Synthesis and Characterization of a High Flux Nanocellulose–Cellulose Acetate Nanocomposite Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose Nanofiber in Organic Solvent
2.3. Preparation of Nanocomposite Membranes
2.4. Cellulose Nanofibers (CNF) Characterization
2.5. Membrane Characterization
2.6. Evaluation of the Nanocomposite Membrane Performance
3. Results and Discussion
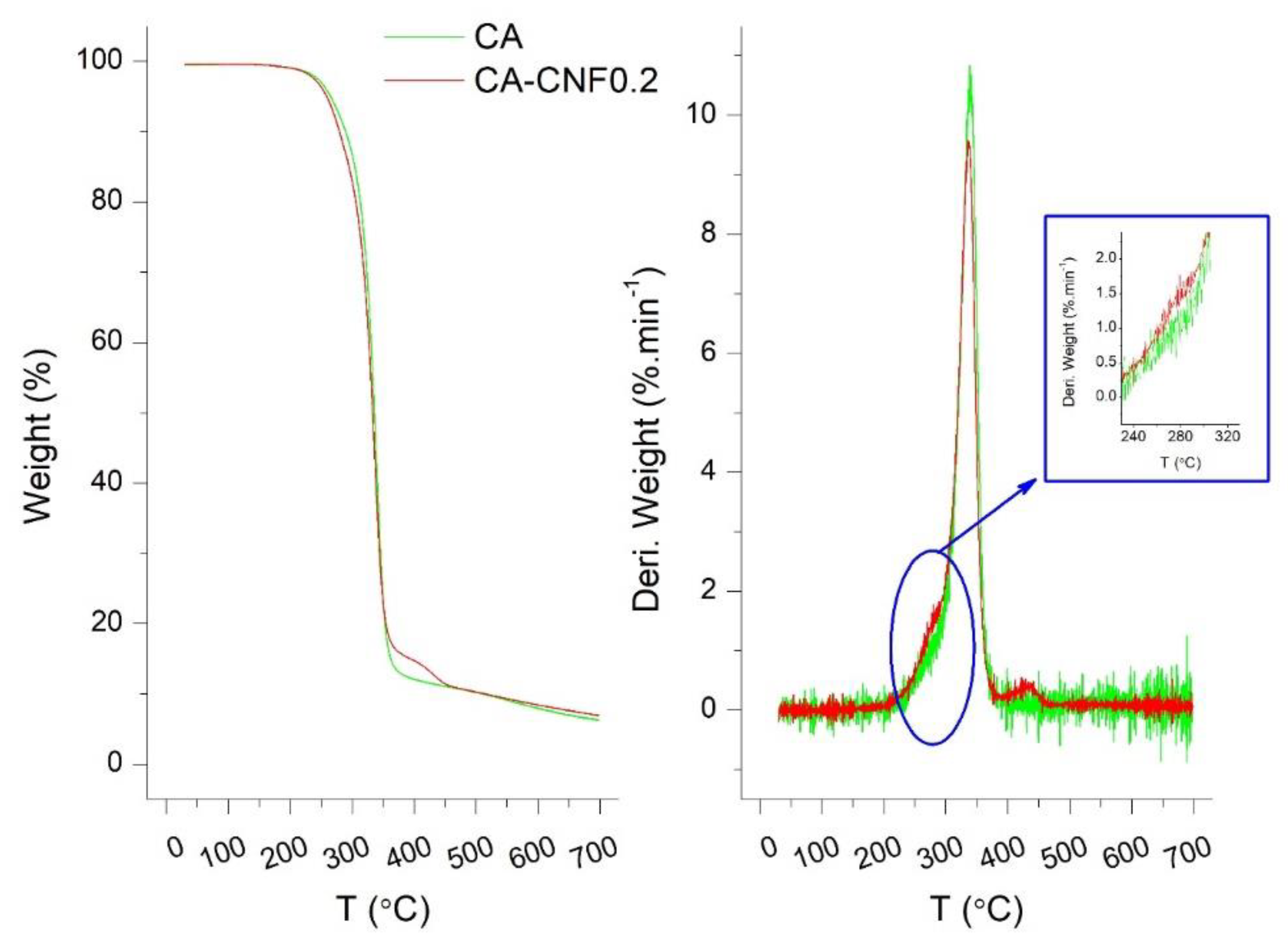
3.1. Properties of Cellulose Nanofibers
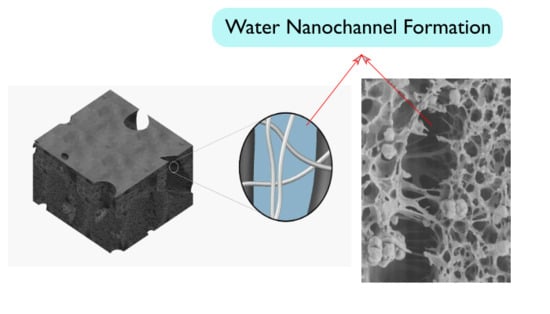
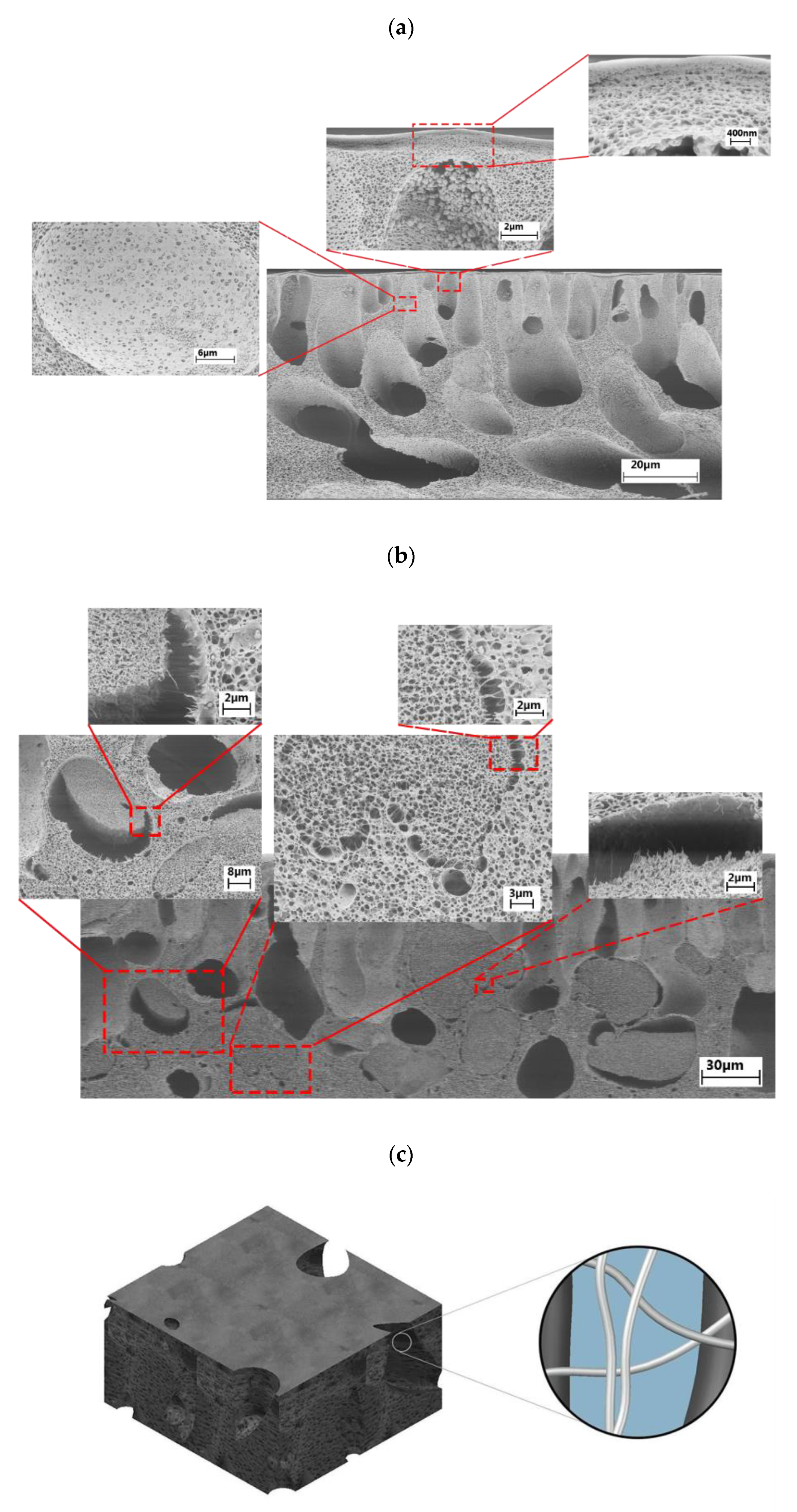
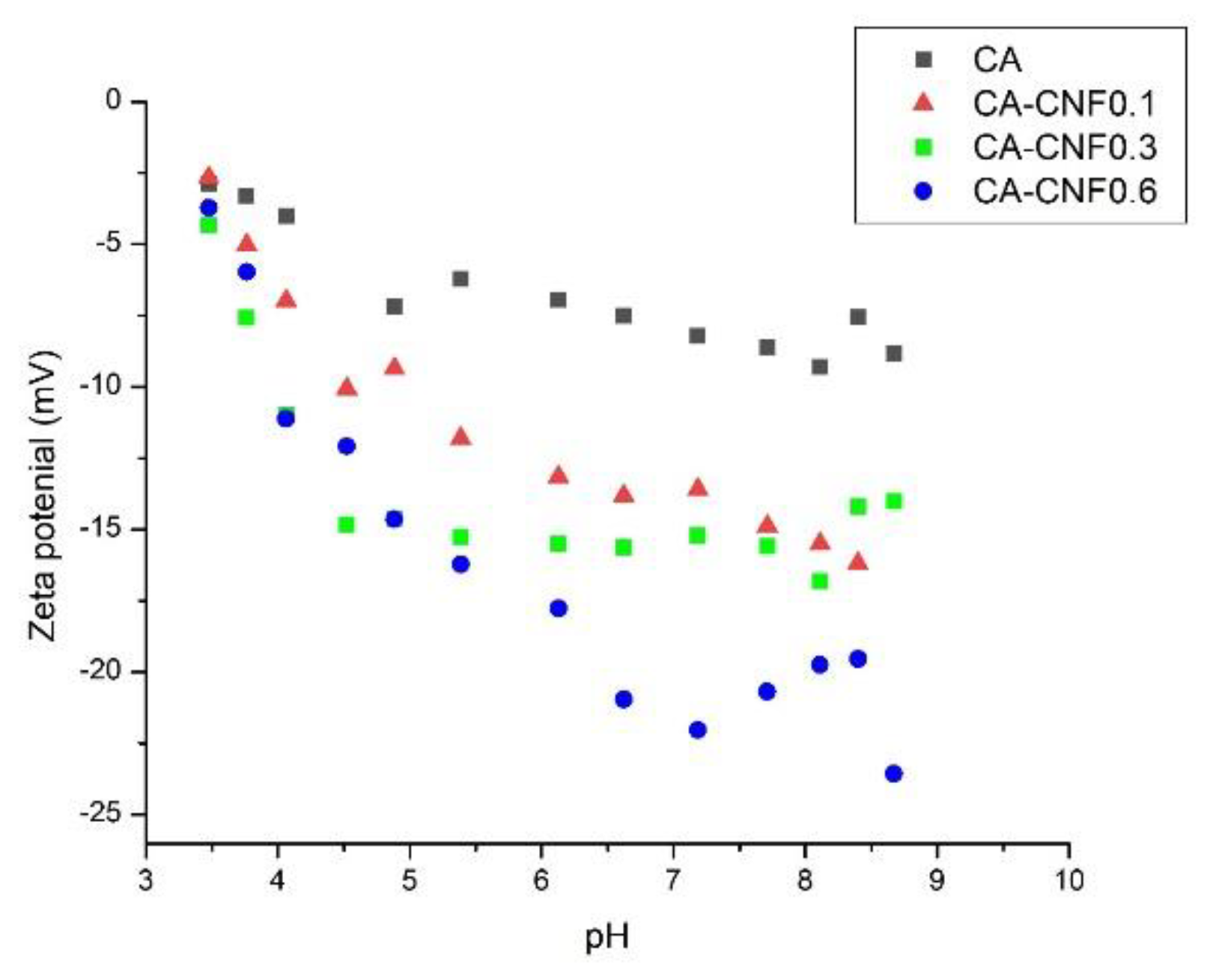
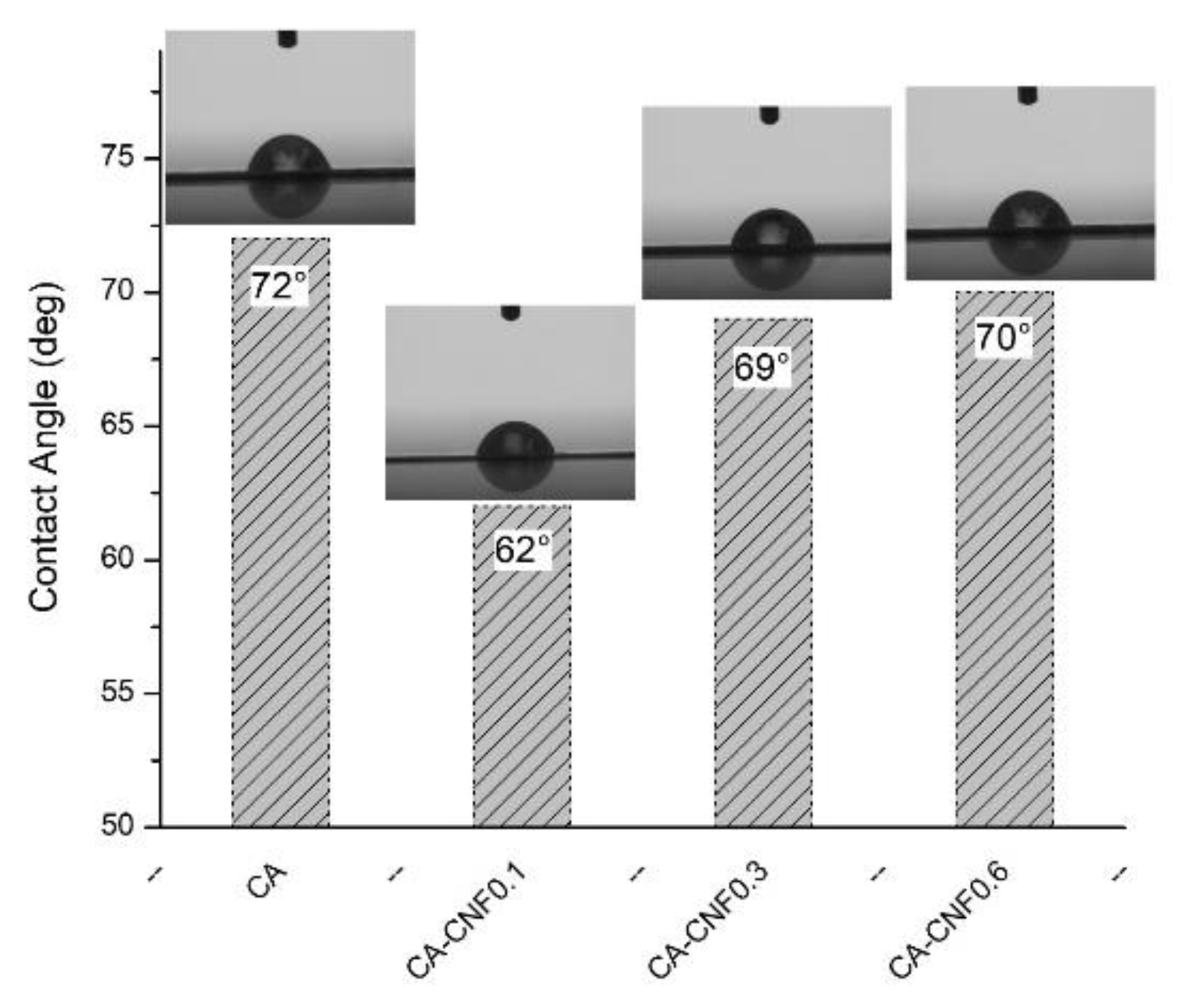
3.2. Ultrafiltration Cellulose Acetate-Cellulose Nanofiber (CA-CNF) Nanocomposite Membranes
3.3. Ultrafiltration Performance of the Nanocomposite Membranes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, W.; Liu, J.; Chu, H.; Dong, B. Pretreatment and membrane hydrophilic modification to reduce membrane fouling. Membranes 2013, 3, 226–241. [Google Scholar] [CrossRef]
- Hu, N.; Xiao, T.; Cai, X.; Ding, L.; Fu, Y.; Yang, X. Preparation and characterization of hydrophilically modified PVDF membranes by a novel nonsolvent thermally induced phase separation method. Membranes 2016, 6, 47. [Google Scholar] [CrossRef]
- Amouamouha, M.; Gholikandi, G.B. Characterization and antibiofouling performance investigation of hydrophobic silver nanocomposite membranes: A comparative study. Membranes 2017, 7, 64. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Wang, Q.; Ma, J.; Cao, J.; Hu, W.; Wu, Z. Relationship between polymers compatibility and casting solution stability in fabricating PVDF/PVA membranes. J. Membr. Sci. 2017, 537, 263–271. [Google Scholar] [CrossRef]
- Zeng, G.; Ye, Z.; He, Y.; Yang, X.; Ma, J.; Shi, H.; Feng, Z. Application of dopamine-modified halloysite nanotubes/PVDF blend membranes for direct dyes removal from wastewater. Chem. Eng. J. 2017, 323, 572–583. [Google Scholar] [CrossRef]
- Deng, B.; Yu, M.; Yang, X.; Zhang, B.; Li, L.; Xie, L.; Li, J.; Lu, X. Antifouling microfiltration membranes prepared from acrylic acid or methacrylic acid grafted poly(vinylidene fluoride) powder synthesized via pre-irradiation induced graft polymerization. J. Membr. Sci. 2010, 350, 252–258. [Google Scholar] [CrossRef]
- Yang, X.; Deng, B.; Liu, Z.; Shi, L.; Bian, X.; Yu, M.; Li, L.; Li, J.; Lu, X. Microfiltration membranes prepared from acryl amide grafted poly(vinylidene fluoride) powder and their pH sensitive behaviour. J. Membr. Sci. 2010, 362, 298–305. [Google Scholar] [CrossRef]
- Ding, X.; Hua, M.M.; Zhao, H.; Yang, P.; Chen, X.; Xin, Q.; Zhang, Y. Poly (ethylene oxide) composite membrane synthesized by UV-initiated free radical photopolymerization for CO2separation. J. Membr. Sci. 2017, 531, 129–137. [Google Scholar] [CrossRef]
- Reis, R.; Duke, M.; Merenda, A.; Winther-Jensen, B.; Puskar, L.; Tobin, M.J.; Orbell, J.D.; Dumée, L.F. Customizing the surface charge of thin-film composite membranes by surface plasma thin film polymerization. J. Membr. Sci. 2017, 537, 1–10. [Google Scholar] [CrossRef]
- Li, Q.; Lin, H.H.; Wang, X.L. Preparation of sulfobetaine-grafted PVDF hollow fiber membranes with a stably anti-protein-fouling performance. Membranes 2014, 4, 181–199. [Google Scholar] [CrossRef]
- You, X.; Ma, T.; Su, Y.; Wu, H.; Wu, M.; Cai, H.; Sun, G.; Jiang, Z. Enhancing the permeation flux and antifouling performance of polyamide nanofiltration membrane by incorporation of PEG-POSS nanoparticles. J. Membr. Sci. 2017, 540, 454–463. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Peng, K.; Na, Y.; Xiong, Y.; Liu, W.; Liu, J.; Lu, L.; Li, S. Functional magnetic nanoparticles for enhancing ultrafiltration of waste cutting emulsions by significantly increasing flux and reducing membrane fouling. J. Membr. Sci. 2019, 573, 73–84. [Google Scholar] [CrossRef]
- Kadhom, M.; Yin, J.; Deng, B. A thin film nanocomposite membrane with MCM-41 silica nanoparticles for brackish water purification. Membranes 2016, 6, 50. [Google Scholar] [CrossRef]
- Kadhom, M.; Hu, W.; Deng, B. Thin film nanocomposite membrane filled with metal-organic frameworks UiO-66 and MIL-125 nanoparticles for water desalination. Membranes 2017, 7, 31. [Google Scholar] [CrossRef]
- Wu, M.B.; Lv, Y.; Yang, H.C.; Liu, L.F.; Zhang, X.; Xu, Z.K. Thin film composite membranes combining carbon nanotube intermediate layer and microfiltration support for high nanofiltration performances. J. Membr. Sci. 2016, 515, 238–244. [Google Scholar] [CrossRef]
- Pan, F.; Wang, H.; Li, W.; Zhang, S.; Sun, J.; Yang, H.; Wang, M.; Wang, M.; Zhou, X.; Liu, X.; et al. Constructing rapid diffusion pathways in ultrapermeable hybrid membranes by hierarchical porous nanotubes. Chem. Eng. Sci. 2018, in press. [Google Scholar] [CrossRef]
- Li, M.; Brant, J.A. Synthesis of polyamide thin-film nanocomposite membranes using surface modified imogolite nanotubes. J. Membr. Sci. 2018, 563, 664–675. [Google Scholar] [CrossRef]
- Daramola, M.O.; Hlanyane, P.; Sadare, O.O.; Oluwasina, O.O.; Iyuke, S.E. Performance of carbon nanotube/polysulfone (CNT/Psf) composite membranes during Oil–water mixture separation: Effect of CNT dispersion method. Membranes 2017, 7, 14. [Google Scholar] [CrossRef]
- Altalhi, T.; Ginic-Markovic, M.; Han, N.; Clarke, S.; Losic, D. Synthesis of carbon nanotube (CNT) composite membranes. Membranes 2010, 1, 37–47. [Google Scholar] [CrossRef]
- Akbari, M.; Shariaty-Niassar, M.; Matsuura, T.; Ismail, A.F. Janus graphene oxide nanosheet: A promising additive for enhancement of polymeric membranes performance prepared via phase inversion. J. Colloid Interface Sci. 2018, 527, 10–24. [Google Scholar] [CrossRef]
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Wang, J.; Zhang, Y.; Wu, W.; Liu, J.; Van der Bruggen, B. Zwitterionic functionalized MoS2 nanosheets for a novel composite membrane with effective salt/dye separation performance. J. Membr. Sci. 2018, in press. [Google Scholar] [CrossRef]
- Holt, J.K.; Noy, A.; Huser, T.; Eaglesham, D.; Bakajin, O. Fabrication of a carbon nanotube-embedded silicon nitride membrane for studies of nanometer-scale mass transport. Nano Lett. 2004, 4, 2245–2250. [Google Scholar] [CrossRef]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast Mass Transport Through Sub–2-Nanometer Carbon Nanotubes. Science 2006, 312, 1034–1038. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Novel ultrafiltration membranes prepared from a multi-walled carbon nanotubes/polymer composite. J. Membr. Sci. 2010, 362, 374–383. [Google Scholar] [CrossRef]
- Yang, D.; Tian, D.; Xue, C.; Gao, F.; Liu, Y.; Li, H.; Bao, Y.; Liang, J.; Zhao, Z.; Qiu, J. Tuned Fabrication of the Aligned and Opened CNT Membrane with Exceptionally High Permeability and Selectivity for Bioalcohol Recovery. Nano Lett. 2018, 18, 6150–6156. [Google Scholar] [CrossRef]
- Goh, P.S.; Ng, B.C.; Ismail, A.F.; Aziz, M.; Sanip, S.M. Surfactant dispersed multi-walled carbon nanotube/polyetherimide nanocomposite membrane. Solid State Sci. 2010, 12, 2155–2162. [Google Scholar] [CrossRef]
- Shawky, H.A.; Chae, S.R.; Lin, S.; Wiesner, M.R. Synthesis and characterization of a carbon nanotube/polymer nanocomposite membrane for water treatment. Desalination 2011, 272, 46–50. [Google Scholar] [CrossRef]
- Mansourpanah, Y.; Madaeni, S.S.; Rahimpour, A.; Adeli, M.; Hashemi, M.Y.; Moradian, M.R. Fabrication new PES-based mixed matrix nanocomposite membranes using polycaprolactone modified carbon nanotubes as the additive: Property changes and morphological studies. Desalination 2011, 277, 171–177. [Google Scholar] [CrossRef]
- Carpenter, A.W.; De Lannoy, C.F.; Wiesner, M.R. Cellulose nanomaterials in water treatment technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Cho, B.U.; Won, J.M. Effect of precipitated calcium carbonate - Cellulose nanofibrils composite filler on paper properties. Carbohydr. Polym. 2016, 136, 820–825. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, Z.; Liu, L.; Wang, H. Flexible electrically conductive nanocomposite membrane based on bacterial cellulose and polyaniline. J. Phys. Chem. B 2011, 115, 8453–8457. [Google Scholar] [CrossRef]
- Sharma, P.R.; Joshi, R.; Sharma, S.K.; Hsiao, B.S. A Simple Approach to Prepare Carboxycellulose Nanofibers from Untreated Biomass. Biomacromolecules 2017, 18, 2333–2342. [Google Scholar] [CrossRef]
- Moberg, T.; Sahlin, K.; Yao, K.; Geng, S.; Westman, G.; Zhou, Q.; Oksman, K.; Rigdahl, M. Rheological properties of nanocellulose suspensions: Effects of fibril/particle dimensions and surface characteristics. Cellulose 2017, 24, 2499–2510. [Google Scholar] [CrossRef]
- Saito, T.; Kuramae, R.; Wohlert, J.; Berglund, L.A.; Isogai, A. An ultrastrong nanofibrillar biomaterial: The strength of single cellulose nanofibrils revealed via sonication-induced fragmentation. Biomacromolecules 2013, 14, 248–253. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, K.; Zhan, C.; Geng, L.; Chu, B.; Hsiao, B.S. Characterization of nanocellulose using small-angle neutron, X-ray, and dynamic light scattering techniques. J. Phys. Chem. B 2017, 121, 1340–1351. [Google Scholar] [CrossRef]
- Lin, N.; Bruzzese, C.; Dufresne, A. TEMPO-oxidized nanocellulose participating as crosslinking aid for alginate-based sponges. ACS Appl. Mater. Interfaces 2012, 4, 4948–4959. [Google Scholar] [CrossRef]
- Van Voorthuizen, E.M.; Ashbolt, N.J.; Schäfer, A.I. Role of hydrophobic and electrostatic interactions for initial enteric virus retention by MF membranes. J. Membr. Sci. 2001, 194, 69–79. [Google Scholar] [CrossRef]
- Zhang, P.; Shen, Y.; Guo, J.S.; Li, C.; Wang, H.; Chen, Y.P.; Yan, P.; Yang, J.X.; Fang, F. Extracellular protein analysis of activated sludge and their functions in wastewater treatment plant by shotgun proteomics. Sci. Rep. 2015, 5, 12041. [Google Scholar] [CrossRef] [PubMed]
- Rytwo, G.; Lavi, R.; König, T.N.; Avidan, L. Direct Relationship Between Electrokinetic Surface-charge Measurement of Effluents and Coagulant Type and Dose. Colloids Interface Sci. Commun. 2014, 1, 27–30. [Google Scholar] [CrossRef][Green Version]
- Smolders, C.A.; Reuvers, A.J.; Boom, R.M.; Wienk, I.M. Microstructures in phase-inversion membranes. Part 1. Formation of macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Idris, A.; Man, Z.; Maulud, A.S.; Khan, M.S. Effects of phase separation behavior on morphology and performance of polycarbonate membranes. Membranes 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.A.; Sefain, M.Z.; El-Kalyoubi, S.F. Thermal behaviour of cellulose acetate. Thermochim. Acta 1989, 150, 33–38. [Google Scholar] [CrossRef]
- Kamal, H.; Abd-Elrahim, F.M.; Lotfy, S. Characterization and some properties of cellulose acetate-co-polyethylene oxide blends prepared by the use of gamma irradiation. J. Radiat. Res. Appl. Sci. 2014, 7, 146–153. [Google Scholar] [CrossRef]
- Kannan, R.; Kakade, B.A.; Pillai, V.K. Polymer electrolyte fuel cells using nafion-based composite membranes with functionalized carbon nanotubes. Angew. Chem. Int. Ed. 2008, 47, 2653–2656. [Google Scholar] [CrossRef]
- Malm, C.J.; Tanghe, L.J. Chemical Reactions in the Making of Cellulose Acetate. Ind. Eng. Chem. 1955, 47, 995–999. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, X.; Huang, X.; Waite, T.D.; Wen, X. Combined effect of membrane and foulant hydrophobicity and surface charge on adsorptive fouling during microfiltration. J. Membr. Sci. 2011, 373, 140–151. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 1138–1148. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Zheng, J.; Hadi, P.; Yang, M.; Huang, X.; Ma, H.; Walker, H.W.; Hsiao, B.S. Synthesis and Characterization of a High Flux Nanocellulose–Cellulose Acetate Nanocomposite Membrane. Membranes 2019, 9, 70. https://doi.org/10.3390/membranes9060070
Li N, Zheng J, Hadi P, Yang M, Huang X, Ma H, Walker HW, Hsiao BS. Synthesis and Characterization of a High Flux Nanocellulose–Cellulose Acetate Nanocomposite Membrane. Membranes. 2019; 9(6):70. https://doi.org/10.3390/membranes9060070
Chicago/Turabian StyleLi, Nancy, Jackie Zheng, Pejman Hadi, Mengying Yang, Xiangyu Huang, Hongyang Ma, Harold W. Walker, and Benjamin S. Hsiao. 2019. "Synthesis and Characterization of a High Flux Nanocellulose–Cellulose Acetate Nanocomposite Membrane" Membranes 9, no. 6: 70. https://doi.org/10.3390/membranes9060070
APA StyleLi, N., Zheng, J., Hadi, P., Yang, M., Huang, X., Ma, H., Walker, H. W., & Hsiao, B. S. (2019). Synthesis and Characterization of a High Flux Nanocellulose–Cellulose Acetate Nanocomposite Membrane. Membranes, 9(6), 70. https://doi.org/10.3390/membranes9060070