Real-World Data on Clinical Features, Outcomes, and Prognostic Factors in Multiple Myeloma from Miyazaki Prefecture, Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Definitions and Clinical Outcome Variables
2.3. Statistical Analysis
3. Results
3.1. Patients Characteristics at Diagnosis and Initial Treatments
3.2. The Best Response to Initial Treatment
3.3. Time to Next Treatment (TTNT) and 2nd Line Regimens
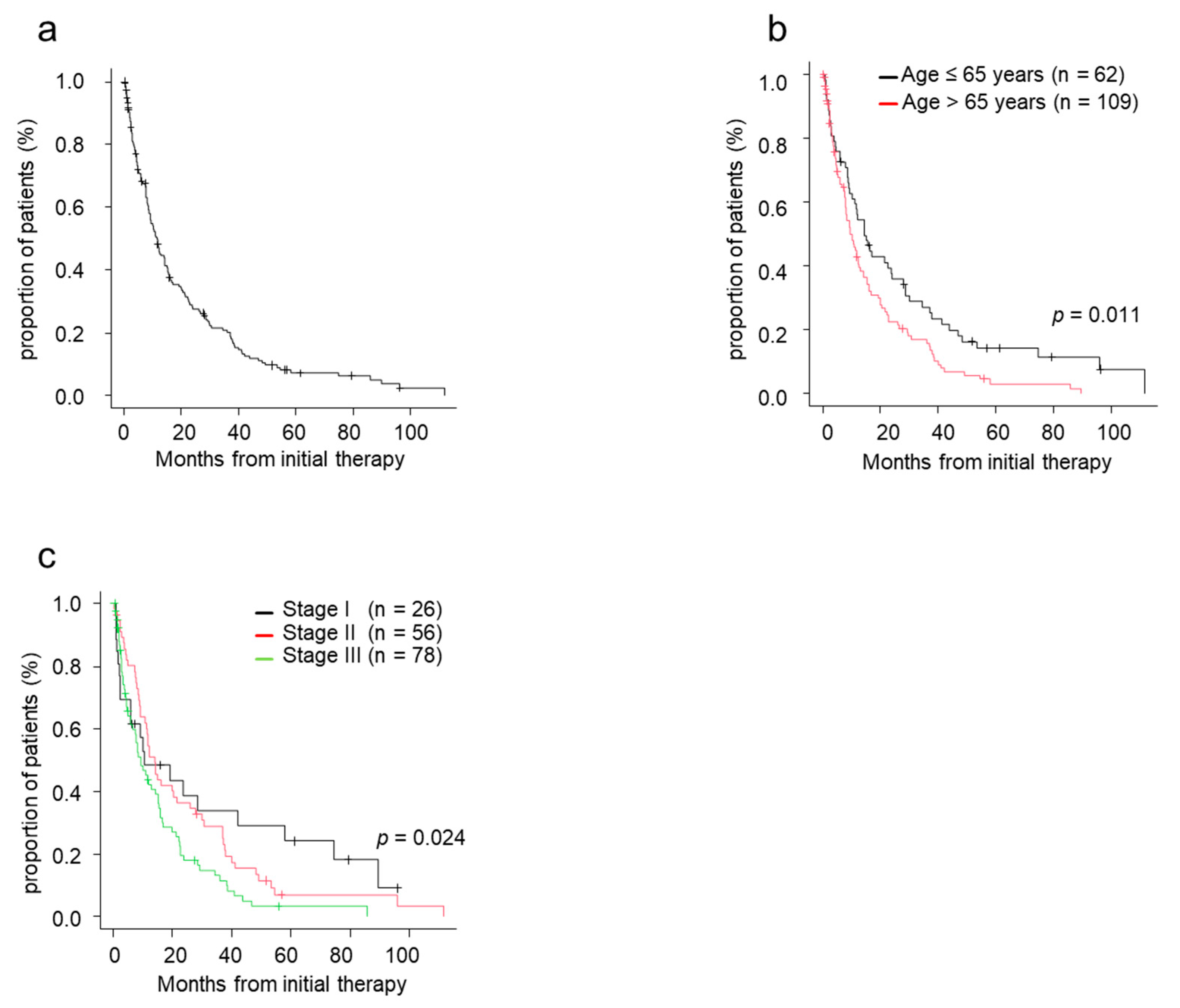
3.4. Overall Survival
3.5. Factors Which Affected on TTNT and OS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASCT | autologous stem cell transplantation |
| CA | chromosomal abnormality |
| CR | complete response |
| ISS | International Staging System |
| JSM | Japanese Society of Myeloma |
| MM | multiple myeloma |
| MST | median survival time |
| OS | overall survival |
| PD | progressive disease |
| PR | partial response |
| sCR | stringent complete response |
| SD | stable disease |
| TTNT | time to next treatment |
| VGPR | very good partial response |
References
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Shida, S.; Takahashi, N.; Niioka, T.; Kitabayashi, A.; Kawabata, Y.; Kume, M.; Saitoh, H.; Hatano, Y.; Ichikawa, Y.; Kuroki, J.; et al. Treatment of multiple myeloma in Akita: Features and outcomes in the era of novel agents. J. Clin. Exp. Hematop. 2014, 54, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.; Delforge, M.; Driessen, C.; Fink, L.; Flinois, A.; Gonzalez-McQuire, S.; Safaei, R.; Karlin, L.; Mateos, M.V.; Raab, M.S.; et al. Multiple myeloma: Patient outcomes in real-world practice. Br. J. Haematol. 2016, 175, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Hajek, R.; Jarkovsky, J.; Maisnar, V.; Pour, L.; Spicka, I.; Minarik, J.; Gregora, E.; Kessler, P.; Sykora, M.; Frankova, H.; et al. Real-world Outcomes of Multiple Myeloma: Retrospective Analysis of the Czech Registry of Monoclonal Gammopathies. Clin. Lymphoma. Myeloma. Leuk. 2018, 18, e219–e240. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Handa, H.; Saitoh, T.; Murakami, H.; Itagaki, M.; Asaoku, H.; Suzuki, K.; Isoda, A.; Matsumoto, M.; Sawamura, M.; et al. Trends of survival in patients with multiple myeloma in Japan: A multicenter retrospective collaborative study of the Japanese Society of Myeloma. Blood Cancer J. 2015, 5, e349. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, S.; Harada, T.; Saitoh, T.; Shimazaki, C.; Itagaki, M.; Asaoku, H.; Kuroda, Y.; Chou, T.; Yoshiki, Y.; Suzuki, K.; et al. Survival of multiple myeloma patients aged 65–70 years in the era of novel agents and autologous stem cell transplantation. A multicenter retrospective collaborative study of the Japanese Society of Myeloma and the European Myeloma Network. Acta Haematol. 2014, 132, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Knauf, W.; Aldaoud, A.; Hutzschenreuter, U.; Klausmann, M.; Dille, S.; Wetzel, N.; Janicke, M.; Marschner, N.; the TLN-Group (Tumour Registry Lymphatic Neoplasms). Survival of non-transplant patients with multiple myeloma in routine care differs from that in clinical trials-data from the prospective German Tumour Registry Lymphatic Neoplasms. Ann. Hematol. 2018, 97, 2437–2445. [Google Scholar] [CrossRef]
- Raab, M.S.; Cavo, M.; Delforge, M.; Driessen, C.; Fink, L.; Flinois, A.; Gonzalez-McQuire, S.; Safaei, R.; Karlin, L.; Mateos, M.V.; et al. Multiple myeloma: Practice patterns across Europe. Br. J. Haematol. 2016, 175, 66–76. [Google Scholar] [CrossRef]
- Bergin, K.; McQuilten, Z.; Moore, E.; Wood, E.; Spencer, A. Myeloma in the Real World: What Is Really Happening? Clin. Lymphoma. Myeloma. Leuk. 2017, 17, 133–144.e131. [Google Scholar] [CrossRef]
- Greipp, P.R.; San Miguel, J.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Blade, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International staging system for multiple myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Durie, B.G.; Harousseau, J.L.; Miguel, J.S.; Blade, J.; Barlogie, B.; Anderson, K.; Gertz, M.; Dimopoulos, M.; Westin, J.; Sonneveld, P.; et al. International uniform response criteria for multiple myeloma. Leukemia 2006, 20, 1467–1473. [Google Scholar] [CrossRef]
- Gay, F.; Larocca, A.; Wijermans, P.; Cavallo, F.; Rossi, D.; Schaafsma, R.; Genuardi, M.; Romano, A.; Liberati, A.M.; Siniscalchi, A.; et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: Analysis of 1175 patients. Blood 2011, 117, 3025–3031. [Google Scholar] [CrossRef]


| Characteristic | Overall | Non-Novel Agent | Novel Agent | p Value # | p Value ☥ | ||
|---|---|---|---|---|---|---|---|
| Total | Age ≤ 65 years | Age > 65 years | |||||
| (n = 284) | (n = 56) | (n = 228) | (n = 84) | (n = 144) | |||
| Age, y | 71.0 (33–93) | 75 (42–90) | 69 (33–93) | 58.5 (33–65) | 77 (66–93) | 0.002 | <0.001 |
| Sex | |||||||
| Male | 144 (50.7) | 25 (44.6) | 119 (52.2) | 45 (53.6) | 74 (51.4) | 0.388 | 0.857 |
| Female | 140 (49.3) | 31 (55.4) | 109 (47.8) | 39 (46.4) | 70 (48.6) | ||
| ISS stage | 0.073 | 0.002 | |||||
| I | 47 (18.4) | 12 (26.1) | 35 (16.7) | 21 (26.2) | 14 (10.9) | ||
| II | 87 (34.1) | 19 (41.3) | 68 (32.5) | 29 (36.2) | 39 (30.2) | ||
| III | 121 (47.5) | 15 (32.6) | 106 (50.7) | 30 (37.5) | 76 (58.9) | ||
| Missing data | 29 | 10 | 19 | 4 | 15 | ||
| Cytogenetic profile | |||||||
| Standard-risk a | 70 (75.4) | 4 (66.7) | 67 (77.0) | 24 (80.0) | 43 (75.4) | 0.987 | 0.687 |
| High-risk b | 23 (24.6) | 2 (33.3) | 20 (23.0) | 6 (20.0) | 14 (24.6) | ||
| Missing data | 191 | 50 | 141 | 54 | 87 | ||
| Initial therapy | <0.001 | <0.001 | |||||
| Triplet | 37 (13.0) | 0 (0.0) | 37 (16.2) | 12 (14.3) | 25 (17.4) | ||
| Bortezomib-containing regimen | 147 (51.8) | 0 (0.0) | 147 (64.5) | 69 (82.1) | 78(54.2) | ||
| Lenalidomide-containing regimen | 26 (9.6) | 0 (0.0) | 26 (11.4) | 3 (3.6) | 23 (16.0) | ||
| Thalidomide-containing regimen | 18 (6.3) | 0 (0.0) | 18 (7.9) | 0 (0.0) | 18 (12.5) | ||
| MP regimen | 37 (13.0) | 37 (66.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Other | 19 (6.7) | 19 (33.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| ASCT | 0.021 | <0.001 | |||||
| Yes | 47 (16.5) | 3 (5.4) | 44 (19.3) | 34 (40.5) | 10 (6.9) | ||
| No | 237 (83.5) | 53 (94.6) | 184 (80.7) | 50 (59.5) | 134 (93.1) | ||
| Best response | 0.004 | <0.001 | |||||
| OR (sCR + CR + VGPR + PR) | 164 (74.9) | 19 (55.9) | 145 (78.4) | 59 (88.1) | 86 (72.9) | ||
| sCR | 11 (5.0) | 0 (0.0) | 11 (5.9) | 4 (6.0) | 7 (5.9) | ||
| CR | 17 (7.8) | 0 (0.0) | 17 (9.2) | 10 (14.9) | 7 (5.9) | ||
| VGPR | 56 (25.5) | 7 (20.6) | 49 (26.5) | 14 (20.9) | 35 (29.7) | ||
| PR | 80 (26.2) | 12 (35.3) | 68 (36.8) | 31 (46.3) | 37 (31.4) | ||
| SD | 41 (18.7) | 12 (35.3) | 29 (15.7) | 6 (9.0) | 23 (19.5) | ||
| PD | 14 (6.4) | 3 (8.8) | 11 (5.9) | 2 (3.0) | 9 (7.6) | ||
| Missing data | 65 | 22 | 43 | 17 | 26 |
| Second Line Treatment Regimen | Initial Treatment Regimen | |||
|---|---|---|---|---|
| Triplet (n = 37) | Bortezomib-Containing (n = 147) | Lenalidomide-Containing (n = 26) | Thalidomide-Containing (n = 18) | |
| Daratuzumab-containing | 3 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Elotuzumab-containing | 4 (19.0) | 3 (3.4) | 0 (0.0) | 0 (0.0) |
| Carfilzomib-containing | 3 (14.3) | 2 (2.2) | 1 (10.0) | 0 (0.0) |
| Ixazomib-containing | 1 (4.8) | 3 (3.4) | 1 (10.0) | 0 (0.0) |
| Bortezomib-containing | 1 (4.8) | 16 (18.0) | 5 (50.0) | 3 (30.0) |
| Pomalidomide-containing | 4 (19.0) | 11 (12.4) | 1 (10.0) | 0 (0.0) |
| Lenalidomide-containing | 3 (14.3) | 35 (39.3) | 0 (0.0) | 6 (60.0) |
| Thalidomide-containing | 0 (0.0) | 1 (1.1) | 0 (0.0) | 1 (10.0) |
| Triplet | 2 (9.5) | 12 (13.5) | 2 (20.0) | 0 (0.0) |
| Conventional chemotherapy | 0 (0.0) | 6 (6.7) | 0 (0.0) | 0 (0.0) |
| No further treatment | 5 | 12 | 3 | 2 |
| due to poor general conditions * | 0 | 3 | 2 | 2 |
| due to unknown reasons | 5 | 9 | 1 | 0 |
| Early death | 3 | 9 | 5 | 1 |
| Missing data | 8 | 37 | 8 | 5 |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (>65 vs. ≤65 years) | 1.55 (1.10–2.18) | 0.011 | 1.22 (0.79–1.89) | 0.367 |
| Stage (III vs. I or II) | 1.58 (1.12–2.26) | 0.005 | 1.37 (0.94–1.99) | 0.090 |
| Cytogenetic profiles (High-risk a vs. standard-risk b) | 1.22 (0.66–2.28) | 0.523 | ||
| ASCT status (performed vs. not performed) | 0.64 (0.42–0.98) | 0.041 | 0.88 (0.52–1.48) | 0.630 |
| Response of initial therapy (PR or better vs. SD or PD) | 0.43 (0.27–0.66) | <0.001 | 0.50 (0.31–0.79) | 0.003 |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (>65 vs. ≤65 years) | 2.45 (1.56–3.84) | <0.001 | 1.30 (0.76–2.22) | 0.332 |
| Stage (III vs. I or II) | 2.57 (1.66–4.00) | <0.001 | 2.19 (1.37–3.49) | 0.001 |
| Cytogenetic profiles (High-risk a vs. standard-risk b) | 1.57 (0.74–3.31) | 0.239 | ||
| ASCT status (performed vs. not performed) | 0.30 (0.16–0.58) | <0.001 | 0.40 (0.18–0.85) | 0.017 |
| Response of initial therapy (PR or better vs. SD or PD) | 0.43 (0.26–0.71) | 0.001 | 0.47 (0.27–0.79) | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akizuki, K.; Matsuoka, H.; Toyama, T.; Kamiunten, A.; Sekine, M.; Shide, K.; Kameda, T.; Kawano, N.; Maeda, K.; Takeuchi, M.; et al. Real-World Data on Clinical Features, Outcomes, and Prognostic Factors in Multiple Myeloma from Miyazaki Prefecture, Japan. J. Clin. Med. 2021, 10, 105. https://doi.org/10.3390/jcm10010105
Akizuki K, Matsuoka H, Toyama T, Kamiunten A, Sekine M, Shide K, Kameda T, Kawano N, Maeda K, Takeuchi M, et al. Real-World Data on Clinical Features, Outcomes, and Prognostic Factors in Multiple Myeloma from Miyazaki Prefecture, Japan. Journal of Clinical Medicine. 2021; 10(1):105. https://doi.org/10.3390/jcm10010105
Chicago/Turabian StyleAkizuki, Keiichi, Hitoshi Matsuoka, Takanori Toyama, Ayako Kamiunten, Masaaki Sekine, Kotaro Shide, Takuro Kameda, Noriaki Kawano, Kouichi Maeda, Masanori Takeuchi, and et al. 2021. "Real-World Data on Clinical Features, Outcomes, and Prognostic Factors in Multiple Myeloma from Miyazaki Prefecture, Japan" Journal of Clinical Medicine 10, no. 1: 105. https://doi.org/10.3390/jcm10010105
APA StyleAkizuki, K., Matsuoka, H., Toyama, T., Kamiunten, A., Sekine, M., Shide, K., Kameda, T., Kawano, N., Maeda, K., Takeuchi, M., Kawano, H., Sato, S., Ishizaki, J., Tahira, Y., Shimoda, H., Hidaka, T., Yamashita, K., Kubuki, Y., & Shimoda, K. (2021). Real-World Data on Clinical Features, Outcomes, and Prognostic Factors in Multiple Myeloma from Miyazaki Prefecture, Japan. Journal of Clinical Medicine, 10(1), 105. https://doi.org/10.3390/jcm10010105





