Hybrid Hollow Fiber Nanofiltration–Calcite Contactor: A Novel Point-of-Entry Treatment for Removal of Dissolved Mn, Fe, NOM and Hardness from Domestic Groundwater Supplies
Abstract
1. Introduction
2. Experimental
2.1. Materials
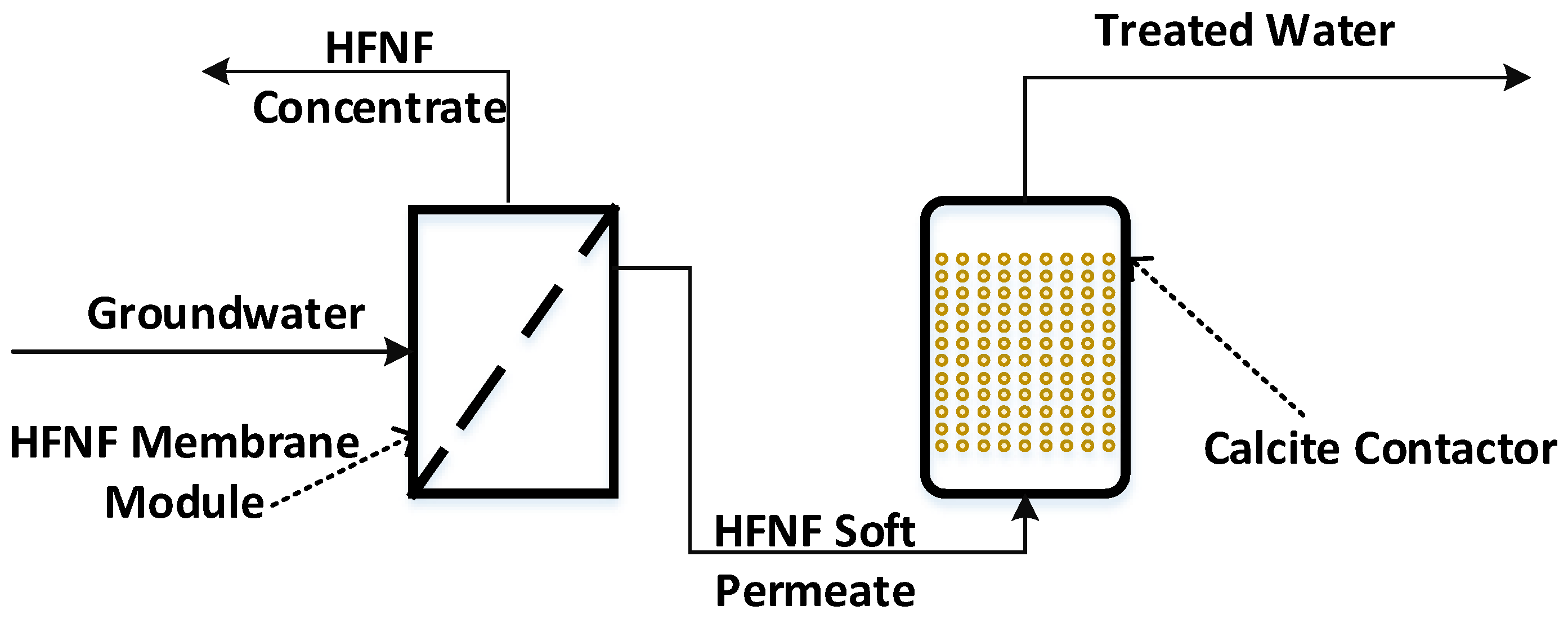
2.2. Experimental Design
2.3. Protocols
2.3.1. Nanofiltration
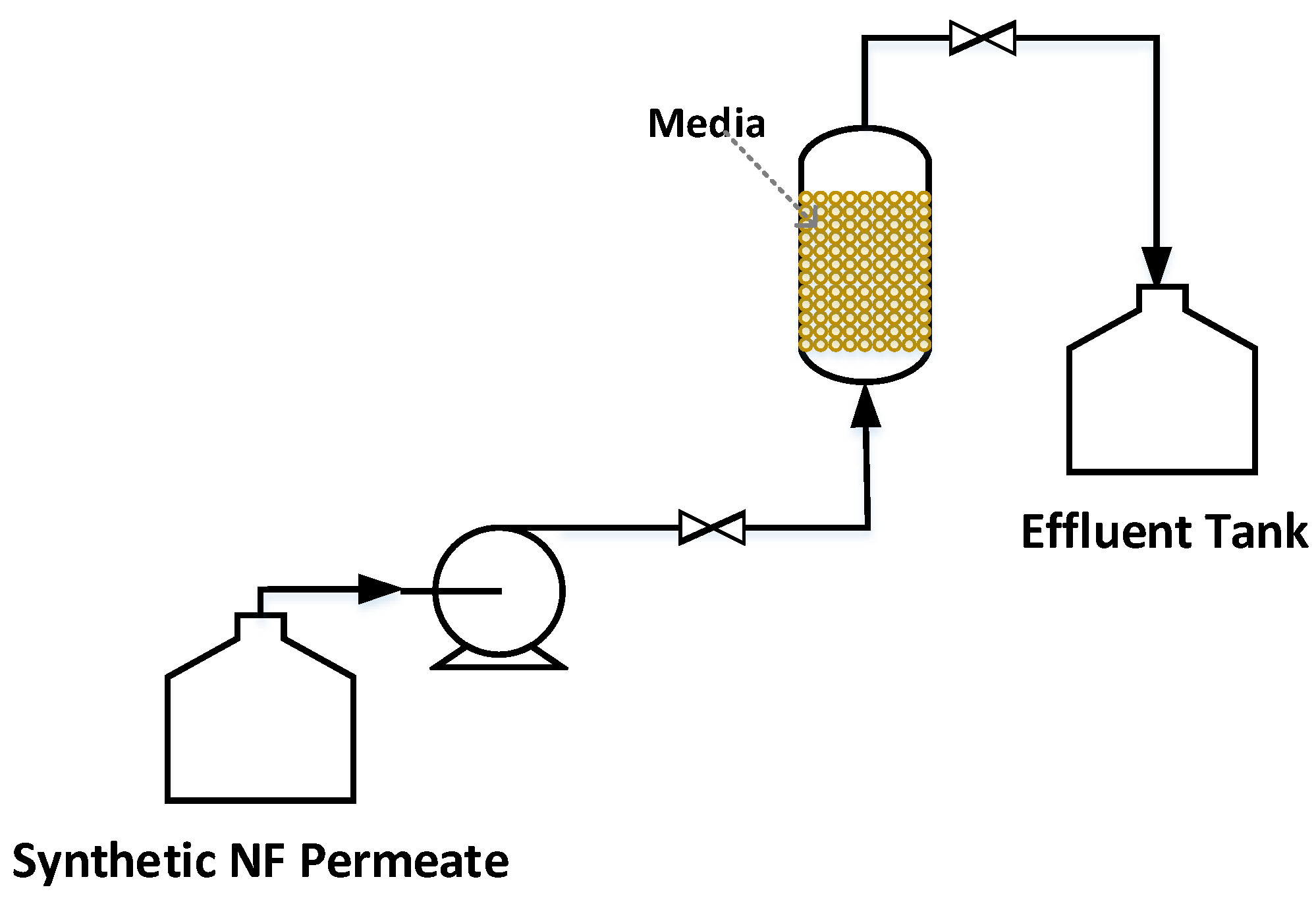
2.3.2. Post-Filtration via a Calcite Contactor
2.4. Analytical Methods
2.5. Statistical Analyses
3. Results and Discussion
3.1. Nanofiltration
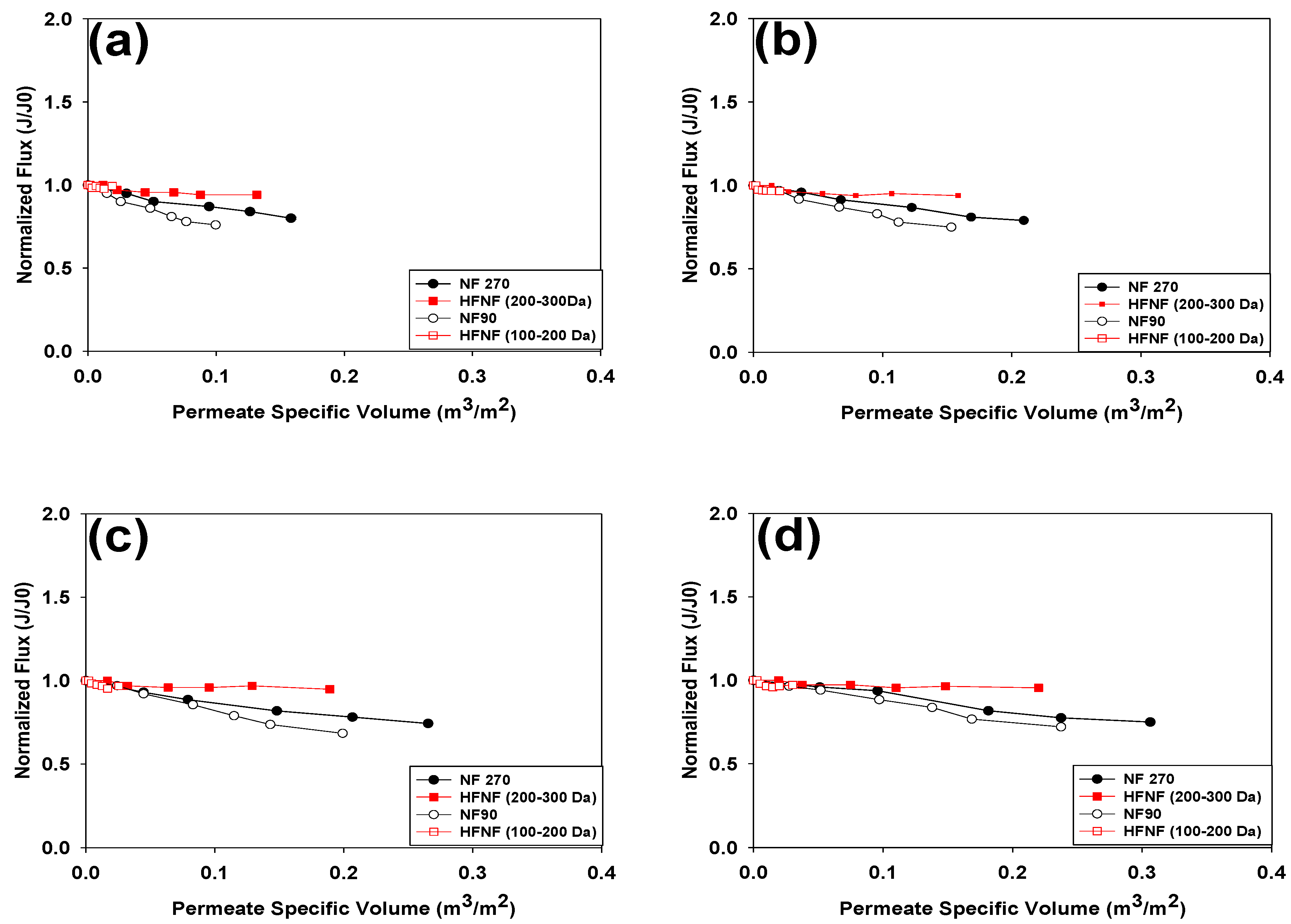
3.1.1. Membrane Permeability
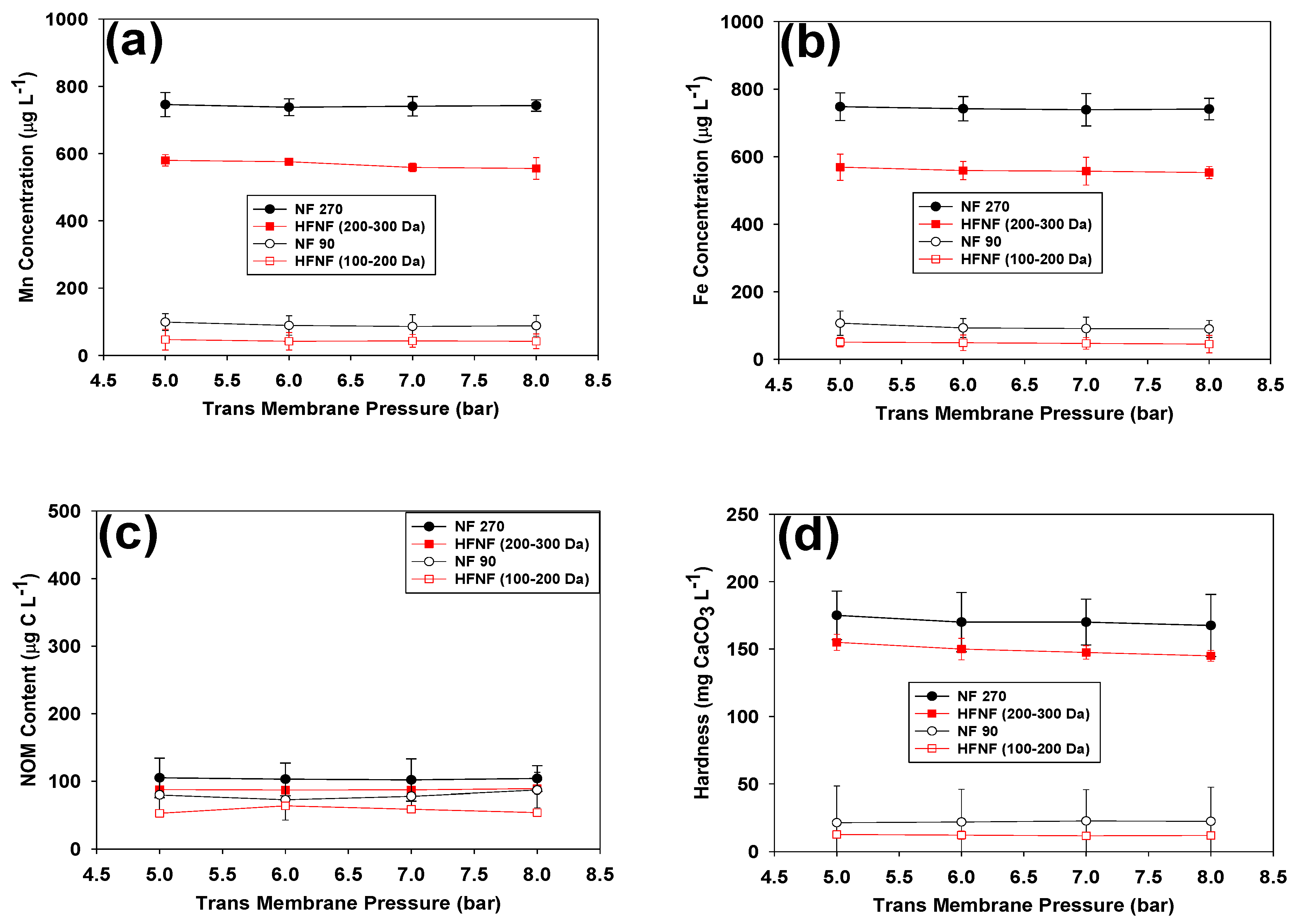
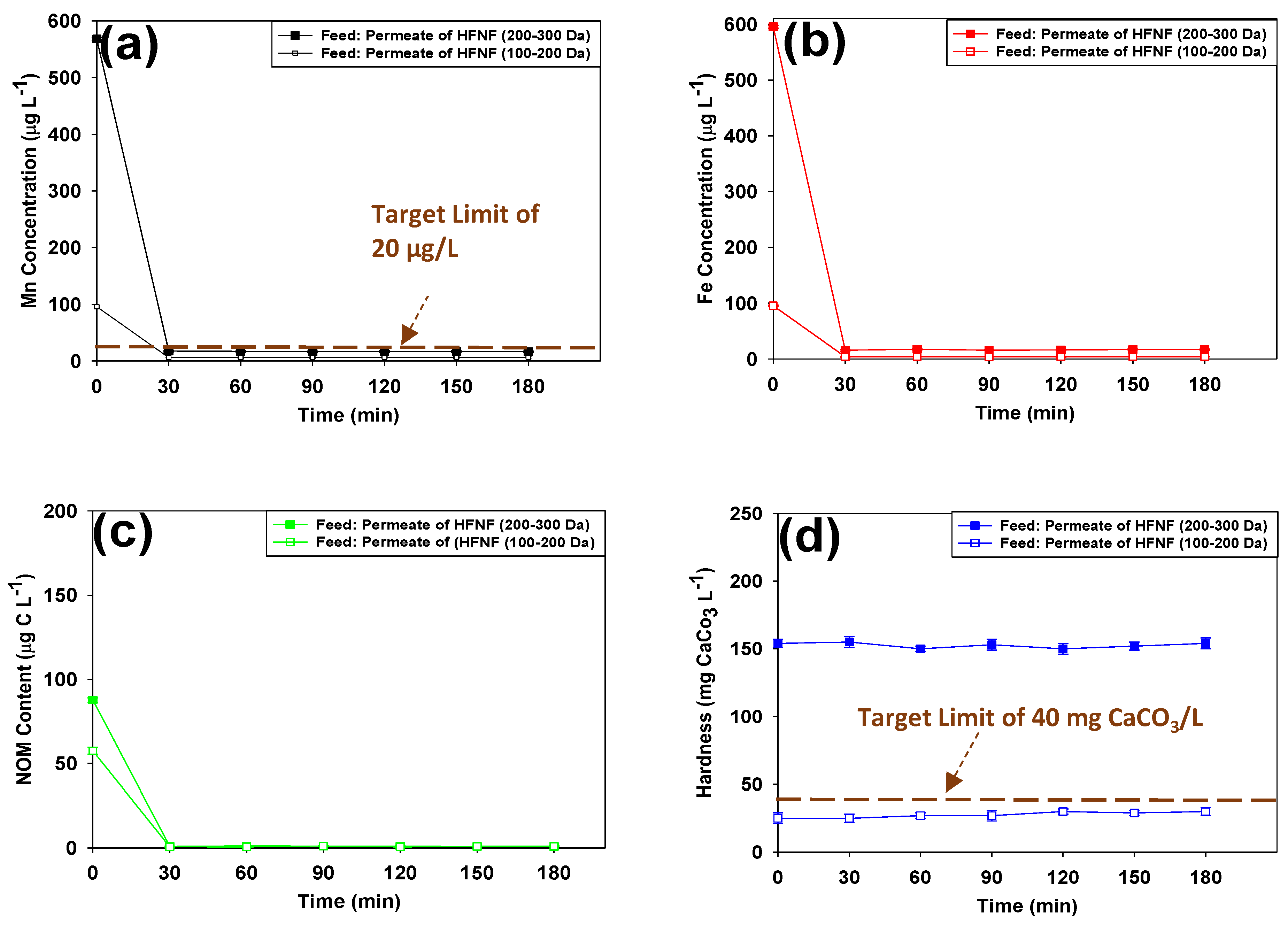
3.1.2. Mn, Fe, NOM and Hardness Removal
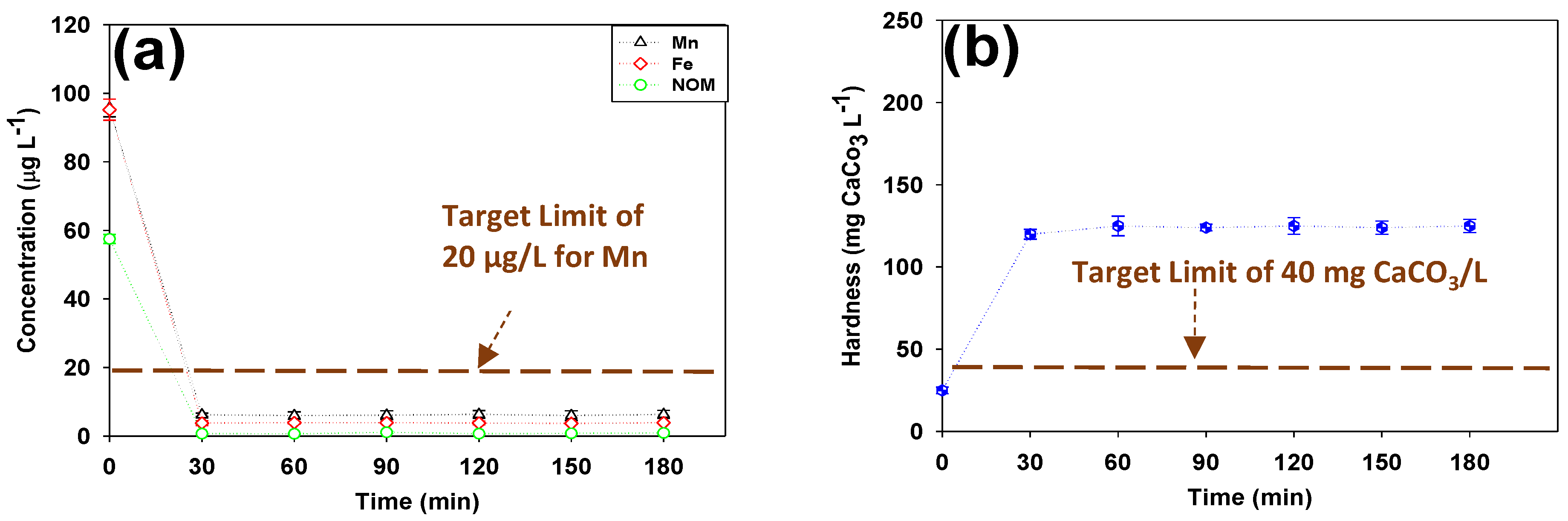
3.2. Post-Filtration via Calcite Contactor
3.3. Implications
4. Conclusions
- The HFNF (200–300 Da) and HFNF (100–200 Da) membranes offered a higher productivity than the NF270 and NF90 membranes which were tested as the reference cases.
- The HFNF (200–300 Da) and HFNF (100–200 Da) membranes exhibited a stable permeate flux during the filtration of the hard GW whereas the permeate flux of the NF270 and NF90 membranes considerably decreased due to the formation of Ca-NOM complexes on the surface of these membranes, regardless of the employed TMP.
- Size exclusion played the main role in retaining the dissolved Mn and Fe during the filtration of the hard GW: the presence of hardness negatively impacted charge exclusion by binding of the Ca and Mg ions to the charged groups on their surface. Thus, higher Mn and Fe removals were achieved when NF90 and HFNF (100–200 Da) were used.
- The surface morphology and type of the ligand groups on the surface of the NF membranes can initiate the membrane fouling phenomenon. The smoother surface of the HFNF membranes and presence of the sulfonic groups on their surface boosted their antifouling properties.
- When the tight NF membranes (i.e., NF90 and HFNF (100–200 Da)) were examined, above 90% of Mn, Fe were removed from the hard GW, while the Mn and Fe removal efficiency of the loose NF270 and HFNF (200–300 Da) was not greater than 42%. The HFNF (100–200 Da) and NF270 membranes exhibited the highest and lowest Mn and Fe retention, respectively.
- All the tested membranes could efficiently retain NOM as a result of charge and size exclusions.
- In the case of high initial concentrations of Mn and Fe in a hard GW, treatment with a standalone tight HFNF membranes might not lead to the desired target limit for these ions (especially the targeted Mn of g/L) and a post-filtration step by means of a calcite-CorosexTM (90/10 wt.%) contactor is required to further decrease the Mn and Fe levels in the finished water and adjust its hardness level.
Author Contributions
Funding
Acknowledgments
References
- Civardi, J.; Tompeck, M. Iron and Manganese Removal Handbook, 2nd ed.; American Water Works Association: Denver, CO, USA, 2015. [Google Scholar]
- Tobiason, J.E.; Bazilio, A.; Goodwill, J.; Mai, X.; Nguyen, C. Manganese removal from drinking water sources. Water Pollut. 2016, 2, 168–177. [Google Scholar] [CrossRef]
- Bouchard, M.F.; Sauve, S.; Barbeau, B.; Legrand, M.; Brodeurand, M.-E.; Bouffard, T.; Limogesand, E.; Bellinger, D.C.; Mergler, D. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ. Health Perspect. 2011, 119, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Oulhote, Y.; Mergler, D.; Barbeau, B.; Bellinger, D.C.; Bouffard, T.; Brodeur, M.-E.; Saint-Amour, D.; Legrand, M.; Sauve, S.; Bouchard, M.F. Changes in water manganese levels and longitudinal assessment of intellectual function in children exposed through drinking water. Environ. Health Perspect. 2014, 122, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Dion, L.-A.; Saint-Amour, D.; Sauve, S.; Barbeau, B.; Mergler, D.; Bouchard, M.F. Neurobehavioral function in school-age children exposed to manganese in drinking water. NeuroToxicology 2018, 64, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Manganese in Drinking Water; Technical Report; Health Canada: Ottawa, ON, Canada, 2016. Available online: https://www.canada.ca/en/health-canada/programs/consultation-manganese-drinking-water/manganese-drinking-water.html (accessed on 16 July 2019).
- Epa: Chemical Contaminants-ccl 4, Final ccl 4 Chemical Contaminants; Technical Report; United States Environmental Protection Agency: Washington, DC, USA, 2018. Available online: https://www.epa.gov/ccl/chemical-contaminants-ccl-4 (accessed on 16 July 2019).
- Carriere, A.; Brouillon, M.; Sauve, S.; Bouchard, M.F.; Barbeau, B. Performance of point-of-use devices to remove manganese from drinking water. J. Environ. Sci. Health Part A 2011, 46, 601–607. [Google Scholar] [CrossRef]
- Barbeau, B.; Carriere, A.; Brouillon, M. Spatial and temporal variations of manganese concentrations in drinking water. J. Environ. Sci. Health Part A 2011, 46, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Nutrients in Drinking Water; Technical Report; Water, Sanitation and Health: Geneva, Switzerland, 2005.
- Haddad, M.; Ohkame, T.; Berube, P.R.; Barbeau, B. Performance of thin-film composite hollow fiber nanofiltration for the removal of dissolved mn, fe and nom from domestic groundwater supplies. Water Res. 2018, 145, 408–417. [Google Scholar] [CrossRef]
- Sheme, H.; Hasson, D.; Semiat, R. Design considerations of a packed calcite bed for hardening desalinated water. Ind. Eng. Chem. Res. 2013, 52, 10549–10553. [Google Scholar] [CrossRef]
- Hasson, D.; Fine, L.; Sagiv, A.; Semiat, R.; Shemer, H. Modeling remineralization of desalinated water by micronized calcite dissolution. Environ. Sci. Technol. 2017, 51, 12481–12488. [Google Scholar] [CrossRef]
- Aziz, H.; Smith, P.G. The influence of ph and coarse media on manganese precipitation from water. Water Res. 1992, 26, 853–855. [Google Scholar] [CrossRef]
- Franklin, M.L.; Morse, J.W. The interaction of manganese(ii) with the surface of calcite in dilute solutions and seawater. Mar. Chem. 1983, 12, 241–254. [Google Scholar] [CrossRef]
- Silva, A.M.; Cunha, E.C.; Silva, F.D.R.; Leao, V.A. Treatment of high-manganese mine water with limestone and sodium carbonate. J. Clean. Product. 2012, 29–30, 11–19. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, J.; She, Q.; Pun Wan, M.; Wang, R.; Chang, V.W.-C.; Tang, C.Y. Role of calcium ions on the removal of haloacetic acids from swimming pool water by nanofiltration: mechanisms and implications. Water Res. 2017, 110, 332–341. [Google Scholar] [CrossRef] [PubMed]
- De-Zuane, J. Handbook of Drinking Water Quality; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Mohammad, A.W.; Teow, H.; Ang, L.; Chung, T.; Oatley-Radcliffe, L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Vrijenhoek, E.M.; Hong, S.; Elimelech, M. Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2001, 181, 115–128. [Google Scholar] [CrossRef]
- Elimelech, M.; Zhu, X.; Childress, A.E.; Hong, S. Role of membrane surface morphology in colloidal fouling of cellulose acetate and composite aromatic polyamide reverse osmosis membranes. J. Membr. Sci. 1997, 127, 101–109. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Darwish, N.A.; Mohammad, A.W. Characterisation of nanofiltration membranes using atomic force microscopy. Desalination 2005, 177, 187–199. [Google Scholar] [CrossRef]
- Boussu, K.; Van der Bruggen, B.; Volodin, A.; Snauwaert, J.; Van Haesendonck, C.; Vandecasteele, C. Roughness and hydrophobicity studies of nanofiltration membranes using different modes of afm. J. Colloid Interface Sci. 2005, 286, 200–638. [Google Scholar] [CrossRef] [PubMed]
- Pontie, M.; Dach, H.; Leparc, J.; Hafsi, M.; Lhassani, A. Novel approach combining physico-chemical characterizations and mass transfer modelling of nanofiltration and low pressure reverse osmosis membranes for brackish water desalination intensification. Desalination 2008, 221, 174–191. [Google Scholar] [CrossRef]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (nom) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Ahn, W.-Y.; Kalinichev, A.G.; Clark, M.M. Effects of background cations on the fouling of polyethersulfone membranes by natural organic matter: Experimental and molecular modeling study. J. Membr. Sci. 2008, 309, 128–140. [Google Scholar] [CrossRef]
- Liao, P.; Li, W.; Jiang, Y.; Wu, J.; Yuan, S.; Fortner, J.D.; Giammar, D.l.E. Formation, aggregation, and deposition dynamics of nom-iron colloids at anoxic-oxic interfaces. Environ. Sci. Technol. 2007, 51, 12235–12245. [Google Scholar] [CrossRef] [PubMed]
- Childress, A.E.; Elimelech, M. Effect of solution chemistry on the surface charge of polymeric reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1996, 119, 253–268. [Google Scholar] [CrossRef]
- Tay, J.-H.; Liu, J.; Sun, D.D. Effect of solution physico-chemistry on the charge property of nanofiltration membranes. Water Res. 2002, 36, 585–598. [Google Scholar] [CrossRef]
- Ribau, M.; Maria, T.; Rosa, J. The impact of water background inorganic matrix on the natural organic matter removal by nanofiltration. J. Membr. Sci. 2006, 279, 513–520. [Google Scholar]
- Teixeira, M.R.; Rosa, M.J.; Nystrom, M. The role of membrane charge on nanofiltration performance. J. Membr. Sci. 2005, 265, 253–268. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Wang, Y.; Shu, X.; Wu, S.; Ran, Q.; Liu, J. Binding of calcium cations with three different types of oxygen-based functional groups of superplasticizers studied by atomistic simulations. J. Mol. Model. 2018, 24, 1–10. [Google Scholar] [CrossRef]
- Zachara, J.M.; Cowan, C.E.; Resch, C.T. Sorption of divalent metals on calcite. Geochim. Cosmochim. Acta 1991, 55, 1549–1562. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
| Membrane | Charged Groups | Membrane Effective Area (m) | Pure Water Flux (LMH/bar) | MWCO (Da) * |
|---|---|---|---|---|
| NF270 | COO | 0.0042 | 16.10 [17] | 226 [17] |
| NF90 | COO | 0.0042 | 7.69 [17] | 118 [17] |
| HFNF | SO | 0.1750 | 5.00 | 200–300 |
| SO | 0.1710 | 0.64 | 100–200 |
| Mn | Fe | Ca | Mg | Na | NOM * | Dissolved O | pH | Hardness | Alkalinity |
|---|---|---|---|---|---|---|---|---|---|
| (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg C/L) | (mg/L) | mg CaCO/L | mg CaCO/L | |
| Media | D (mm) | Purity (%) | Specific Gravity (g/cm) | Bulk Density (kg/cm) | Surface Area (m/g) |
|---|---|---|---|---|---|
| Calcite | 0.4 | 99.7 | 2.68 | 1500 | 0.37 |
| Corosex | 1.6 | 98.0 | 3.60 | 1200 | 0.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddad, M.; Barbeau, B. Hybrid Hollow Fiber Nanofiltration–Calcite Contactor: A Novel Point-of-Entry Treatment for Removal of Dissolved Mn, Fe, NOM and Hardness from Domestic Groundwater Supplies. Membranes 2019, 9, 90. https://doi.org/10.3390/membranes9070090
Haddad M, Barbeau B. Hybrid Hollow Fiber Nanofiltration–Calcite Contactor: A Novel Point-of-Entry Treatment for Removal of Dissolved Mn, Fe, NOM and Hardness from Domestic Groundwater Supplies. Membranes. 2019; 9(7):90. https://doi.org/10.3390/membranes9070090
Chicago/Turabian StyleHaddad, Maryam, and Benoit Barbeau. 2019. "Hybrid Hollow Fiber Nanofiltration–Calcite Contactor: A Novel Point-of-Entry Treatment for Removal of Dissolved Mn, Fe, NOM and Hardness from Domestic Groundwater Supplies" Membranes 9, no. 7: 90. https://doi.org/10.3390/membranes9070090
APA StyleHaddad, M., & Barbeau, B. (2019). Hybrid Hollow Fiber Nanofiltration–Calcite Contactor: A Novel Point-of-Entry Treatment for Removal of Dissolved Mn, Fe, NOM and Hardness from Domestic Groundwater Supplies. Membranes, 9(7), 90. https://doi.org/10.3390/membranes9070090





