Gas Permeation of Sulfur Thin-Films and Potential as a Barrier Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Synthesis of the Sulfur Membrane
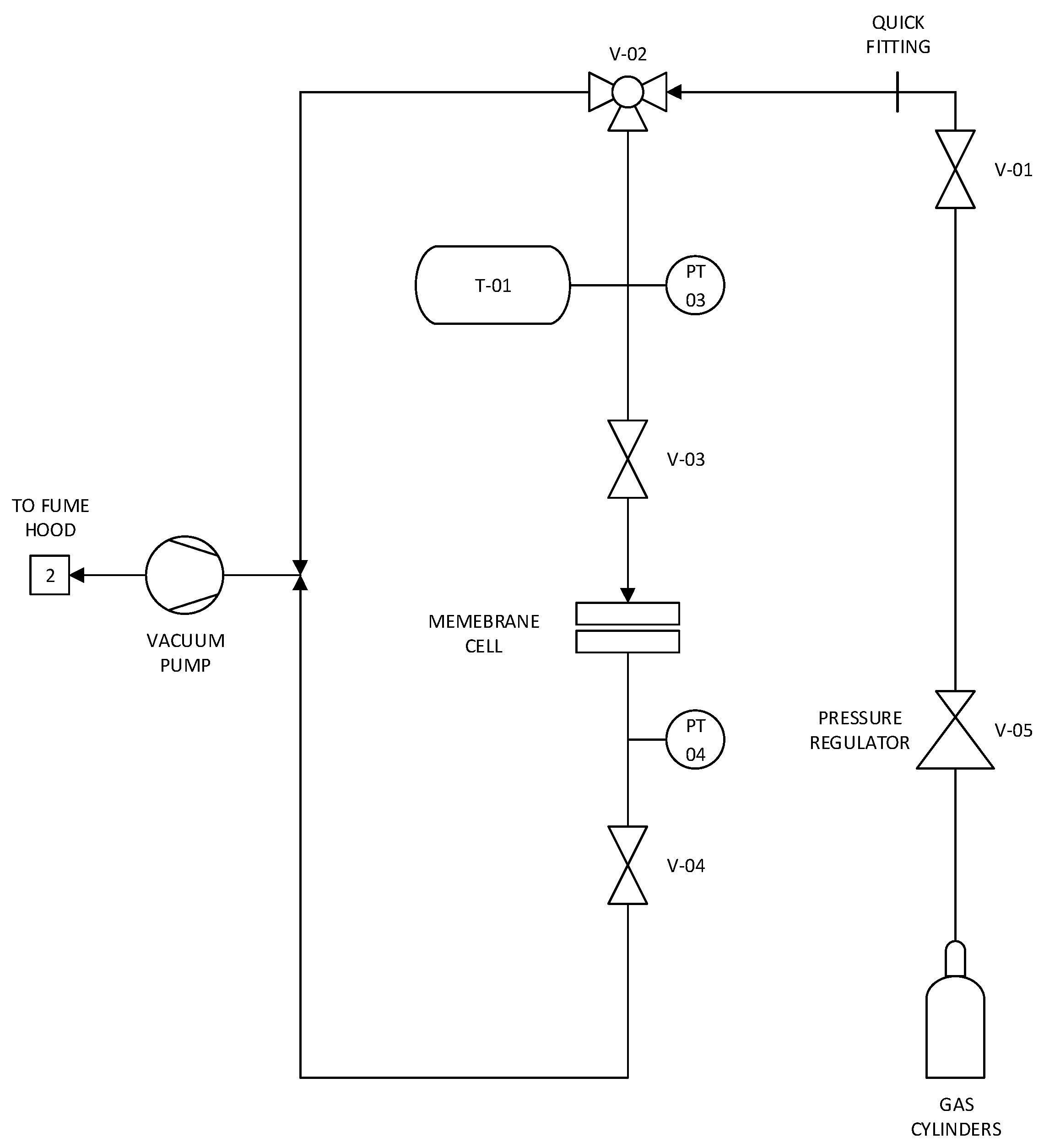
2.3. Single-Gas Permeability Test
3. Results and Discussion
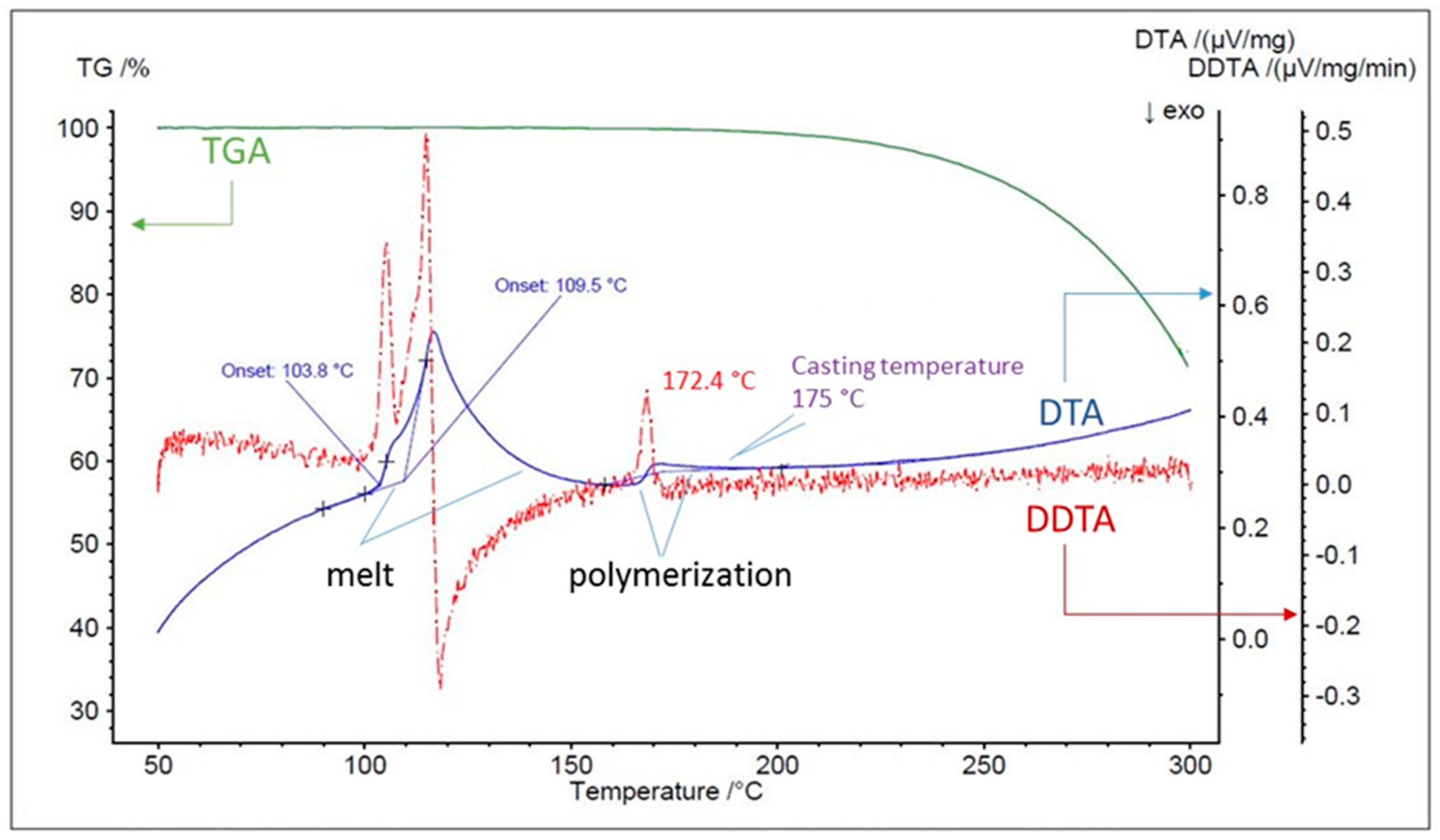
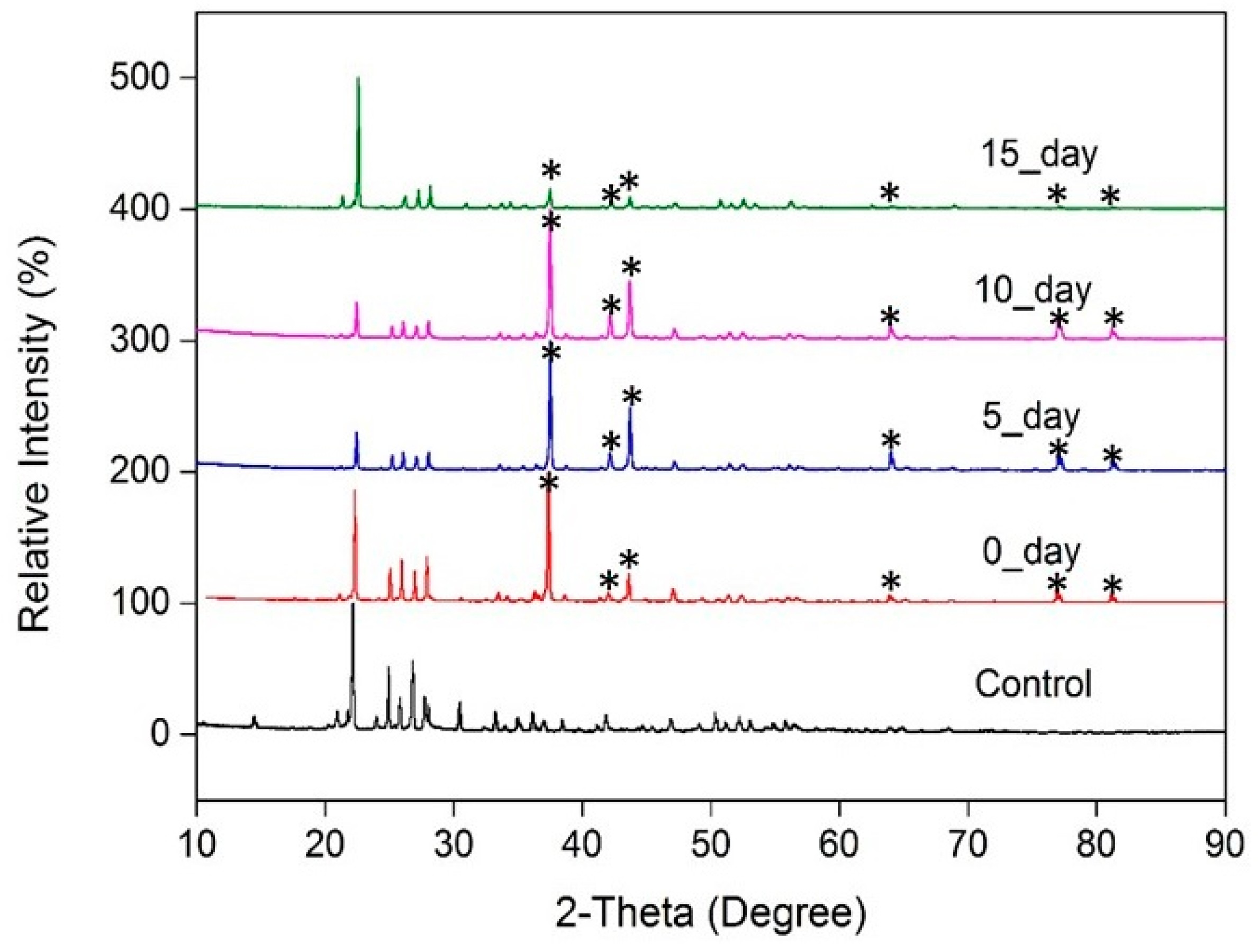
3.1. Check of Sulfur Physio-Chemical Properties
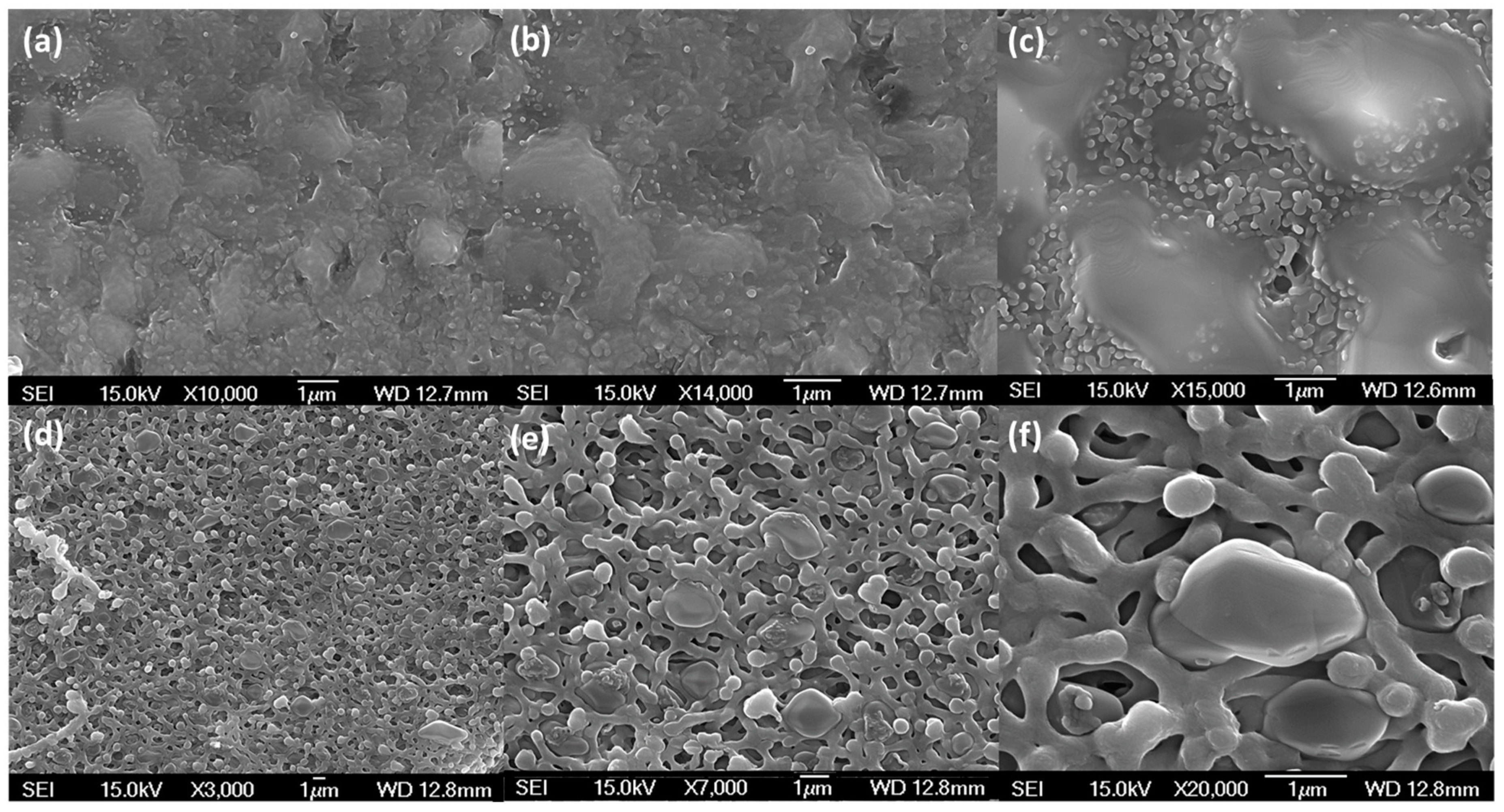
3.2. Preparation of Thin-Film Sulfur Membranes
3.3. Single-Gas Permeation Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Smith, J.A.; Petcher, S.; Zhang, B.; Parker, D.J.; Griffin, J.M.; Hasell, T. Catalytic inverse vulcanization. Nat. Commun. 2019, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Kutney, G. Sulfur: History, Technology, Applications & Industry; ChemTec Publishing: Toronto, ON, Canada, 2007. [Google Scholar]
- Merchant Research and Consulting. Sulfur: 2019 World Market Review and Forecast to 2028; Merchant Research & Consulting Ltd.: Birmingham, UK, 2019. [Google Scholar]
- Lagaron, J.M.; Catalá, R.; Gavara, R. Structural characteristics defining high barrier properties in polymeric materials. Mater. Sci. Technol. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- United States Geological Survey. Sulfur Statistics and Information. Available online: https://www.usgs.gov/centers/nmic/sulfur-statistics-and-information (accessed on 20 May 2019).
- Wooding, A.; Kavale, S.; MacRitchie, F.; Stoddard, F.; Wallace, A. Effects of nitrogen and sulfur fertilizer on protein composition, mixing requirements, and dough strength of four wheat cultivars. Cereal Chem. 2000, 77, 798–807. [Google Scholar] [CrossRef]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium–sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Katare, S.; Patkar, P.; Caruthers, J.M.; Venkatasubramanian, V.; Walker, K.A. Sulfur vulcanization of natural rubber for benzothiazole accelerated formulations: From reaction mechanisms to a rational kinetic model. Rubber Chem. Technol. 2003, 76, 592–693. [Google Scholar] [CrossRef]
- Meyer, B. Elemental sulfur. Chem. Rev. 1976, 76, 367–388. [Google Scholar] [CrossRef]
- Lim, J.; Pyun, J.; Char, K. Recent approaches for the direct use of elemental sulfur in the synthesis and processing of advanced materials. Angew. Chem. Int. Ed. 2015, 54, 3249–3258. [Google Scholar] [CrossRef]
- Worthington, M.J.; Kucera, R.L.; Chalker, J.M. Green chemistry and polymers made from sulfur. Green Chem. 2017, 19, 2748–2761. [Google Scholar] [CrossRef]
- Boyd, D.A. Sulfur and its role in modern materials science. Angew. Chem. Int. Ed. 2016, 55, 15486–15502. [Google Scholar] [CrossRef]
- Nguyen, T.B. Recent advances in organic reactions involving elemental sulfur. Adv. Synth. Catal. 2017, 359, 1066–1130. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Griebel, J.J.; Glass, R.S.; Char, K.; Pyun, J. Polymerizations with elemental sulfur: A novel route to high sulfur content polymers for sustainability, energy and defense. Prog. Polym. Sci. 2016, 58, 90–125. [Google Scholar] [CrossRef]
- Griebel, J.J.; Nguyen, N.A.; Namnabat, S.; Anderson, L.E.; Glass, R.S.; Norwood, R.A.; Mackay, M.E.; Char, K.; Pyun, J. Dynamic Covalent Polymers via Inverse Vulcanization of Elemental Sulfur for Healable Infrared Optical Materials. ACS Macro Lett. 2015, 4, 862–866. [Google Scholar] [CrossRef]
- Xin, Y.; Peng, H.; Xu, J.; Zhang, J. Ultrauniform Embedded Liquid Metal in Sulfur Polymers for Recyclable, Conductive, and Self-Healable Materials. Adv. Funct. Mater. 2019, 29, 1808989. [Google Scholar] [CrossRef]
- Parker, D.J.; Chong, S.T.; Hasell, T. Sustainable inverse-vulcanised sulfur polymers. RSC Adv. 2018, 8, 27892–27899. [Google Scholar] [CrossRef]
- Eftekhari, A.; Kim, D.-W. Cathode materials for lithium–sulfur batteries: A practical perspective. J. Mater. Chem. A 2017, 5, 17734–17776. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, P.; Yan, C.; Dong, X.; Zhang, X. Recent progress in polymer materials for advanced lithium-sulfur batteries. Prog. Polym. Sci. 2019, 90, 118–163. [Google Scholar] [CrossRef]
- Chalker, J.M.; Worthington, M.J.H.; Lundquist, N.A.; Esdaile, L.J. Synthesis and Applications of Polymers Made by Inverse Vulcanization. Top. Curr. Chem. 2019, 377, 16. [Google Scholar] [CrossRef]
- Hasell, T.; Parker, D.J.; Jones, H.A.; McAllister, T.; Howdle, S.M. Porous inverse vulcanised polymers for mercury capture. Chem. Commun. 2016, 52, 5383–5386. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Albuquerque, I.S.; Gibson, C.T.; Sibley, A.; Slattery, A.D.; Campbell, J.A.; Alboaiji, S.F.K.; Muller, K.A.; Young, J.; et al. Laying Waste to Mercury: Inexpensive Sorbents Made from Sulfur and Recycled Cooking Oils. Chem. Eur. J. 2017, 23, 16219–16230. [Google Scholar] [CrossRef]
- Crockett, M.P.; Evans, A.M.; Worthington, M.J.H.; Albuquerque, I.S.; Slattery, A.D.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Bernardes, G.J.L.; Chalker, J.M. Sulfur-Limonene Polysulfide: A Material Synthesized Entirely from Industrial By-Products and Its Use in Removing Toxic Metals from Water and Soil. Angew. Chem. Int. Ed. 2016, 55, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, N.A.; Worthington, M.J.H.; Adamson, N.; Gibson, C.T.; Johnston, M.R.; Ellis, A.V.; Chalker, J.M. Polysulfides made from re-purposed waste are sustainable materials for removing iron from water. RSC Adv. 2018, 8, 1232–1236. [Google Scholar] [CrossRef]
- Deng, Z.; Hoefling, A.; Théato, P.; Lienkamp, K. Surface Properties and Antimicrobial Activity of Poly(sulfur-co-1,3-diisopropenylbenzene) Copolymers. Macromol. Chem. Phys. 2018, 219, 1700497. [Google Scholar] [CrossRef]
- Sandrolini, F.; Manzi, S.; Andrucci, A. Sulfur-polymer matrix composites from particulate wastes: A sustainable route to advanced materials. Compos. Part A Appl. Sci. Manuf. 2006, 37, 695–702. [Google Scholar] [CrossRef]
- Griebel, J.J.; Namnabat, S.; Kim, E.T.; Himmelhuber, R.; Moronta, D.H.; Chung, W.J.; Simmonds, A.G.; Kim, K.-J.; van der Laan, J.; Nguyen, N.A.; et al. New Infrared Transmitting Material via Inverse Vulcanization of Elemental Sulfur to Prepare High Refractive Index Polymers. Adv. Mater. 2014, 26, 3014–3018. [Google Scholar] [CrossRef]
- Salman, M.K.; Karabay, B.; Karabay, L.C.; Cihaner, A. Elemental sulfur-based polymeric materials: Synthesis and characterization. J. Appl. Polym. Sci. 2016, 133, 43655. [Google Scholar] [CrossRef]
- Namnabat, S.; Gabriel, J.J.; Pyun, J.; Norwood, R.A.; Dereniak, E.L.; Van Der Laan, J. Sulfur Copolymers for Infrared Optical Imaging; SPIE: San Diego, CA, USA, 2014; Volume 9070. [Google Scholar]
- Mann, M.; Kruger, J.E.; Andari, F.; McErlean, J.; Gascooke, J.R.; Smith, J.A.; Worthington, M.J.H.; McKinley, C.C.C.; Campbell, J.A.; Lewis, D.A.; et al. Sulfur polymer composites as controlled-release fertilisers. Org. Biomol. Chem. 2019, 17, 1929–1936. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Shearer, C.J.; Esdaile, L.J.; Campbell, J.A.; Gibson, C.T.; Legg, S.K.; Yin, Y.; Lundquist, N.A.; Gascooke, J.R.; Albuquerque, I.S.; et al. Sustainable Polysulfides for Oil Spill Remediation: Repurposing Industrial Waste for Environmental Benefit. Adv. Sustain. Syst. 2018, 2, 1800024. [Google Scholar] [CrossRef]
- Massey, L.K. Introduction. In Permeability Properties of Plastics and Elastomers, 2nd ed.; Massey, L.K., Ed.; William Andrew Publishing: Norwich, NY, USA, 2003; pp. 1–56. [Google Scholar]
- Lee, W.M. Selection of barrier materials from molecular structure. Polym. Eng. Sci. 1980, 20, 65–69. [Google Scholar] [CrossRef]
- Je, S.H.; Buyukcakir, O.; Kim, D.; Coskun, A. Direct Utilization of Elemental Sulfur in the Synthesis of Microporous Polymers for Natural Gas Sweetening. Chem 2016, 1, 482–493. [Google Scholar] [CrossRef]
- Zhou, J.; Mok, M.M.; Cowan, M.G.; McDanel, W.M.; Carlisle, T.K.; Gin, D.L.; Noble, R.D. High-permeance room-temperature ionic-liquid-based membranes for CO2/N2 separation. Ind. Eng. Chem. Res. 2014, 53, 20064–20067. [Google Scholar] [CrossRef]
- Cowan, M.G.; Masuda, M.; McDanel, W.M.; Kohno, Y.; Gin, D.L.; Noble, R.D. Phosphonium-based poly(Ionic liquid) membranes: The effect of cation alkyl chain length on light gas separation properties and Ionic conductivity. J. Membr. Sci. 2016, 498, 408–413. [Google Scholar] [CrossRef]
- Crapanzano, L. Polymorphism of Sulfur: Structural and Dynamical Aspects. Ph.D. Thesis, Université Joseph-Fourier, Grenoble, France, 2006. [Google Scholar]
- Cataldo, F. A study on the structure and properties of polymeric sulfur. Die Angew. Makromol. Chem. 1997, 249, 137–149. [Google Scholar] [CrossRef]
- Morris, B.A. 4-Commonly Used Resins and Substrates in Flexible Packaging. In The Science and Technology of Flexible Packaging; Morris, B.A., Ed.; William Andrew Publishing: Oxford, UK, 2017; pp. 69–119. [Google Scholar]
- Sebők, B.; Schülke, M.; Réti, F.; Kiss, G. Diffusivity, permeability and solubility of H2, Ar, N2, and CO2 in poly(tetrafluoroethylene) between room temperature and 180 °C. Polym. Test. 2016, 49, 66–72. [Google Scholar] [CrossRef]


| Gas | Run 1 | Run 2 | Run 3 | |||
|---|---|---|---|---|---|---|
| Barrer | 10−16 mol·m/(m3·s·Pa) | Barrer | 10−16 mol·m/(m3·s·Pa) | Barrer | 10−16 mol·m/(m3·s·Pa) | |
| C2H4 | 0.26 | 0.9 | 0.902 | 32 | 0.842 | 2.82 |
| CO2 | 0.29 | 1.0 | 0.852 | 2.82 | 0.982 | 3.32 |
| H2 | 1.72 | 4.42 | 1.62 | 5.42 | 1.32 | 4.42 |
| He | 0.55 | 1.8 | 0.50 | 1.7 | 1.562 | 5.22 |
| N2 | 0.38 | 1.3 | 0.41 | 1.4 | 0.602 | 2.02 |
| Gas | Sulfur | Cellophane | Poly(tetrafluoroethylene) | |
|---|---|---|---|---|
| Reference | Pre-Discontinuity | Post-Discontinuity | [40] | [41] |
| H2 | 0.71 | 1.6 | 0.43 | 13.27 ± 4.84 |
| He | 0.55 | 1.7 | 1.7 | |
| O2 | 0.14 | |||
| N2 | 0.38 | 0.6 | 0.21 | 1.64 ± 0.23 |
| CO2 | 0.29 | 0.9 | 0.31 | 13.0 ± 2.4 |
| C2H4 | 0.26 | 0.9 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Bennett, T.D.; Cowan, M.G. Gas Permeation of Sulfur Thin-Films and Potential as a Barrier Material. Membranes 2019, 9, 72. https://doi.org/10.3390/membranes9060072
Jia X, Bennett TD, Cowan MG. Gas Permeation of Sulfur Thin-Films and Potential as a Barrier Material. Membranes. 2019; 9(6):72. https://doi.org/10.3390/membranes9060072
Chicago/Turabian StyleJia, Xicheng, Thomas D. Bennett, and Matthew G. Cowan. 2019. "Gas Permeation of Sulfur Thin-Films and Potential as a Barrier Material" Membranes 9, no. 6: 72. https://doi.org/10.3390/membranes9060072
APA StyleJia, X., Bennett, T. D., & Cowan, M. G. (2019). Gas Permeation of Sulfur Thin-Films and Potential as a Barrier Material. Membranes, 9(6), 72. https://doi.org/10.3390/membranes9060072





