Heterotopic Ossification of the Vascular Pedicle after Maxillofacial Reconstructive Surgery Using Fibular Free Flap: Introducing New Classification and Retrospective Analysis
Abstract
1. Introduction
- to estimate the radiological and clinical form and frequency of HO,
- to define and compare different radio-morphological HO types introducing a new classification,
- to report the surgical intervention rate for removing calcified structures,
- and to investigate whether there is a correlation between: analog vs. digital planning, reconstruction methods (immediate vs. delayed), fibular segments and the occurrence of HO.
2. Material and Methods
2.1. Study Design and Patient Population
2.2. Study Parameters and Evaluator Calibration
2.3. Inclusion and Exclusion Criteria for Study Subjects
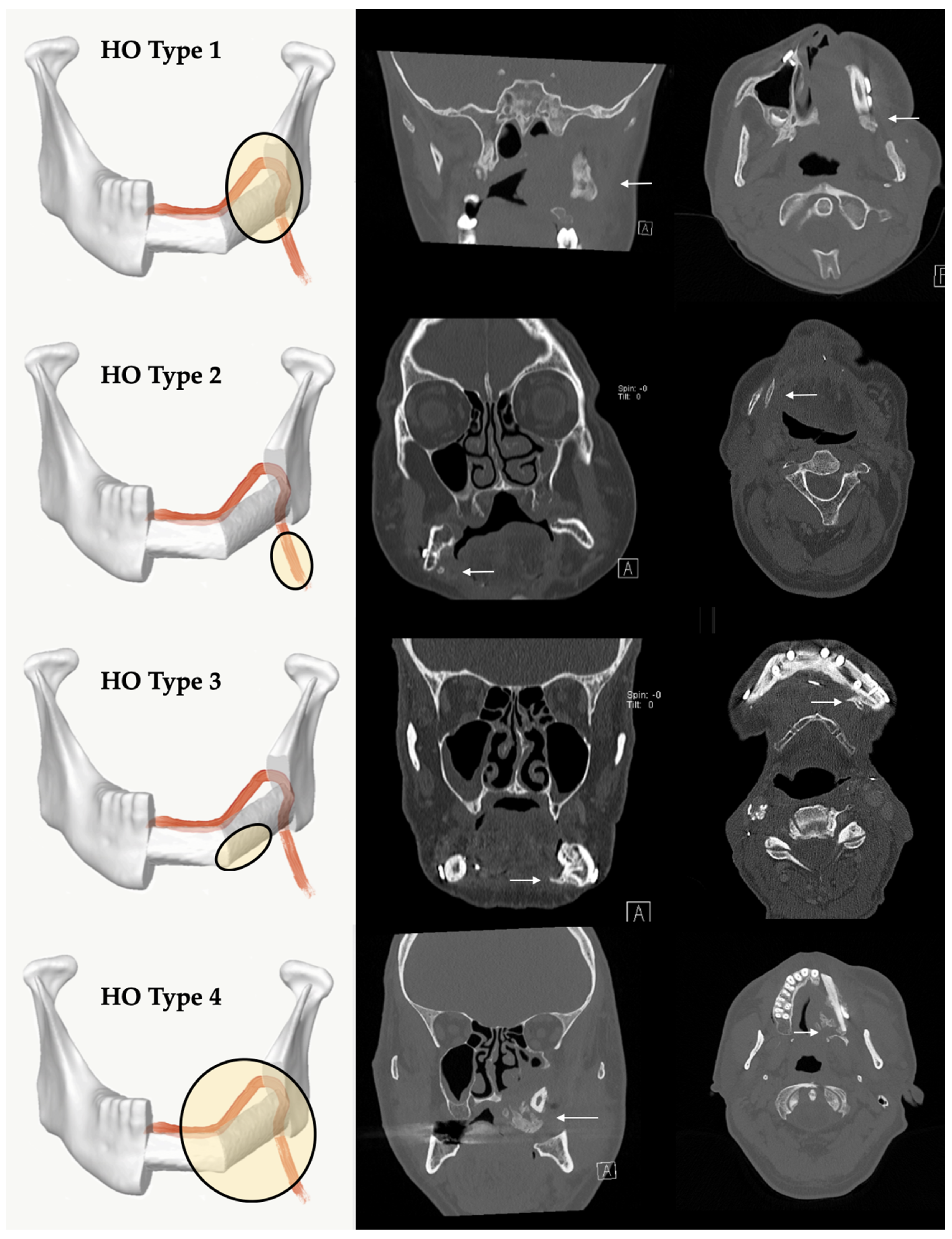
2.4. A New Classification of HO of the Vascular Pedicle and Periosseous Tissue Based on Radiological and Clinical Follow-Up
2.5. Statistical Analyses
2.6. Ethics Statement/Confirmation of Patients’ Permission
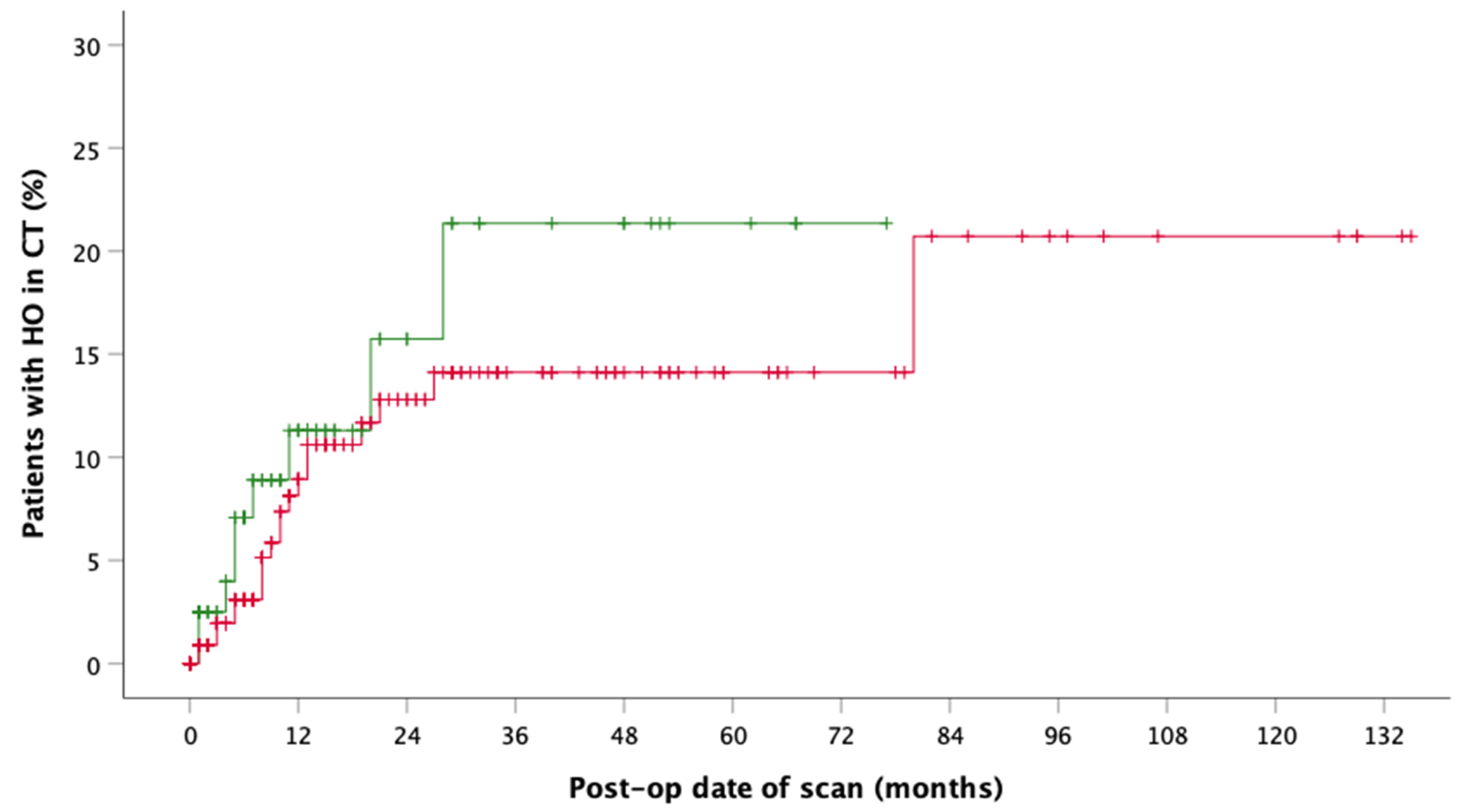
3. Results
4. Discussion
4.1. HO after FFF in Literature
4.2. Classification of Four Different HO Patterns and Biological Etiology
4.3. Impact of Analogous and Virtual Planning
4.4. Frequency of Clinical Symptoms and Surgical Removal of HO
4.5. Effect of Irradiation on HO Occurrence
4.6. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hayden, R.E.; Mullin, D.P.; Patel, A.K. Reconstruction of the segmental mandibular defect: Current state of the art. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 231–236. [Google Scholar] [CrossRef]
- Hidalgo, D.A. Fibula free flap: A new method of mandible reconstruction. Plast. Reconstr. Surg. 1989, 84, 71–79. [Google Scholar] [CrossRef]
- Brown, J.S.; Barry, C.; Ho, M.; Shaw, R. A new classification for mandibular defects after oncological resection. Lancet Oncol. 2016, 17, e23–e30. [Google Scholar] [CrossRef]
- Hakim, S.G.; Kimmerle, H.; Trenkle, T.; Sieg, P.; Jacobsen, H.C. Masticatory rehabilitation following upper and lower jaw reconstruction using vascularised free fibula flap and enossal implants-19 years of experience with a comprehensive concept. Clin. Oral Investig. 2015, 19, 525–534. [Google Scholar] [CrossRef]
- Attia, S.; Wiltfang, J.; Streckbein, P.; Wilbrand, J.F.; El Khassawna, T.; Mausbach, K.; Howaldt, H.P.; Schaaf, H. Functional and aesthetic treatment outcomes after immediate jaw reconstruction using a fibula flap and dental implants. J. Craniomaxillofac. Surg. 2019, 47, 786–791. [Google Scholar] [CrossRef]
- Attia, S.; Wiltfang, J.; Pons-Kuhnemann, J.; Wilbrand, J.F.; Streckbein, P.; Kahling, C.; Howaldt, H.P.; Schaaf, H. Survival of dental implants placed in vascularised fibula free flaps after jaw reconstruction. J. Craniomaxillofac. Surg. 2018, 46, 1205–1210. [Google Scholar] [CrossRef]
- Bluebond-Langner, R.; Rodriguez, E.D. Application of skeletal buttress analogy in composite facial reconstruction. Craniomaxillofac. Trauma Reconstr. 2009, 2, 19–25. [Google Scholar] [CrossRef]
- Attia, S.; Diefenbach, J.; Schmermund, D.; Böttger, S.; Pons-Kühnemann, J.; Scheibelhut, C.; Heiss, C.; Howaldt, H.-P. Donor-Site Morbidity after Fibula Transplantation in Head and Neck Tumor Patients: A Split-Leg Retrospective Study with Focus on Leg Stability and Quality of Life. Cancers 2020, 12, 2217. [Google Scholar] [CrossRef]
- Schusterman, M.A.; Reece, G.P.; Miller, M.J.; Harris, S. The osteocutaneous free fibula flap: Is the skin paddle reliable? Plast. Reconstr. Surg. 1992, 90, 787–793. [Google Scholar] [CrossRef]
- Jones, N.F.; Monstrey, S.; Gambier, B.A. Reliability of the fibular osteocutaneous flap for mandibular reconstruction: Anatomical and surgical confirmation. Plast. Reconstr. Surg. 1996, 97, 707–716. [Google Scholar] [CrossRef]
- Wong, C.H.; Tan, B.K.; Wei, F.C.; Song, C. Use of the soleus musculocutaneous perforator for skin paddle salvage of the fibula osteoseptocutaneous flap: Anatomical study and clinical confirmation. Plast. Reconstr. Surg. 2007, 120, 1576–1584. [Google Scholar] [CrossRef]
- Winters, H.A.; de Jongh, G.J. Reliability of the proximal skin paddle of the osteocutaneous free fibula flap: A prospective clinical study. Plast. Reconstr. Surg. 1999, 103, 846–849. [Google Scholar] [CrossRef]
- Schusterman, M.A.; Harris, S.W.; Raymond, A.K.; Goepfert, H. Immediate free flap mandibular reconstruction: Significance of adequate surgical margins. Head Neck 1993, 15, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Chana, J.S.; Chang, Y.M.; Wei, F.C.; Shen, Y.F.; Chan, C.P.; Lin, H.N.; Tsai, C.Y.; Jeng, S.F. Segmental mandibulectomy and immediate free fibula osteoseptocutaneous flap reconstruction with endosteal implants: An ideal treatment method for mandibular ameloblastoma. Plast. Reconstr. Surg. 2004, 113, 80–87. [Google Scholar] [CrossRef]
- Schaaf, H.; Wahab-Gothe, T.; Kerkmann, H.; Streckbein, P.; Obert, M.; Pons-Kuehnemann, J.; Ahrens, M.; Howaldt, H.P.; Attia, S. Comparison between flat-panel volume computed tomography and histologic assessments of bone invasion of maxillofacial tumors: Utility of an instantaneous radiologic diagnostic tool. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 191–198. [Google Scholar] [CrossRef]
- Iizuka, T.; Hafliger, J.; Seto, I.; Rahal, A.; Mericske-Stern, R.; Smolka, K. Oral rehabilitation after mandibular reconstruction using an osteocutaneous fibula free flap with endosseous implants. Factors affecting the functional outcome in patients with oral cancer. Clin. Oral Implants Res. 2005, 16, 69–79. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Verhelst, P.J.; Dons, F.; Van Bever, P.J.; Schoenaers, J.; Nanhekhan, L.; Politis, C. Fibula Free Flap in Head and Neck Reconstruction: Identifying Risk Factors for Flap Failure and Analysis of Postoperative Complications in a Low Volume Setting. Craniomaxillofac. Trauma Reconstr. 2019, 12, 183–192. [Google Scholar] [CrossRef]
- Zavattero, E.; Fasolis, M.; Garzino-Demo, P.; Berrone, S.; Ramieri, G.A. Evaluation of plate-related complications and efficacy in fibula free flap mandibular reconstruction. J. Craniofac. Surg. 2014, 25, 397–399. [Google Scholar] [CrossRef]
- Rendenbach, C.; Steffen, C.; Hanken, H.; Schluermann, K.; Henningsen, A.; Beck-Broichsitter, B.; Kreutzer, K.; Heiland, M.; Precht, C. Complication rates and clinical outcomes of osseous free flaps: A retrospective comparison of CAD/CAM versus conventional fixation in 128 patients. Int. J. Oral Maxillofac. Surg. 2019, 48, 1156–1162. [Google Scholar] [CrossRef]
- Tarsitano, A.; Sgarzani, R.; Betti, E.; Oranges, C.M.; Contedini, F.; Cipriani, R.; Marchetti, C. Vascular pedicle ossification of free fibular flap: Is it a rare phenomenon? Is it possible to avoid this risk? Acta Otorhinolaryngol. Ital. 2013, 33, 307–310. [Google Scholar] [PubMed]
- DeConde, A.S.; Vira, D.; Blackwell, K.E.; Moriarty, J.M.; Sercarz, J.A.; Nabili, V. Neck mass due to pedicle ossification after oromandibular reconstruction. Laryngoscope 2011, 121, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.S.; Clasper, J.C. Heterotopic ossification: A systematic review. J. R. Army Med. Corps 2015, 161, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Wheatley, B.M.; Cholok, D.; Agarwal, S.; Yu, P.B.; Levi, B.; Davis, T.A. The traumatic bone: Trauma-induced heterotopic ossification. Transl. Res. 2017, 186, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, R.; Manzano, D.; Ruiz-Laza, L.; Moreno-Garcia, C.; Monje, F. The rare phenomenon of vascular pedicle ossification of free fibular flap in mandibular reconstruction. J. Craniomaxillofac. Surg. 2011, 39, 114–118. [Google Scholar] [CrossRef]
- Autelitano, L.; Colletti, G.; Bazzacchi, R.; Biglioli, F. Ossification of vascular pedicle in fibular free flaps: A report of four cases. Int. J. Oral Maxillofac. Surg. 2008, 37, 669–671. [Google Scholar] [CrossRef]
- Smith, R.B.; Funk, G.F. Severe trismus secondary to periosteal osteogenesis after fibula free flap maxillary reconstruction. Head Neck 2003, 25, 406–411. [Google Scholar] [CrossRef]
- Baserga, C.; Massarelli, O.; Bolzoni, A.R.; Rossi, D.S.; Beltramini, G.A.; Baj, A.; Gianni, A.B. Fibula free flap pedicle ossification: Experience of two centres and a review of the literature. J. Craniomaxillofac. Surg. 2018, 46, 1674–1678. [Google Scholar] [CrossRef]
- Glastonbury, C.M.; van Zante, A.; Knott, P.D. Ossification of the vascular pedicle in microsurgical fibular free flap reconstruction of the head and neck. AJNR Am. J. Neuroradiol. 2014, 35, 1965–1969. [Google Scholar] [CrossRef]
- Karagozoglu, K.H.; Winters, H.A.; Forouzanfar, T.; Schulten, E.A. Periosteal ossification of the vascular pedicle after reconstruction of continuity defects of the mandible and the maxilla with fibular free flaps: A retrospective study. Br. J. Oral Maxillofac. Surg. 2013, 51, 965–967. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Lieu, S.; Lu, C.; Colnot, C. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone 2010, 47, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.B.; Kaleem, A.; Alzahrani, S.; Yeoh, M.; Zaid, W. Modified fibula free flap harvesting technique for prevention of heterotopic pedicle ossification. Head Neck 2019, 41, E104–E112. [Google Scholar] [CrossRef] [PubMed]
- Jehn, P.; Zimmerer, R.; Dittmann, J.; Fedchenko, M.; Gellrich, N.C.; Spalthoff, S. Ossification of the Vascular Pedicle After Microsurgical Soft Tissue Transfer of the Lateral Upper Arm Free Flap. Ann. Plast. Surg. 2019, 83, e39–e42. [Google Scholar] [CrossRef] [PubMed]
- Gangidi, S.R.; Courtney, D. “You reap what you sow”—A case of heterotopic ossification within a fasciocutaneous radial forearm free flap reconstruction. Int. J. Oral Maxillofac. Surg. 2013, 42, 458–459. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Shaw, R.J. Reconstruction of the maxilla and midface: Introducing a new classification. Lancet Oncol. 2010, 11, 1001–1008. [Google Scholar] [CrossRef]
- Myon, L.; Ferri, J.; Genty, M.; Raoul, G. Consequences of bony free flap’s pedicle calcification after jaw reconstruction. J. Craniofac. Surg. 2012, 23, 872–877. [Google Scholar] [CrossRef]
- Deschler, D.G.; Hayden, R.E. Bone spur presenting as a submandibular mass following free fibula reconstruction of the mandible. Am. J. Otolaryngol. 1997, 18, 425–427. [Google Scholar] [CrossRef]
- Colletti, G.; Autelitano, L.; Rabbiosi, D.; Biglioli, F.; Chiapasco, M.; Mandala, M.; Allevi, F. Technical refinements in mandibular reconstruction with free fibula flaps: Outcome-oriented retrospective review of 99 cases. Acta Otorhinolaryngol. Ital. 2014, 34, 342–348. [Google Scholar]
- Acarturk, T.O.; Aslaner, E.E. Periosteal ossification from the vascular pedicle of a free fibular flap. J. Craniofac. Surg. 2011, 22, e29–e32. [Google Scholar] [CrossRef]
- Gilbert, A. Vascularised transfer of the fibula shaft. Int. J. Microsurg. 1979, 1, 100. [Google Scholar]
- Mays, A.C.; Gillenwater, A.M.; Garvey, P.B. Rare presentation of heterotopic ossification along a fibula free flap pedicle in a high-volume microvascular reconstruction practice. Head Neck 2018, 40, E21–E24. [Google Scholar] [CrossRef] [PubMed]
- Kwong, F.N.; Harris, M.B. Recent developments in the biology of fracture repair. J. Am. Acad. Orthop. Surg. 2008, 16, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R.; Mikulski, A.; Lietze, A. Solubilized and insolubilized bone morphogenetic protein. Proc. Natl. Acad. Sci. USA 1979, 76, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Urist, M.R. The reaction of the dura to bone morphogenetic protein (BMP) in repair of skull defects. Ann. Surg. 1982, 196, 100–109. [Google Scholar] [CrossRef]
- Lounev, V.Y.; Ramachandran, R.; Wosczyna, M.N.; Yamamoto, M.; Maidment, A.D.; Shore, E.M.; Glaser, D.L.; Goldhamer, D.J.; Kaplan, F.S. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J. Bone Joint Surg. Am. 2009, 91, 652–663. [Google Scholar] [CrossRef]
- Liu, X.; Kang, H.; Shahnazari, M.; Kim, H.; Wang, L.; Larm, O.; Adolfsson, L.; Nissenson, R.; Halloran, B. A novel mouse model of trauma induced heterotopic ossification. J. Orthop. Res. 2014, 32, 183–188. [Google Scholar] [CrossRef]
- Uchibe, K.; Son, J.; Larmour, C.; Pacifici, M.; Enomoto-Iwamoto, M.; Iwamoto, M. Genetic and pharmacological inhibition of retinoic acid receptor gamma function promotes endochondral bone formation. J. Orthop. Res. 2017, 35, 1096–1105. [Google Scholar] [CrossRef]
- Tian, X.B.; Sun, L.; Yang, S.H.; Fu, R.Y.; Wang, L.; Lu, T.S.; Zhang, Y.K.; Fu, D.H. Ectopic osteogenesis of mouse bone marrow stromal cells transfected with BMP 2/VEGF(165) genes in vivo. Orthop. Surg. 2009, 1, 322–325. [Google Scholar] [CrossRef]
- Peterson, J.R.; De La Rosa, S.; Sun, H.; Eboda, O.; Cilwa, K.E.; Donneys, A.; Morris, M.; Buchman, S.R.; Cederna, P.S.; Krebsbach, P.H.; et al. Burn injury enhances bone formation in heterotopic ossification model. Ann. Surg. 2014, 259, 993–998. [Google Scholar] [CrossRef]
- Shirley, D.; Marsh, D.; Jordan, G.; McQuaid, S.; Li, G. Systemic recruitment of osteoblastic cells in fracture healing. J. Orthop. Res. 2005, 23, 1013–1021. [Google Scholar] [CrossRef]
- Burstein, F.D.; Canalis, R.F. Studies on the osteogenic potential of vascularized periosteum: Behavior of periosteal flaps transferred onto soft tissues. Otolaryngol. Head Neck Surg. 1985, 93, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Ortak, T.; Ozdemir, R.; Uysal, A.; Ulusoy, M.G.; Sungur, N.; Sahin, B.; Kocer, U.; Sensoz, O. Osteogenic capacities of periost grafts, periost flaps and prefabricated periosteal flaps: Experimental study. J. Craniofac. Surg. 2005, 16, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Takato, T.; Harii, K.; Nakatsuka, T.; Ueda, K.; Ootake, T. Vascularized periosteal grafts: An experimental study using two different forms of tibial periosteum in rabbits. Plast. Reconstr. Surg. 1986, 78, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A.R.; Jin, Z.; Morrison, M.D.; Arita, E.S.; Song, J.; Tamimi, F. Mandibular tori are associated with mechanical stress and mandibular shape. J. Oral Maxillofac. Surg. 2014, 72, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.D.; Tamimi, F. Oral tori are associated with local mechanical and systemic factors: A case-control study. J. Oral Maxillofac. Surg. 2013, 71, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Eckert-Hubner, K.; Augat, P. The effect of mechanical stability on local vascularization and tissue differentiation in callus healing. J. Orthop. Res. 2002, 20, 1099–1105. [Google Scholar] [CrossRef]
- McCarthy, C.M.; Cordeiro, P.G. Microvascular reconstruction of oncologic defects of the midface. Plast. Reconstr. Surg. 2010, 126, 1947–1959. [Google Scholar] [CrossRef]
- Pellini, R.; Mercante, G.; Spriano, G. Step-by-step mandibular reconstruction with free fibula flap modelling. Acta Otorhinolaryngol. Ital. 2012, 32, 405–409. [Google Scholar]
- Wilde, F.; Cornelius, C.P.; Schramm, A. Computer-Assisted Mandibular Reconstruction using a Patient-Specific Reconstruction Plate Fabricated with Computer-Aided Design and Manufacturing Techniques. Craniomaxillofac. Trauma Reconstr. 2014, 7, 158–166. [Google Scholar] [CrossRef]
- Cornelius, C.P.; Smolka, W.; Giessler, G.A.; Wilde, F.; Probst, F.A. Patient-specific reconstruction plates are the missing link in computer-assisted mandibular reconstruction: A showcase for technical description. J. Craniomaxillofac. Surg. 2015, 43, 624–629. [Google Scholar] [CrossRef]
- Wilde, F.; Hanken, H.; Probst, F.; Schramm, A.; Heiland, M.; Cornelius, C.P. Multicenter study on the use of patient-specific CAD/CAM reconstruction plates for mandibular reconstruction. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 2035–2051. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Gui, L.; Mao, C.; Peng, X.; Yu, G.Y. Applying computer techniques in maxillofacial reconstruction using a fibula flap: A messenger and an evaluation method. J. Craniofac. Surg. 2009, 20, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Modabber, A.; Ayoub, N.; Mohlhenrich, S.C.; Goloborodko, E.; Sonmez, T.T.; Ghassemi, M.; Loberg, C.; Lethaus, B.; Ghassemi, A.; Holzle, F. The accuracy of computer-assisted primary mandibular reconstruction with vascularized bone flaps: Iliac crest bone flap versus osteomyocutaneous fibula flap. Med. Devices 2014, 7, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Wilde, F.; Winter, K.; Kletsch, K.; Lorenz, K.; Schramm, A. Mandible reconstruction using patient-specific pre-bent reconstruction plates: Comparison of standard and transfer key methods. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 129–140. [Google Scholar] [CrossRef]
- Raith, S.; Rauen, A.; Mohlhenrich, S.C.; Ayoub, N.; Peters, F.; Steiner, T.; Holzle, F.; Modabber, A. Introduction of an algorithm for planning of autologous fibular transfer in mandibular reconstruction based on individual bone curvatures. Int. J. Med. Robot. 2018, 14. [Google Scholar] [CrossRef]
- Mucke, T.; Wolff, K.D.; Rau, A.; Kehl, V.; Mitchell, D.A.; Steiner, T. Autonomization of free flaps in the oral cavity: A prospective clinical study. Microsurgery 2012, 32, 201–206. [Google Scholar] [CrossRef]
- Robinson, C.G.; Polster, J.M.; Reddy, C.A.; Lyons, J.A.; Evans, P.J.; Lawton, J.N.; Graham, T.J.; Suh, J.H. Postoperative single-fraction radiation for prevention of heterotopic ossification of the elbow. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1493–1499. [Google Scholar] [CrossRef]
- Heyd, R.; Strassmann, G.; Schopohl, B.; Zamboglou, N. Radiation therapy for the prevention of heterotopic ossification at the elbow. J. Bone Joint Surg. Br. 2001, 83, 332–334. [Google Scholar] [CrossRef]
- Seegenschmiedt, M.H.; Makoski, H.B.; Micke, O.; the German Cooperative Group on Radiotherapy for Benign Diseases. Radiation prophylaxis for heterotopic ossification about the hip joint—A multicenter study. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 756–765. [Google Scholar] [CrossRef]





| n = 102 | HO− n = 73 (71.57%) | HO+ n = 29 (28.43%) | |
|---|---|---|---|
| Age (year), SD | 58.69 ± 11.92 | 52.30 ± 14.39 | p = 0.0236 |
| Follow-up (months), SD | 45.16 ± 44.49 | 66.59 ± 45.04 | |
| HO duration until observation (months) | mean 13.48, median 9 ± 16.54 | ||
| Surgical intervention | 5 | ||
| Sex | |||
| Male | 41 (40.20%) | 25 (24.51%) | |
| Female | 32 (31.37%) | 4 (3.92%) | p = 0.5096 |
| Preoperative planning/osteosynthesis | |||
| Analog/hand-bent | 45 (44.12%) | 15 (14.71%) | |
| Virtual/custom-made | 28 (27.45%) | 14 (13.73%) | p = 0.3806 |
| Reconstruction | |||
| Immediately | 60 (58.82%) | 19 (18.63%) | |
| Delayed | 13 (12.75%) | 10 (9.80%) | p= 0.1128 |
| Diagnosis | |||
| Malignant | 69 (67.65%) | 22 (21.57%) | |
| Benign | 4 (3.92%) | 4 (3.92%) | |
| Other (ORN, BPONJ, OM) | 3 (2.94%) | ||
| Location | |||
| Maxilla | 17 (16.67%) | 9 (8.82%) | |
| Mandibula | 56 (54.90%) | 20 (19.61%) | p = 0.4553 |
| Maxilla defect type (Brown et Shaw 2010) [35] | |||
| II | 15 (57.69%) | 8 (30.77%) | |
| III | 2 (7.69%) | 1 (3.85%) | |
| Mandible defect type (Brown et al. 2016) [3] | |||
| I | 15 (19.74%) | 7 (9.21%) | |
| Ic | 4 (5.26%) | ||
| II | 13 (17.11%) | 3 (3.95%) | |
| IIc | 1 (1.32%) | 1 (1.32%) | |
| III | 22 (28.95%) | 7 (9.21%) | |
| IV | 1 (1.32%) | 2 (2.63%) | |
| Reconstruction | |||
| Maxilla 1 FS | 14 (53.85%) | 7 (26.92%) | |
| Maxilla 2 FS | 3 (11.54%) | 2 (7.69%) | |
| Mandibula 1 FS | 24 (31.58%) | 8 (10.53%) | |
| Mandibula 2 FS | 19 (25.00%) | 6 (7.89%) | |
| Mandibula 3 FS | 13 (17.11%) | 6 (7.89%) | |
| Neck dissection (ND) | |||
| None | 14 (13.73%) | 12 (11.76%) | |
| Selective ND | 23 (22.55%) | 4 (3.92%) | |
| MR ND | 36 (35.29%) | 13 (12.75%) | n.s. |
| Neck surgery | |||
| One side | 54 (52.94%) | 23 (22.55%) | |
| Both sides | 19 (18.63%) | 6 (5.66%) | p = 0.6213 |
| Postoperative irradiation | |||
| None | 31 (30.39%) | 16 (15.69%) | |
| < 60 Gy | 20 (19.61%) | 10 (9.80%) | |
| ≥ 60 Gy | 20 (19.61%) | 2 (1.96%) | |
| Dosage unknown | 2 (1.96%) | 1 (0.98%) | n.s. |
| HO Type | Maxilla | Mandible | Cumulative | Complaints | Surgery | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Age (Year) (Min/Max) | n | Age (Year) (Min/Max) | n | Age (Year) ± SD | Max. | Mand. | Max. | Mand. | |
| 0 | 17 | 58.65 (32.58/79.08) | 56 | 58.70 (32.83/82.75) | 73 | 58.69 ± 11.92 | ||||
| 1 | 4 (13.79%) | 47.63 (14.75/68.25) | 10 (34.48%) | 54.59 (37.75/76.83) | 14 | 52.60 ± 15.85 | 2 | 1 | 1 | - |
| 2 | 2 (6.90%) | 63.5 (53.92/73.08) | 7 (24.14%) | 53.81 (30.0/65.66) | 9 | 55.96 ± 13.61 | 1 | 2 | 1 | - |
| 3 | 2 (6.90%) | 51.21 (46.08/56.33) | 2 | 51.20 ± 7.25 | 1 | - | ||||
| 4 | 3 (10.34%) | 40.25 (24.41/54.0) | 1 (3.45%) | 53.58 | 4 | 43.58 ± 13.87 | 3 | 3 | - | |
| 9 (31.03%) | 48.69 (14.75/73.08) | 20 (68.97%) | 53.92 (30.0/76.83) | 29 | 52.30 ± 14.39 | |||||
| Authors | Flap (n) | Incidence on Radiographs | Imaging | Clinical Symptoms/Surgical Removal |
|---|---|---|---|---|
| Deschler et al., 1997 [37] | FFF (n = 38) | No data available | OPT | 8% (n = 3) |
| Smith et al., 2003 [27] | FFF, CR | CT | (n = 1) | |
| Autelitano et al., 2008 [26] | FFF (n~100) | No data available | ~4% (n = 4) | |
| Gonzales-Garcia et al., 2011 [25] | FFF, CR | CT | (n = 1) | |
| Colletti et al., 2014 [38] | FFF (n = 92) | No data available | 4.3% (n = 4) | |
| Acarturk et al., 2011 [39] | FFF, CR | CT | (n = 1) | |
| DeConde et al., 2011 [22] | FFF (n = 520) * | 65% (n = 43 out of 66) | CT | 2.6% (n = 14 out of 520) |
| Myon et al., 2012 [36] | FFF (n = 149) SF (n = 13) | 9.2% (n = 15) (n = 14) (n = 1) | OPT, CT | 3.7% (n = 6) |
| Karagozoglu et al., 2013 [30] | FFF (n = 74) | 27% (n = 20) | OPT | No data |
| Tarsitano et al., 2013 [21] | FFF sp (n= 41) mp (n = 20) | 17% (n = 7) 0% | OPT, CT | 4.9% (n = 2) |
| Glastonbury et al., 2014 [29] | FFF (n = 32) | 50% (n = 16) | CT | 3.13% (n = 1) |
| Baserga et al., 2016 [28] | FFF (n = 68) | No data | 4.4% (n = 3) | |
| This study | FFF (n=102) | 28.4% (n = 29) | CT, CBCT | 9.8% (n = 10)/ 4.9% (n = 5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knitschke, M.; Siu, K.; Bäcker, C.; Attia, S.; Howaldt, H.-P.; Böttger, S. Heterotopic Ossification of the Vascular Pedicle after Maxillofacial Reconstructive Surgery Using Fibular Free Flap: Introducing New Classification and Retrospective Analysis. J. Clin. Med. 2021, 10, 109. https://doi.org/10.3390/jcm10010109
Knitschke M, Siu K, Bäcker C, Attia S, Howaldt H-P, Böttger S. Heterotopic Ossification of the Vascular Pedicle after Maxillofacial Reconstructive Surgery Using Fibular Free Flap: Introducing New Classification and Retrospective Analysis. Journal of Clinical Medicine. 2021; 10(1):109. https://doi.org/10.3390/jcm10010109
Chicago/Turabian StyleKnitschke, Michael, Kelly Siu, Christina Bäcker, Sameh Attia, Hans-Peter Howaldt, and Sebastian Böttger. 2021. "Heterotopic Ossification of the Vascular Pedicle after Maxillofacial Reconstructive Surgery Using Fibular Free Flap: Introducing New Classification and Retrospective Analysis" Journal of Clinical Medicine 10, no. 1: 109. https://doi.org/10.3390/jcm10010109
APA StyleKnitschke, M., Siu, K., Bäcker, C., Attia, S., Howaldt, H.-P., & Böttger, S. (2021). Heterotopic Ossification of the Vascular Pedicle after Maxillofacial Reconstructive Surgery Using Fibular Free Flap: Introducing New Classification and Retrospective Analysis. Journal of Clinical Medicine, 10(1), 109. https://doi.org/10.3390/jcm10010109







