Dynamic Changes of Generic Quality of Life after Different Treatments for Localized Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. QoL Questionnaire
2.3. Trajectories of QoL Change after Treatments
2.4. Statistical Analyses
3. Results
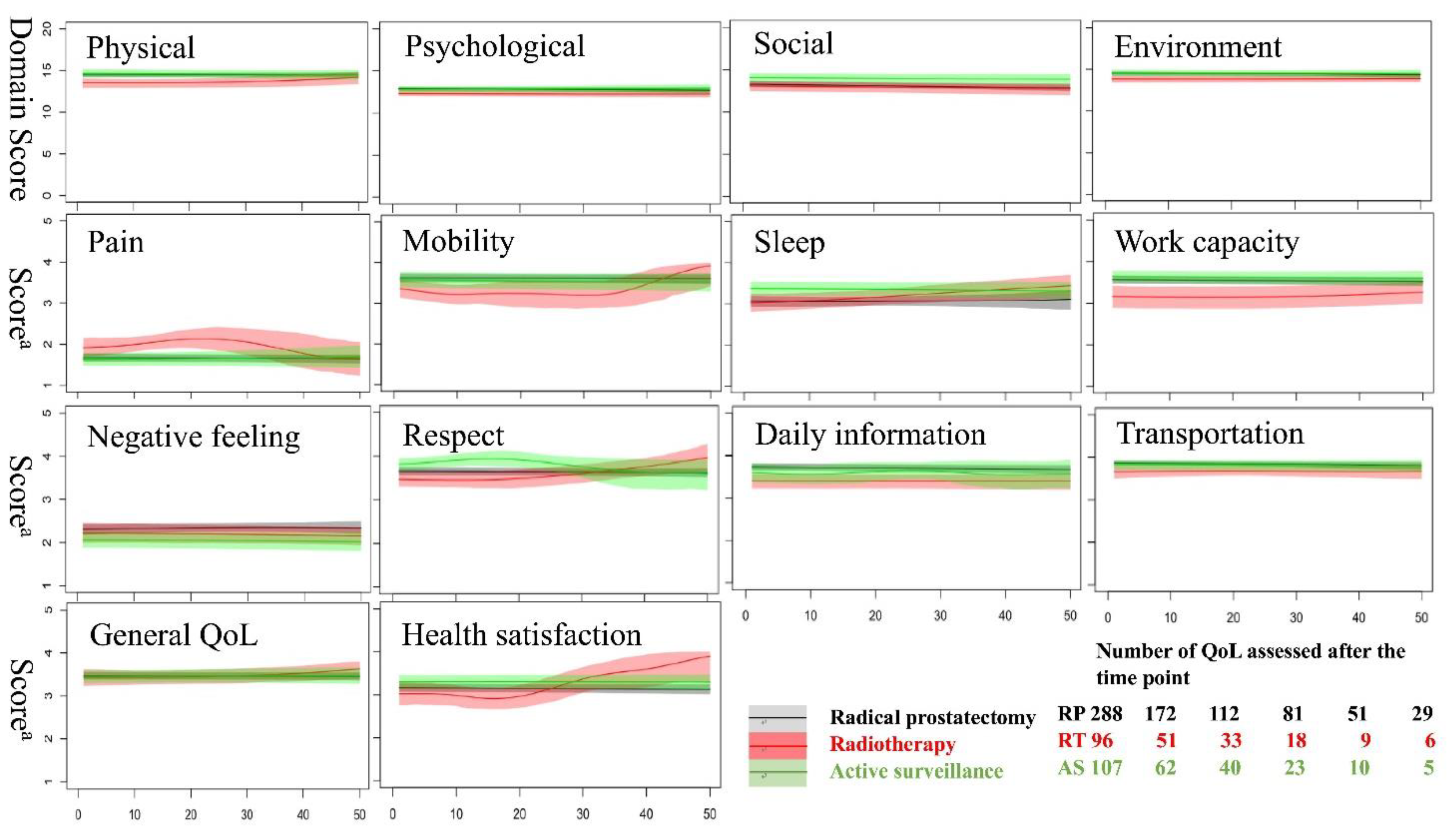
3.1. Changes in QoL after Different Prostate Cancer Treatment
3.2. Determinants of QoL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.; Siegel, R.; Jemal, A. Global Cancer Facts & Figures; American Cancer Society: Atlanta, GA, USA, 2015; Volume 2. [Google Scholar]
- Ladjevardi, S.; Sandblom, G.; Berglund, A.; Varenhorst, E. Tumour Grade, Treatment, and Relative Survival in a Population-based Cohort of Men with Potentially Curable Prostate Cancer. Eur. Urol. 2010, 57, 631–640. [Google Scholar] [CrossRef]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Sanda, M.G.; Dunn, R.L.; Michalski, J.; Sandler, H.M.; Northouse, L.; Hembroff, L.; Lin, X.; Greenfield, T.K.; Litwin, M.S.; Saigal, C.S.; et al. Quality of Life and Satisfaction with Outcome among Prostate-Cancer Survivors. N. Engl. J. Med. 2008, 358, 1250–1261. [Google Scholar] [CrossRef]
- Bergman, J.; Litwin, M.S. Quality of Life in Men Undergoing Active Surveillance for Localized Prostate Cancer. J. Natl. Cancer Inst. Monogr. 2012, 2012, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; Gibbons, E.; Fitzpatrick, R. A Structured Review of Patient-Reported Outcome Measures for Men with Prostate Cancer; University of Oxford: Oxford, UK, 2013. [Google Scholar]
- Velikova, G.; Stark, D.; Selby, P. Quality of life instruments in oncology. Eur. J. Cancer 1999, 35, 1571–1580. [Google Scholar] [CrossRef]
- Lardas, M.; Liew, M.; van den Bergh, R.C.; De Santis, M.; Bellmunt, J.; Van den Broeck, T.; Cornford, P.; Cumberbatch, M.G.; Fossati, N.; Gross, T.; et al. Quality of Life Outcomes after Primary Treatment for Clinically Localised Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 869–885. [Google Scholar] [CrossRef]
- Kao, Y.-L.; Tsai, Y.-S.; Ou, F.-Y.; Syu, Y.-J.; Ou, C.-H.; Yang, W.-H.; Cheng, H.-L.; Tzai, T.-S.; Wang, J.-D. Determinants of quality of life in prostate cancer patients: A single institute analysis. Urol. Sci. 2015, 26, 254–258. [Google Scholar] [CrossRef]
- Potosky, A.L.; Legler, J.; Albertsen, P.C.; Stanford, J.L.; Gilliland, F.D.; Hamilton, A.S.; Eley, J.W.; Stephenson, R.A.; Harlan, L.C. Health Outcomes After Prostatectomy or Radiotherapy for Prostate Cancer: Results From the Prostate Cancer Outcomes Study. J. Natl. Cancer Inst. 2000, 92, 1582–1592. [Google Scholar] [CrossRef]
- Ramachandra, P.; Booth, S.; Pieters, T.; Vrotsou, K.; Huppert, F.A. A brief self-administered psychological intervention to improve well-being in patients with cancer: Results from a feasibility study. Psycho-Oncology 2009, 18, 1323–1326. [Google Scholar] [CrossRef]
- Boisen, S.; Krägeloh, C.; Shepherd, D.; Ryan, C.; Masters, J.; Osborne, S.; MacLeod, R.D.; Gray, M.; Keogh, J.W.L. A Cross-Sectional Comparison of Quality of Life between Physically Active and Underactive Older Men with Prostate Cancer. J. Aging Phys. Act. 2016, 24, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Skevington, S.M. Advancing cross-cultural research on quality of life: Observations drawn from the WHOQOL development. Qual. Life Res. 2002, 11, 135–144. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Hwang, J.-S.; Wang, W.-C.; Lai, W.-W.; Su, W.-C.; Wu, T.-Y.; Yao, G.; Wang, J. Psychometric evaluation of the WHOQOL-BREF, Taiwan version, across five kinds of Taiwanese cancer survivors: Rasch analysis and confirmatory factor analysis. J. Formos. Med. Assoc. 2019, 118, 215–222. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Wang, J. Integrating health profile with survival for quality of life assessment. Qual. Life Res. 2004, 13, 1–10. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lai, W.-W.; Hsiue, T.-R.; Su, W.-C.; Lin, C.-K.; Hwang, J.-S.; Wang, J. Health-related quality of life after first-line anti-cancer treatments for advanced non-small cell lung cancer in clinical practice. Qual. Life Res. 2015, 25, 1441–1449. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-C.; Lai, W.-W.; Chang, S.-M.; Hwang, J.-S.; Su, W.-C.; Wang, J. Dynamic changes in quality of life after three first-line therapies for EGFR mutation-positive advanced non-small-cell lung cancer. Ther. Adv. Med. Oncol. 2018, 10, 1758834018755072. [Google Scholar] [CrossRef]
- Shevach, J.; Weiner, A.; Morgans, A.K. Quality of Life–Focused Decision-Making for Prostate Cancer. Curr. Urol. Rep. 2019, 20, 57. [Google Scholar] [CrossRef]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.M.; Peters, T.J.; Holding, P.; Bonnington, S.; et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Barocas, D.A.; Alvarez, J.; Resnick, M.J.; Koyama, T.; Hoffman, K.E.; Tyson, M.D.; Conwill, R.; Mccollum, D.; Cooperberg, M.R.; Goodman, M.; et al. Association Between Radiation Therapy, Surgery, or Observation for Localized Prostate Cancer and Patient-Reported Outcomes After 3 Years. JAMA 2017, 317, 1126–1140. [Google Scholar] [CrossRef] [PubMed]
- Dickey, S.L.; Grayson, C.J. The Quality of Life among Men Receiving Active Surveillance for Prostate Cancer: An Integrative Review. Healthcare 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiang, W. Income gradient in health-related quality of life—The role of social networking time. Int. J. Equity Health 2019, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Thommasen, H.V.; Zhang, W. Impact of chronic disease on quality of life in the Bella Coola Valley. Rural Remote Health 2006, 6, 528. [Google Scholar] [PubMed]
- Chang, Y.-C.; Ouyang, W.-C.; Lu, M.-C.; Wang, J.; Hu, S.C. Levels of depressive symptoms may modify the relationship between the WHOQOL-BREF and its determining factors in community-dwelling older adults. Int. Psychogeriatr. 2015, 28, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Adam, S.; Feller, A.; Rohrmann, S.; Arndt, V. Health-related quality of life among long-term (≥5 years) prostate cancer survivors by primary intervention: A systematic review. Health Qual. Life Outcomes 2018, 16, 22. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yang, Z.; Qi, L.; Chen, M. Robot-assisted and laparoscopic vs open radical prostatectomy in clinically localized prostate cancer. Medicine 2019, 98, e15770. [Google Scholar] [CrossRef]
- Keogh, J.; Krägeloh, C.U.; Shepherd, D.; Ryan, C.; Osborne, S.; Masters, J.; MacLeod, R.D. Quantitative assessment of quality of life in New Zealand prostate cancer survivors: The effect of androgen deprivation therapy. J. Cancer Res. Ther. 2013, 1, 105–110. [Google Scholar]
| Disease Status | Radical Prostatectomy | Radiotherapy | Active Surveillance | p |
|---|---|---|---|---|
| Number of subjects (n) | 104 | 37 | 55 | |
| Number of assessments (n) | 288 | 96 | 107 | |
| Age at diagnosis, mean (SD) years | 65.3 (5.66) | 71.7 (4.96) | 71.4 (8.02) | 0.001 |
| Education, years, mean (SD) years | 12.4 (4.51) | 10.8 (4.32) | 10.5 (5.10) | 0.02 |
| Incomes (per 104 NTD per month) | 5.74 (5.95) | 3.09 (2.99) | 4.90 (5.75) | 0.04 |
| Employment, n (%) | ||||
| Employed | 50 (48) | 10 (27) | 16 (29) | 0.02 |
| Unemployed | 54 (51) | 27 (73) | 39 (71) | |
| Comorbidities, n (%) | ||||
| Cardiovascular disease | 18 (17) | 8 (21) | 8 (14) | 0.68 |
| Hypertension | 35 (33) | 12 (32) | 21 (38) | 0.81 |
| Diabetes | 26 (25) | 6 (16) | 8 (14) | 0.23 |
| Arthritis | 8 (8) | 7 (18) | 7 (12) | 0.16 |
| Anxiety † (first assessment) | 15 (14) | 6 (16) | 4 (7) | 0.34 |
| Anxiety † (later assessment) | 32 (17) | 9 (15) | 8 (15) | 0.89 |
| Peptic ulcer | 3 (3) | 2 (5) | 4 (7) | 0.44 |
| Risk group, n (%) | ||||
| Low | 19 (18) | 5 (13) | 19 (34) | 0.02 |
| Intermediate | 47 (45) | 8 (22) | 17 (31) | |
| High | 38 (37) | 24 (65) | 19 (34) | |
| Prostate size at diagnosis, mean (SD) mL | 39.2 (19.2) | 44.4 (24.9) | 43.1 (24.0) | 0.36 |
| ADL score (1st assessment), mean (SD) | 66.6 (39.1) | 67.7 (39.8) | 79.5 (34.8) | 0.11 |
| Domain score (1st assessment), mean (SD) | ||||
| Physical | 14.4 (2.02) | 14.1 (2.26) | 14.4 (2.43) | 0.72 |
| Psychology | 12.8 (1.85) | 12.5 (1.52) | 12.6 (1.82) | 0.66 |
| Social | 13.7 (2.30) | 13.7 (2.85) | 14.0 (2.60) | 0.81 |
| Environment | 14.4 (1.95) | 14.1 (2.26) | 14.2 (2.19) | 0.73 |
| NCKUH (n = 196) | TCR (n = 31,183) | p | |
|---|---|---|---|
| Age at diagnosis, mean (SD) years | 68.24 (7.00) | 72.82 (9.16) | <0.01 |
| Comorbidities, n (%) | |||
| Cardiovascular disease | 34 (17) | 6667 (21) | 0.17 |
| Hypertension | 89 (45) | 15,968 (51) | 0.10 |
| Diabetes | 40 (20) | 5610 (18) | 0.38 |
| Arthritis | 22 (11) | 48 (0.1) | <0.01 |
| Anxiety/depression | 18 (9) | 2742 (9) | 0.84 |
| Peptic ulcer | 23 (12) | 4215 (14) | 0.46 |
| Risk group a, n (%) b | |||
| Low | 43 (22) | 3983 (25) | 0.62 |
| Intermediate | 72 (37) | 5643 (35) | |
| High | 81 (41) | 6321 (40) | |
| Primary prostate treatment, n (%) b | |||
| Radical prostatectomy | 104 (53) | 12,942 (46) | 0.11 |
| Radiotherapy | 37 (19) | 5311 (19) | |
| Active surveillance | 55 (28) | 9670 (35) |
| Fixed Effects | Income (per 104 NTD) | DM (Yes/No) | Arthritis (Yes/No) | Anxiety a (Yes/No) | Risk Group b (Int. vs. High) | Uro. Med. (yes/no) | |
|---|---|---|---|---|---|---|---|
| QoL Score | |||||||
| Overall QoL | −0.43 (0.12) * | ||||||
| Overall health | −0.30 (0.13) * | ||||||
| Physical domain | 0.07 (0.03) † | −0.88 (0.36) * | −1.11 (0.39) * | −0.46 (0.21) * | |||
| Pain and discomfort | −0.20 (0.09) * | ||||||
| Depend on medical | 0.02 (0.01) * | −0.56 (0.14) ‡ | −0.53 (0.17) * | ||||
| Energy and fatigue | 0.03 (0.01) * | ||||||
| Mobility | −0.35 (0.14) * | −0.42 (0.18) * | |||||
| Sleep and rest | −0.62 (0.15) * | ||||||
| Daily activities | 0.02 (0.01) * | ||||||
| Work capacity | 0.02 (0.01) * | −0.31 (0.11) † | −0.31 (0.14) * | −0.19 (0.09) * | |||
| Psychological domain | 0.07 (0.02) † | −0.66 (0.27) * | |||||
| Spirituality | 0.03 (0.01) † | −0.34 (0.12) † | |||||
| Bodily image | 0.02 (0.01) † | ||||||
| Self-esteem | 0.03 (0.01) † | −0.28 (0.11) * | −0.38 (0.13) * | ||||
| Negative feelings | −0.58 (0.17) * | ||||||
| Social domain | 0.09 (0.03) † | 1.13 (0.39) † | |||||
| Personal rel. | 0.02 (0.01) † | 0.24 (0.11) * | |||||
| Sexual activity | 0.42 (0.19) * | ||||||
| Social support | 0.02 (0.01) † | 0.24 (0.10) * | |||||
| Environment domain | 0.10 (0.03) ‡ | ||||||
| Eating/food | 0.02 (0.01) * | −0.34 (0.11) † | |||||
| Home | 0.03 (0.01) ‡ | ||||||
| Financial | 0.05 (0.01) ‡ | ||||||
| Information | 0.03 (0.01) † | ||||||
| Physical env. | 0.03 (0.01) † | ||||||
| Transportation | 0.03 (0.01) * | ||||||
| Treatment | Radiotherapy vs. Radical Prostatectomy | Active Surveillance vs. Radical Prostatectomy | Radiotherapy vs. Active Surveillance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time b | before Treatment | after Treatment ≤ 1 Year | after Treatment > 1 Year | before Treatment | after Treatment ≤ 1 Year | after Treatment > 1 Year | before Treatment | after Treatment ≤ 1 Year | after Treatment > 1 Year | |
| QoL Items a | ||||||||||
| Physical domain | 1.11 (0.53) * | −1.53 (0.64) * | ||||||||
| Sleep and rest | 0.63 (0.21) † | |||||||||
| Work capacity | −0.44 (0.20) * | −0.75 (0.24) † | ||||||||
| Psychological domain | −0.93 (0.42) * | |||||||||
| Spirituality | −0.40 (0.20) * | |||||||||
| Self-esteem | −0.34 (0.17) * | |||||||||
| Social domain | 1.62 (0.66) * | −1.74 (0.80) * | ||||||||
| Personal rel. | 0.34 (0.17) * | |||||||||
| Social support | −0.49 (0.19) † | |||||||||
| Respect | −0.53 (0.23) * | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, Y.-L.; Ou, C.-H.; Lin, S.-H.; Chang, S.-M.; Wang, J.-D.; Tsai, Y.-S. Dynamic Changes of Generic Quality of Life after Different Treatments for Localized Prostate Cancer. J. Clin. Med. 2021, 10, 158. https://doi.org/10.3390/jcm10010158
Kao Y-L, Ou C-H, Lin S-H, Chang S-M, Wang J-D, Tsai Y-S. Dynamic Changes of Generic Quality of Life after Different Treatments for Localized Prostate Cancer. Journal of Clinical Medicine. 2021; 10(1):158. https://doi.org/10.3390/jcm10010158
Chicago/Turabian StyleKao, Yao-Lin, Chien-Hui Ou, Sheng-Hsiang Lin, Sheng-Mao Chang, Jung-Der Wang, and Yuh-Shyan Tsai. 2021. "Dynamic Changes of Generic Quality of Life after Different Treatments for Localized Prostate Cancer" Journal of Clinical Medicine 10, no. 1: 158. https://doi.org/10.3390/jcm10010158
APA StyleKao, Y.-L., Ou, C.-H., Lin, S.-H., Chang, S.-M., Wang, J.-D., & Tsai, Y.-S. (2021). Dynamic Changes of Generic Quality of Life after Different Treatments for Localized Prostate Cancer. Journal of Clinical Medicine, 10(1), 158. https://doi.org/10.3390/jcm10010158






