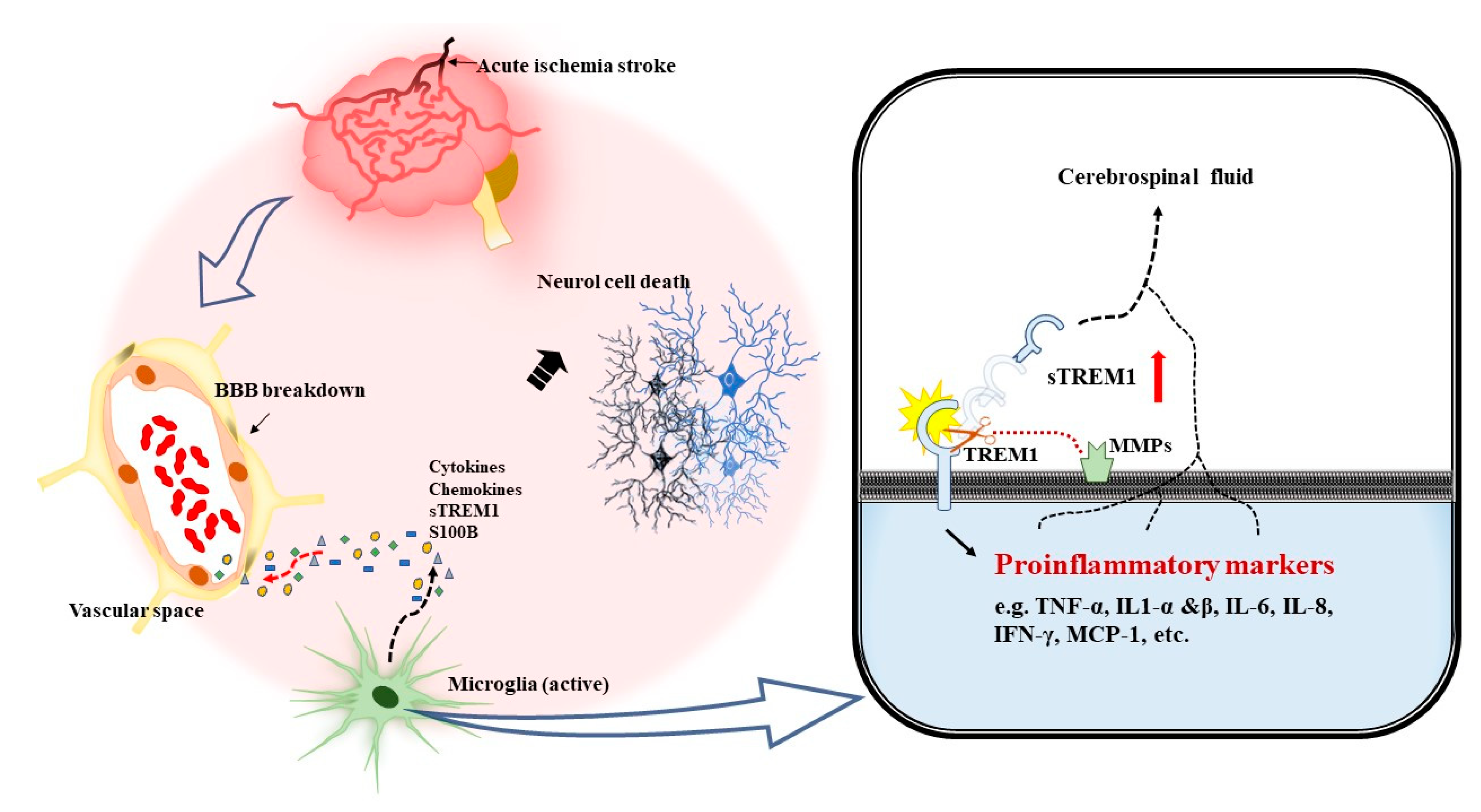
1. Introduction
2. Experimental Section
2.1. Patients and Study Design
2.2. Assessment of Blood Leukocyte and Serum Levels of Soluble Triggering Receptor Expressed on Myeloid Cells-1 and Human S100 Calcium-Binding Protein B
2.3. Cytokine Multiplex Bead Immunoassay
2.4. Statistical Analyses
3. Results
3.1. Demographic Data of 60 Patients with Acute Ischemic Stroke
3.2. Serum Levels of the Soluble Triggering Receptor Expressed on Myeloid Cells-1, Proinflammatory Cytokines, and Human S100 Calcium-Binding Protein B Correlated with the Infarct Size of Acute Ischemic Stroke
3.3. Serum Levels of the Soluble Triggering Receptor Expressed on Myeloid Cells-1, Proinflammatory Cytokines, and Human S100 Calcium-Binding Protein B Correlated with the Severity of Acute Ischemic Stroke
3.4. Outcome of Stroke Is Associated with the Serum Levels of the Soluble Triggering Receptor Expressed on Myeloid Cells-1 and Human S100 Calcium-Binding Protein B and the Severity of Acute Ischemic Stroke
3.5. Predictive Odds Ratios of the Serum Levels of the Soluble Triggering Receptor Expressed on Myeloid Cells-1 and Severity of Ischemic Stroke
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, R.D.; Whisnant, J.P.; Sicks, J.D.; O’Fallon, W.M.; Wiebers, D.O. Stroke incidence, prevalence, and survival: Secular trends in Rochester, Minnesota, through 1989. Stroke 1996, 27, 373–380. [Google Scholar] [PubMed]
- Powers, W.J. Acute ischemic stroke. N. Engl. J. Med. 2020, 383, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.A.; Lo, E.H.; Iadecola, C. The science of stroke: Mechanisms in search of treatments. Neuron 2010, 67, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Lallukka, T.; Ervasti, J.; Lundstrom, E.; Mittendorfer-Rutz, E.; Friberg, E.; Virtanen, M.; Alexanderson, K. Trends in diagnosis-specific work disability before and after stroke: A longitudinal population-based study in Sweden. J. Am. Heart Assoc. 2018, 7. [Google Scholar] [CrossRef]
- Ganesh, A.; Goyal, M. Thrombectomy for acute ischemic stroke: Recent insights and future directions. Curr. Neurol. Neurosci. Rep. 2018, 18, 59. [Google Scholar] [CrossRef]
- Meurer, W.J.; Barth, B.; Abraham, M.; Hoffman, J.R.; Vilke, G.M.; DeMers, G. Intravenous recombinant tissue plasminogen activator and ischemic stroke: Focused update of 2010 clinical practice advisory from the american academy of emergency medicine. J. Emerg. Med. 2018, 54, 723–730. [Google Scholar] [CrossRef]
- Adeoye, O.; Hornung, R.; Khatri, P.; Kleindorfer, D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: A doubling of treatment rates over the course of 5 years. Stroke 2011, 42, 1952–1955. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef]
- Papanagiotou, P.; Ntaios, G. Endovascular thrombectomy in acute ischemic stroke. Circ. Cardiovasc Interv. 2018, 11, e005362. [Google Scholar] [CrossRef]
- Enzmann, G.; Kargaran, S.; Engelhardt, B. Ischemia-reperfusion injury in stroke: Impact of the brain barriers and brain immune privilege on neutrophil function. Adv. Neurol. Disord. 2018, 11, 1756286418794184. [Google Scholar] [CrossRef]
- Mizuma, A.; Yenari, M.A. Anti-inflammatory targets for the treatment of reperfusion injury in stroke. Front. Neurol. 2017, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.S.; Jin, H.; Sun, X.; Huang, S.; Zhang, F.L.; Guo, Z.N.; Yang, Y. Free radical damage in ischemia-reperfusion injury: An obstacle in acute ischemic stroke after revascularization therapy. Oxid. Med. Cell. Longev. 2018, 2018, 3804979. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Huang, J.; Liang, B.; Chen, Q.; Xie, J.; Yang, J.; Yan, Y.; Tang, Q. TLR4 polymorphisms affect stroke risk and inflammatory response in Chinese ischemic stroke patients. Neurol. Sci. 2018, 39, 127–133. [Google Scholar] [CrossRef]
- Chen, H.; He, Y.; Chen, S.; Qi, S.; Shen, J. Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: Applications for natural product efficacy with omics and systemic biology. Pharm. Res. 2020, 158, 104877. [Google Scholar] [CrossRef]
- Gori, A.M.; Giusti, B.; Piccardi, B.; Nencini, P.; Palumbo, V.; Nesi, M.; Nucera, A.; Pracucci, G.; Tonelli, P.; Innocenti, E.; et al. Inflammatory and metalloproteinases profiles predict three-month poor outcomes in ischemic stroke treated with thrombolysis. J. Cereb. Blood Flow Metab. 2017, 37, 3253–3261. [Google Scholar] [CrossRef]
- Xu, P.; Hong, Y.; Xie, Y.; Yuan, K.; Li, J.; Sun, R.; Zhang, X.; Shi, X.; Li, R.; Wu, J.; et al. TREM-1 exacerbates neuroinflammatory injury via NLRP3 inflammasome-mediated pyroptosis in experimental subarachnoid hemorrhage. Transl. Stroke Res. 2020. [Google Scholar] [CrossRef]
- Froyshov, H.M.; Bjornerem, A.; Engstad, T.; Halvorsen, D.S. Elevated inflammatory markers predict mortality in long-term ischemic stroke-survivors: A population-based prospective study. Aging Clin. Exp. Res. 2017, 29, 379–385. [Google Scholar] [CrossRef]
- Vila, N.; Castillo, J.; Davalos, A.; Chamorro, A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 2000, 31, 2325–2329. [Google Scholar] [CrossRef]
- Bouchon, A.; Dietrich, J.; Colonna, M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 2000, 164, 4991–4995. [Google Scholar] [CrossRef]
- Cao, C.; Gu, J.; Zhang, J. Soluble triggering receptor expressed on myeloid cell-1 (sTREM-1): A potential biomarker for the diagnosis of infectious diseases. Front. Med. 2017, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Pelham, C.J.; Agrawal, D.K. Emerging roles for triggering receptor expressed on myeloid cells receptor family signaling in inflammatory diseases. Expert Rev. Clin. Immunol. 2014, 10, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Derive, M.; Massin, F.; Gibot, S. Triggering receptor expressed on myeloid cells-1 as a new therapeutic target during inflammatory diseases. Self Nonself 2010, 1, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Bosco, M.C.; Raggi, F.; Varesio, L. Therapeutic potential of targeting TREM-1 in inflammatory diseases and cancer. Curr. Pharm. Des. 2016, 22, 6209–6233. [Google Scholar] [CrossRef] [PubMed]
- Adly, A.A.; Ismail, E.A.; Andrawes, N.G.; El-Saadany, M.A. Circulating soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) as diagnostic and prognostic marker in neonatal sepsis. Cytokine 2014, 65, 184–191. [Google Scholar] [CrossRef]
- Sandquist, M.; Wong, H.R. Biomarkers of sepsis and their potential value in diagnosis, prognosis and treatment. Expert Rev. Clin. Immunol. 2014, 10, 1349–1356. [Google Scholar] [CrossRef]
- Tammaro, A.; Derive, M.; Gibot, S.; Leemans, J.C.; Florquin, S.; Dessing, M.C. TREM-1 and its potential ligands in non-infectious diseases: From biology to clinical perspectives. Pharmacol. Ther. 2017, 177, 81–95. [Google Scholar] [CrossRef]
- Kouassi, K.T.; Gunasekar, P.; Agrawal, D.K.; Jadhav, G.P. TREM-1; Is it a pivotal target for cardiovascular diseases? J. Cardiovasc. Dev. Dis. 2018, 5. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, X.; Liu, Q.; Xie, Y.; Shi, X.; Chen, J.; Li, Y.; Guo, H.; Sun, R.; Hong, Y.; et al. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 2019, 10, 555. [Google Scholar] [CrossRef]
- Liu, Q.; Johnson, E.M.; Lam, R.K.; Wang, Q.; Bo Ye, H.; Wilson, E.N.; Minhas, P.S.; Liu, L.; Swarovski, M.S.; Tran, S.; et al. Peripheral TREM1 responses to brain and intestinal immunogens amplify stroke severity. Nat. Immunol. 2019, 20, 1023–1034. [Google Scholar] [CrossRef]
- Roselli, F.; Huber-Lang, M. TREM1-ors shake the brain and gut after stroke. Nat. Immunol. 2019, 20, 950–952. [Google Scholar] [CrossRef]
- Sun, X.G.; Ma, Q.; Jing, G.; Wang, G.Q.; Hao, X.D.; Wang, L. Increased levels of soluble triggering receptor expressed on myeloid cells-1 in cerebrospinal fluid of subarachnoid hemorrhage patients. J. Clin. Neurosci. 2017, 35, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.G.; Ma, Q.; Jing, G.; Wang, L.; Hao, X.D.; Wang, G.Q. Early elevated levels of soluble triggering receptor expressed on myeloid cells-1 in subarachnoid hemorrhage patients. Neurol. Sci. 2017, 38, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Biagioni, F.; Busceti, C.L.; Gambardella, S.; Limanaqi, F.; Fornai, F. TREM receptors connecting bowel inflammation to neurodegenerative disorders. Cells 2019, 8, 1124. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Gong, P.Y.; Tan, M.S.; Xue, X.; Huang, S.; Zhou, J.S.; Tan, L.; Zhang, Y.D. Soluble TREM1 concentrations are increased and positively correlated with total tau levels in the plasma of patients with Alzheimer’s disease. Aging Clin. Exp. Res. 2019, 31, 1801–1805. [Google Scholar] [CrossRef] [PubMed]
- Saadipour, K. TREM1: A potential therapeutic target for Alzheimer’s disease. Neurotox. Res. 2017, 32, 14–16. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V.; et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Potter, G.M.; Marlborough, F.J.; Wardlaw, J.M. Wide variation in definition, detection, and description of lacunar lesions on imaging. Stroke 2011, 42, 359–366. [Google Scholar] [CrossRef]
- Sulter, G.; Steen, C.; De Keyser, J. Use of the barthel index and modified rankin scale in acute stroke trials. Stroke 1999, 30, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.V.; Dewis, L.S.; Peters, N.C.; Sherwood, C.C.; Barrett, J.E. Stroke rehabilitation: Analysis of repeated barthel index measures. Arch. Phys. Med. Rehabil. 1979, 60, 14–17. [Google Scholar] [PubMed]
- Pelham, C.J.; Pandya, A.N.; Agrawal, D.K. Triggering receptor expressed on myeloid cells receptor family modulators: A patent review. Expert Opin. Ther. Patients 2014, 24, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Genua, M.; Rutella, S.; Correale, C.; Danese, S. The triggering receptor expressed on myeloid cells (TREM) in inflammatory bowel disease pathogenesis. J. Transl. Med. 2014, 12, 293. [Google Scholar] [CrossRef] [PubMed]
- Tessarz, A.S.; Cerwenka, A. The TREM-1/DAP12 pathway. Immunol. Lett. 2008, 116, 111–116. [Google Scholar] [CrossRef]
- Roe, K.; Gibot, S.; Verma, S. Triggering receptor expressed on myeloid cells-1 (TREM-1): A new player in antiviral immunity? Front. Microbiol. 2014, 5, 627. [Google Scholar] [CrossRef]
- De Oliveira Matos, A.; Dos Santos Dantas, P.H.; Figueira Marques Silva-Sales, M.; Sales-Campos, H. The role of the triggering receptor expressed on myeloid cells-1 (TREM-1) in non-bacterial infections. Crit. Rev. Microbiol. 2020, 46, 237–252. [Google Scholar] [CrossRef]
- Gibot, S.; Kolopp-Sarda, M.N.; Bene, M.C.; Bollaert, P.E.; Lozniewski, A.; Mory, F.; Levy, B.; Faure, G.C. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J. Exp. Med. 2004, 200, 1419–1426. [Google Scholar] [CrossRef]
- Patoulias, D.; Kalogirou, M.S.; Patoulias, I. Triggering receptor expressed on myeloid Cells-1 (TREM-1) and its soluble in the plasma form (sTREM-1) as a diagnostic biomarker in neonatal sepsis. Folia Med. Crac. 2018, 58, 15–19. [Google Scholar] [CrossRef]
- Yuan, Z.; Syed, M.A.; Panchal, D.; Joo, M.; Colonna, M.; Brantly, M.; Sadikot, R.T. Triggering receptor expressed on myeloid cells 1 (TREM-1)-mediated Bcl-2 induction prolongs macrophage survival. J. Biol. Chem. 2014, 289, 15118–15129. [Google Scholar] [CrossRef]
- Dantas, P.; Matos, A.O.; da Silva Filho, E.; Silva-Sales, M.; Sales-Campos, H. Triggering receptor expressed on myeloid cells-1 (TREM-1) as a therapeutic target in infectious and noninfectious disease: A critical review. Int. Rev. Immunol. 2020, 39, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Kuemmel, A.; Alflen, A.; Schmidt, L.H.; Sebastian, M.; Wiewrodt, R.; Schulze, A.B.; Buhl, R.; Radsak, M. Soluble triggering receptor expressed on myeloid Cells 1 in lung cancer. Sci. Rep. 2018, 8, 10766. [Google Scholar] [CrossRef] [PubMed]
- Intiso, D.; Zarrelli, M.M.; Lagioia, G.; Di Rienzo, F.; Checchia De Ambrosio, C.; Simone, P.; Tonali, P.; Cioffi Dagger, R.P. Tumor necrosis factor alpha serum levels and inflammatory response in acute ischemic stroke patients. Neurol. Sci. 2004, 24, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Huang, N.N.; Zhao, Y.X.; Li, Y.S.; Wang, D.; Fan, Y.C.; Li, X.H. Elevated tumor necrosis factor-a-induced protein 8-like 2 mRNA from peripheral blood mononuclear cells in patients with acute ischemic stroke. Int. J. Med. Sci. 2018, 15, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Beridze, M.; Sanikidze, T.; Shakarishvili, R.; Intskirveli, N.; Bornstein, N.M. Selected acute phase CSF factors in ischemic stroke: Findings and prognostic value. BMC Neurol. 2011, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Gibot, S.; Massin, F.; Alauzet, C.; Montemont, C.; Lozniewski, A.; Bollaert, P.E.; Levy, B. Effects of the TREM-1 pathway modulation during mesenteric ischemia-reperfusion in rats. Crit. Care Med. 2008, 36, 504–510. [Google Scholar] [CrossRef]
- Astrand, R.; Unden, J.; Romner, B. Clinical use of the calcium-binding S100B protein. Methods Mol. Biol. 2013, 963, 373–384. [Google Scholar] [CrossRef]
- Koh, S.X.; Lee, J.K. S100B as a marker for brain damage and blood-brain barrier disruption following exercise. Sports Med. 2014, 44, 369–385. [Google Scholar] [CrossRef]
- Dassan, P.; Keir, G.; Brown, M.M. Criteria for a clinically informative serum biomarker in acute ischaemic stroke: A review of S100B. Cereb. Dis. 2009, 27, 295–302. [Google Scholar] [CrossRef]
- Foerch, C.; Singer, O.C.; Neumann-Haefelin, T.; du Mesnil de Rochemont, R.; Steinmetz, H.; Sitzer, M. Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch. Neurol. 2005, 62, 1130–1134. [Google Scholar] [CrossRef]
- Tanaka, Y.; Koizumi, C.; Marumo, T.; Omura, T.; Yoshida, S. Serum S100B is a useful surrogate marker for long-term outcomes in photochemically-induced thrombotic stroke rat models. Life Sci. 2007, 81, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Saposnik, G.; Cote, R.; Phillips, S.; Gubitz, G.; Bayer, N.; Minuk, J.; Black, S.; Stroke Outcome Research Canada (SORCan) Working Group. Stroke outcome in those over 80: A multicenter cohort study across Canada. Stroke 2008, 39, 2310–2317. [Google Scholar] [CrossRef] [PubMed]

| Small Infarction | Large Infarction | ||
|---|---|---|---|
| Small-Artery Occlusion (Lacune) | Large-Artery Atherosclerosis | Cardioembolism | |
| Sex (female/male; %) | 11/22; 50% | 6/10; 60% | 3/8; 37.5% |
| Age | 66.48 ± 9.81 | 66.25 ± 11.69 | 70.90 ± 10.03 |
| Hypertension (n) | 31 | 13 | 10 |
| Diabetes (n) | 9 | 5 | 6 |
| Hyperlipidemia (n) | 21 | 11 | 2 |
| Ischemic heart disease (n) | 9 | 4 | 9 |
| Atrial fibrillation (n) | 0 | 0 | 11 |
| Smoking (n) | 11 | 5 | 3 |
| Controls (n = 24) | Small Infarction (n = 33) | Large Infarction (n = 27) | |
|---|---|---|---|
| WBC (106/mL) | 6.28 ± 1.64 | 7.32 ± 2.32 | 7.87 ±1.84 |
| sTREM-1 (pg/mL) | 65.25 ± 20.96 | 89.20 ± 35.61 | 120.61± 52.39 *,‡ |
| IL-1β (pg/mL) | 0.36 ± 0.13 | 0.28 ± 0.07 | 0.32 ± 0.16 |
| IL-6 (pg/mL) | 0.05 ± 0.04 | 0.06 ± 0.04 | 0.56 ± 1.21 * |
| IL-8 (pg/mL) | 4.53 ± 2.64 | 3.69 ± 4.10 | 7.53 ± 6.52 |
| TNFα (pg/mL) | 5.81 ± 5.66 | 6.77 ± 2.57 | 9.44 ± 6.83 * |
| IFN-γ (pg/mL) | 0.63 ± 0.56 | 0.40 ± 0.20 | 0.41 ± 0.20 |
| S100B (pg/mL) | 29.96 ± 6.86 | 30.13 ± 15.01 | 59.87 ± 32.27 *,‡ |
| Controls (n = 24) | Group 1 (NIHSS ≤ 5) (n = 25) | Group 2 (NIHSS: 6–16) (n = 18) | Group 3 (NIHSS ≥ 17) (n = 17) | |
|---|---|---|---|---|
| WBC (106/mL) | 6.28 ± 1.64 | 6.98 ± 2.27 | 7.32 ± 1.59 | 8.25 ± 2.17 |
| sTREM-1 (pg/mL) | 65.25 ± 20.96 | 88.78 ± 33.94 | 90.64 ± 47.25 | 138.18 ± 44.83 *,‡,# |
| IL-1β (pg/mL) | 0.36 ± 0.13 | 0.32 ± 0.14 | 0.26 ± 0.007 | 0.33 ± 0.17 |
| IL-6 (pg/ml) | 0.05 ± 0.04 | 0.07 ± 0.04 | 0.10 ± 0.08 | 1.80 ± 3.09 *,‡,# |
| IL-8 (pg/mL) | 4.53 ± 2.64 | 5.76 ± 7.89 | 4.18 ± 2.20 | 8.43 ± 3.17 |
| TNFα (pg/mL) | 5.81 ± 2.66 | 7.27 ± 2.95 | 7.68 ± 7.33 | 11.35 ± 6.89 * |
| IFN-γ (pg/mL) | 0.63 ± 0.56 | 0.40 ± 0.20 | 0.40 ± 0.16 | 0.43 ± 3.17 |
| S100B (pg/mL) | 29.96 ± 6.86 | 31.04 ± 15.24 | 45.52 ± 19.52 | 89.18 ± 6.86 *,‡,# |
| Barthel Index | ||
|---|---|---|
| Correlation | p Value | |
| Age (years) | −0.231 | 0.246 |
| sTREM-1 (pg/mL) | −0.525 | 0.005 * |
| IL-6 (pg/mL) | −0.362 | 0.106 |
| TNFα (pg/mL) | −0.043 | 0.848 |
| S100B (pg/mL) NIHSS | −0.574 −0.686 | <0.002 * <0.001 * |
| Odds Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|
| sTREM-1 | 1.327 | 1.020–1.726 | 0.035 * |
| NIHSS | 1.043 | 1.001–1.087 | 0.042 * |
| Constant | 0.001 | 0.205 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-B.; Chen, N.-C.; Chen, C.-L.; Fu, M.-H.; Pan, H.-Y.; Hsu, C.-Y.; Chen, S.-D.; Chuang, Y.-C. Serum Levels of Soluble Triggering Receptor Expressed on Myeloid Cells-1 Associated with the Severity and Outcome of Acute Ischemic Stroke. J. Clin. Med. 2021, 10, 61. https://doi.org/10.3390/jcm10010061
Huang J-B, Chen N-C, Chen C-L, Fu M-H, Pan H-Y, Hsu C-Y, Chen S-D, Chuang Y-C. Serum Levels of Soluble Triggering Receptor Expressed on Myeloid Cells-1 Associated with the Severity and Outcome of Acute Ischemic Stroke. Journal of Clinical Medicine. 2021; 10(1):61. https://doi.org/10.3390/jcm10010061
Chicago/Turabian StyleHuang, Jyun-Bin, Nai-Ching Chen, Chien-Liang Chen, Mu-Hui Fu, Hsiu-Yung Pan, Chung-Yao Hsu, Shang-Der Chen, and Yao-Chung Chuang. 2021. "Serum Levels of Soluble Triggering Receptor Expressed on Myeloid Cells-1 Associated with the Severity and Outcome of Acute Ischemic Stroke" Journal of Clinical Medicine 10, no. 1: 61. https://doi.org/10.3390/jcm10010061
APA StyleHuang, J.-B., Chen, N.-C., Chen, C.-L., Fu, M.-H., Pan, H.-Y., Hsu, C.-Y., Chen, S.-D., & Chuang, Y.-C. (2021). Serum Levels of Soluble Triggering Receptor Expressed on Myeloid Cells-1 Associated with the Severity and Outcome of Acute Ischemic Stroke. Journal of Clinical Medicine, 10(1), 61. https://doi.org/10.3390/jcm10010061