New Insight to the Effects of Heat Treatment in Air on the Permeation Properties of Thin Pd77%Ag23% Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Preparation
2.2. Hydrogen Stabilization/Permeation
2.3. Heat Treatment in Air (HTA)
2.4. Hydrogen Solubility
2.5. AFM Imaging
3. Results and Discussion
3.1. Permeability
3.2. Hydrogen Solubility and Diffusivity
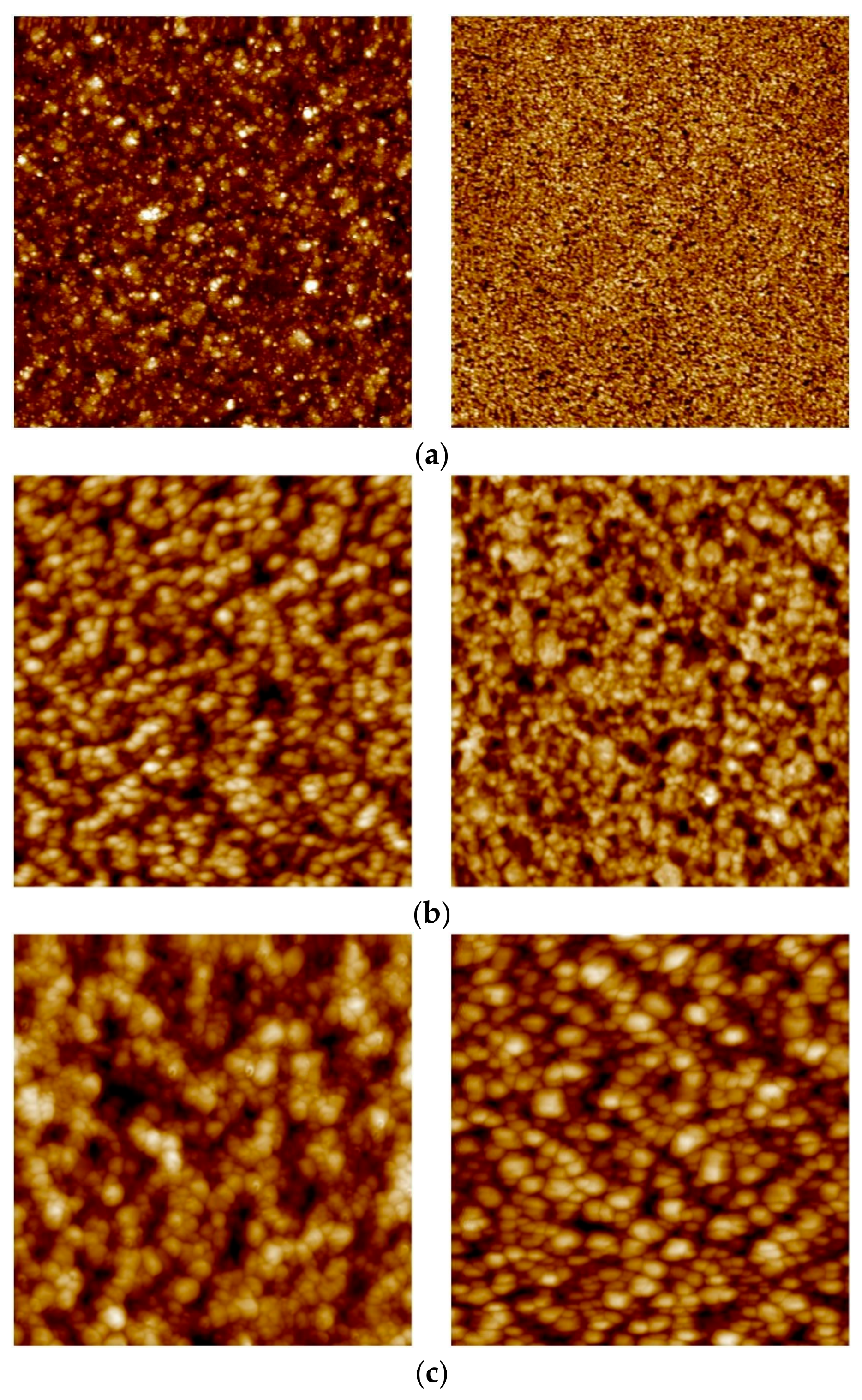
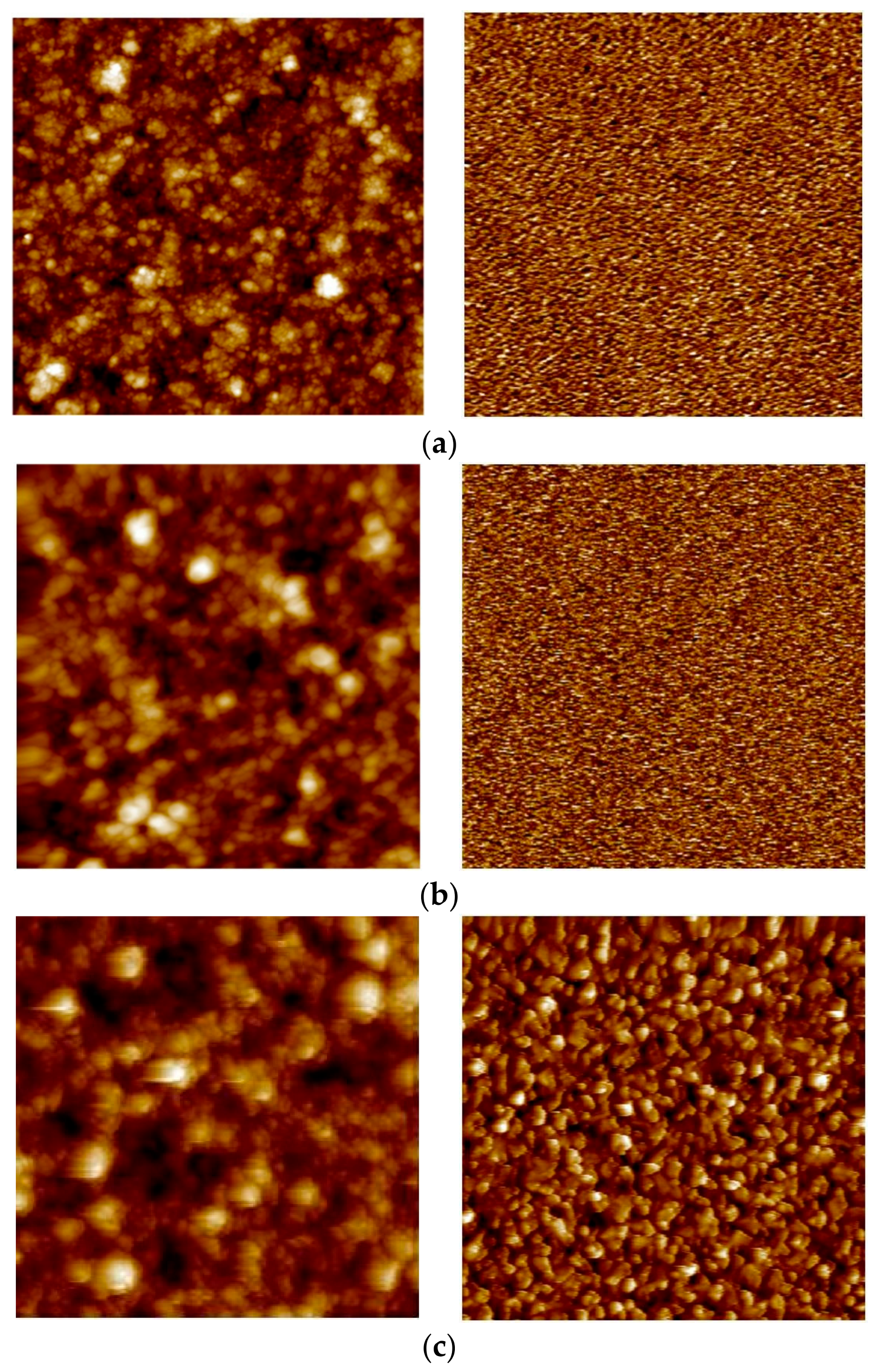
3.3. Surface Topography
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paglieri, S.N.; Way, J.D. Innovations in palladium membrane research. Sep. Purif. Methods 2002, 31, 1–169. [Google Scholar] [CrossRef]
- Bredesen, R.; Peters, T.A.; Stange, M.; Vicinanza, N.; Venvik, H.J. Chapter 11 Palladium-based Membranes in Hydrogen Production. In Membrane Engineering for the Treatment of Gases: Volume 2: Gas-Separation Problems Combined with Membrane Reactors; The Royal Society of Chemistry: Cambridge, UK, 2011; pp. 40–86. [Google Scholar]
- Grashoff, G.J.; Pilkington, C.E.; Corti, C.W. The purification of hydrogen. A review of the technology emphasizing the current status of palladium membrane diffusion. Platinum. Met. Rev. 1983, 27, 157–169. [Google Scholar]
- Kibria, A.K.M.F.; Sakamoto, Y. The effect of alloying of palladium with silver and rhodium on the hydrogen solubility, miscibility gap and hysteresis. Int. J. Hydrogen Energy 2000, 25, 853–859. [Google Scholar] [CrossRef]
- Itoh, N.; Xu, W.C. Selective Hydrogenation of Phenol to Cyclohexanone Using Palladium-Based Membranes as Catalysts. Appl. Catal. A Gen. 1993, 107, 83–100. [Google Scholar] [CrossRef]
- Uemiya, S.; Matsuda, T.; Kikuchi, E. Hydrogen Permeable Palladium Silver Alloy Membrane Supported on Porous Ceramics. J. Membr. Sci. 1991, 56, 315–325. [Google Scholar] [CrossRef]
- Fort, D.; Farr, J.P.G.; Harris, I.R. A comparison of palladium-silver and palladium-yttrium alloys as hydrogen separation membranes. J. Less Common Met. 1975, 39, 293–308. [Google Scholar] [CrossRef]
- Keuler, J.N.; Lorenzen, L. Developing a heating procedure to optimise hydrogen permeance through Pd–Ag membranes of thickness less than 2.2 μm. J. Membr. Sci. 2002, 195, 203–213. [Google Scholar] [CrossRef]
- Mejdell, A.L.; Chen, D.; Peters, T.A.; Bredesen, R.; Venvik, H.J. The effect of heat treatment in air on CO inhibition of a ~3 μm Pd–Ag (23 wt.%) membrane. J. Membr. Sci. 2010, 350, 371–377. [Google Scholar] [CrossRef]
- Mejdell, A.L.; Klette, H.; Ramachandran, A.; Borg, A.; Bredesen, R. Hydrogen permeation of thin, free-standing Pd/Ag23% membranes before and after heat treatment in air. J. Membr. Sci. 2008, 307, 96–104. [Google Scholar] [CrossRef]
- Peters, T.A.; Stange, M.; Bredesen, R. On the high pressure performance of thin supported Pd–23%Ag membranes—Evidence of ultrahigh hydrogen flux after air treatment. J. Membr. Sci. 2011, 378, 28–34. [Google Scholar] [CrossRef]
- Pizzi, D.; Worth, R.; Baschetti, M.G.; Sarti, G.C.; Noda, K.-I. Hydrogen permeability of 2.5 μm palladium–silver membranes deposited on ceramic supports. J. Membr. Sci. 2008, 325, 446–453. [Google Scholar] [CrossRef]
- Ramachandran, A.; Tucho, W.M.; Mejdell, A.L.; Stange, M.; Venvik, H.J.; Walmsley, J.C.; Holmestad, R.; Bredesen, R.; Borg, A. Surface characterization of Pd/Ag23 wt.% membranes after different thermal treatments. Appl. Surf. Sci. 2010, 256, 6121–6132. [Google Scholar] [CrossRef]
- Roa, F.; Way, J.D. The effect of air exposure on palladium–copper composite membranes. Appl. Surf. Sci. 2005, 240, 85–104. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Z.; Gao, X.; Guo, Y.; Wang, B.; Sakai, O.; Sakai, H.; Takahashi, T. Changes in hydrogen permeability and surface state of Pd–Ag/ceramic composite membranes after thermal treatment. J. Membr. Sci. 2005, 252, 145–154. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Z.; Yao, B.; Gao, X.; Sakai, H.; Takahashi, T. Hydrogen permeance and surface states of Pd-Ag/ceramic composite membranes. AIChE J. 2006, 52, 2783–2791. [Google Scholar] [CrossRef]
- Zhang, K.; Gade, S.K.; Hatlevik, Ø.; Way, J.D. A sorption rate hypothesis for the increase in H2 permeability of palladium-silver (Pd–Ag) membranes caused by air oxidation. Int. J. Hydrogen Energy 2012, 37, 583–593. [Google Scholar] [CrossRef]
- Zhang, K.; Gade, S.K.; Way, J.D. Effects of heat treatment in air on hydrogen sorption over Pd–Ag and Pd–Au membrane surfaces. J. Membr. Sci. 2012, 403–404, 78–83. [Google Scholar] [CrossRef]
- Tucho, W.M.; Venvik, H.J.; Walmsley, J.C.; Stange, M.; Ramachandran, A.; Mathiesen, R.H.; Borg, A.; Bredesen, R.; Holmestad, R. Microstructural studies of self-supported (1.5–10 μm) Pd/23 wt.%Ag hydrogen separation membranes subjected to different heat treatments. J. Mater. Sci. 2009, 44, 4429–4442. [Google Scholar] [CrossRef]
- Ali, J.K.; Newson, E.J.; Rippin, D.W.T. Deactivation and regeneration of Pd-Ag membranes for dehydrogenation reactions. J. Membr. Sci. 1994, 89, 171–184. [Google Scholar] [CrossRef]
- Peters, T.A.; Kaleta, T.; Stange, M.; Bredesen, R. Hydrogen transport through a selection of thin Pd-alloy membranes: Membrane stability, H2S inhibition, and flux recovery in hydrogen and simulated WGS mixtures. Catal. Today 2012, 193, 8–19. [Google Scholar] [CrossRef]
- Hideo, U.; Tsugio, O.; Kazutaka, S. New Technique of Activating Palladium Surface for Absorption of Hydrogen or Deuterium. Jpn. J. Appl. Phys. 1993, 32, 5095. [Google Scholar]
- Wang, D.; Clewley, J.D.; Flanagan, T.B.; Balasubramaniam, R.; Shanahan, K.L. Enhanced rates of hydrogen absorption resulting from oxidation of Pd or internal oxidation of Pd-Al alloys. J. Alloys Compd. 2000, 298, 261–273. [Google Scholar] [CrossRef]
- Bryden, K.J.; Ying, J.Y. Nanostructured palladium–iron membranes for hydrogen separation and membrane hydrogenation reactions. J. Membr. Sci. 2002, 203, 29–42. [Google Scholar] [CrossRef]
- Natter, H.; Wettmann, B.; Heisel, B.; Hempelmann, R. Hydrogen in nanocrystalline palladium. J. Alloys Compd. 1997, 253, 84–86. [Google Scholar] [CrossRef]
- Stuhr, U.; Striffler, T.; Wipf, H.; Natter, H.; Wettmann, B.; Janssen, S.; Hempelmann, R.; Hahn, H. An investigation of hydrogen diffusion in nanocrystalline Pd by neutron spectroscopy. J. Alloys Compd. 1997, 253–254, 393–396. [Google Scholar] [CrossRef]
- McCool, B.A.; Lin, Y.S. Nanostructured thin palladium-silver membranes: Effects of grain size on gas permeation properties. J. Mater. Sci. 2001, 36, 3221–3227. [Google Scholar] [CrossRef]
- Okazaki, J.; Ikeda, T.; Tanaka, D.A.P.; Suzuki, T.M.; Mizukami, F. In situ high-temperature X-ray diffraction study of thin palladium/α-alumina composite membranes and their hydrogen permeation properties. J. Membr. Sci. 2009, 335, 126–132. [Google Scholar] [CrossRef]
- Vicinanza, N.; Svenum, I.-H.; Næss, L.N.; Peters, T.A.; Bredesen, R.; Borg, A.; Venvik, H.J. Thickness dependent effects of solubility and surface phenomena on the hydrogen transport properties of sputtered Pd77%Ag23% thin film membranes. J. Membr. Sci. 2015, 476, 602–608. [Google Scholar] [CrossRef]
- Løvvik, O.M.; Opalka, S.M. Reversed surface segregation in palladium-silver alloys due to hydrogen adsorption. Surf. Sci. 2008, 602, 2840–2844. [Google Scholar] [CrossRef]
- Shu, J.; Bongondo, B.E.W.; Grandjean, B.P.A.; Adnot, A.; Kaliaguine, S. Surface segregation of PdAg membranes upon hydrogen permeation. Surf. Sci. 1993, 291, 129–138. [Google Scholar] [CrossRef]
- Svenum, I.H.; Herron, J.A.; Mavrikakis, M.; Venvik, H.J. Adsorbate-induced segregation in a PdAg membrane model system: Pd3Ag(1 1 1). Catal. Today 2012, 193, 111–119. [Google Scholar] [CrossRef]
- Bredesen, R.; Klette, H. Method of Manufacturing Thin Metal Membranes. U.S. Patent 6,086,729, 11 July 2000. [Google Scholar]
- Klette, H.; Bredesen, R. Sputtering of very thin palladium-alloy hydrogen separation membranes. Membr. Technol. 2005, 2005, 7–9. [Google Scholar] [CrossRef]
- Mejdell, A.L.; Jondahl, M.; Peters, T.A.; Bredesen, R.; Venvik, H.J. Experimental investigation of a microchannel membrane configuration with a 1.4 μm Pd/Ag 23 wt.% membrane—Effects of flow and pressure. J. Membr. Sci. 2009, 327, 6–10. [Google Scholar] [CrossRef]
- Collins, J.P.; Way, J.D. Preparation and characterization of a composite palladium-ceramic membrane. Ind. Eng. Chem. Res. 1993, 32, 3006–3013. [Google Scholar] [CrossRef]
- Hurlbert, R.C.; Konecny, J.O. Diffusion of hydrogen through palladium. J. Chem. Phys. 1961, 34, 655–658. [Google Scholar] [CrossRef]
- Keurentjes, J.T.F.; Gielens, F.C.; Tong, H.D.; van Rijn, C.J.M.; Vorstman, M.A.G. High-Flux Palladium Membranes Based on Microsystem Technology. Ind. Eng. Chem. Res. 2004, 43, 4768–4772. [Google Scholar] [CrossRef]
- Moss, T.S.; Peachey, N.M.; Snow, R.C.; Dye, R.C. Multilayer metal membranes for hydrogen separation. Int. J. Hydrogen Energy 1998, 23, 99–106. [Google Scholar] [CrossRef]
- Nam, S.-E.; Lee, S.-H.; Lee, K.-H. Preparation of a palladium alloy composite membrane supported in a porous stainless steel by vacuum electrodeposition. J. Membr. Sci. 1999, 153, 163–173. [Google Scholar] [CrossRef]
- Ward, T.L.; Dao, T. Model of hydrogen permeation behavior in palladium membranes. J. Membr. Sci. 1999, 153, 211–231. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Höllein, V.; Daub, K. Membrane reactors for hydrogenation and dehydrogenation processes based on supported palladium. J. Mol. Catal. A Chem. 2001, 173, 135–184. [Google Scholar] [CrossRef]
- Uemiya, S. State-of-the-art of supported metal membranes for gas separation. Sep. Purif. Methods 1999, 28, 51–85. [Google Scholar] [CrossRef]
- Wu, L.-Q.; Xu, N.; Shi, J. Novel method for preparing palladium membranes by photocatalytic deposition. AIChE J. 2000, 46, 1075–1083. [Google Scholar] [CrossRef]
- Mekonnen, W.; Arstad, B.; Klette, H.; Walmsley, J.C.; Bredesen, R.; Venvik, H.; Holmestad, R. Microstructural characterization of self-supported 1.6 μm Pd/Ag membranes. J. Membr. Sci. 2008, 310, 337–348. [Google Scholar] [CrossRef]
- Peters, T.A.; Tucho, W.M.; Ramachandran, A.; Stange, M.; Walmsley, J.C.; Holmestad, R.; Borg, A.; Bredesen, R. Thin Pd–23%Ag/stainless steel composite membranes: Long-term stability, life-time estimation and post-process characterization. J. Membr. Sci. 2009, 326, 572–581. [Google Scholar] [CrossRef]
- Padama, A.A.B.; Kasai, H.; Budhi, Y.W.; Arboleda, N.B. Ab initio Investigation of Hydrogen Atom Adsorption and Absorption on Pd(110) Surface. J. Phys. Soc. Jpn. 2012, 81, 114705. [Google Scholar] [CrossRef]
- Fernandes, V.R.; Bossche, M.V.d.; Knudsen, J.; Farstad, M.H.; Gustafson, J.; Venvik, H.J.; Gronbeck, H.; Borg, A. Reversed Hysteresis during CO Oxidation over Pd75Ag25(100). ACS Catal. 2016, 6, 4154–4161. [Google Scholar] [CrossRef]
- Arstad, B.; Venvik, H.; Klette, H.; Walmsley, J.C.; Tucho, W.M.; Holmestad, R.; Holmen, A.; Bredesen, R. Studies of self-supported 1.6 mu m Pd/23 wt.% Ag membranes during and after hydrogen production in a catalytic membrane reactor. Catal. Today 2006, 118, 63–72. [Google Scholar] [CrossRef]
- Sieverts, A. Absorption of gases by metals. Z. Metallkd. 1929, 21, 37–46. [Google Scholar]
- Hughes, D.T.; Harris, I.R. A comparative study of hydrogen permeabilities and solubilities in some palladium solid solution alloys. J. Less Common Met. 1978, 61, P9–P21. [Google Scholar] [CrossRef]
- Picard, C.; Kleppa, O.J.; Boureau, G. High-temperature thermodynamics of the solutions of hydrogen in palladium-silver alloys. J. Chem. Phys. 1979, 70, 2710–2719. [Google Scholar] [CrossRef]
- Bhargav, A.; Jackson, G.S. Thermokinetic modeling and parameter estimation for hydrogen permeation through Pd0.77Ag0.23 membranes. Int. J. Hydrogen Energy 2009, 34, 5164–5173. [Google Scholar] [CrossRef]
- Fort, D.; Harris, I.R. Physical properties of some palladium alloy hydrogen diffusion membrane materials. J. Less Common Met. 1975, 41, 313–327. [Google Scholar] [CrossRef]
- Voelkl, J.; Alefeld, G. Diffusion of hydrogen in metals. Top. Appl. Phys. 1978, 28, 321–348. [Google Scholar]
- Holleck, G.L. Diffusion and solubility of hydrogen in palladium and palladium—Silver alloys. J. Phys. Chem. 1970, 74, 503–511. [Google Scholar] [CrossRef]
- Tucho, W.M.; Venvik, H.J.; Stange, M.; Walmsley, J.C.; Holmestad, R.; Bredesen, R. Effects of thermal activation on hydrogen permeation properties of thin, self-supported Pd/Ag membranes. Sep. Purif. Technol. 2009, 68, 403–410. [Google Scholar] [CrossRef]
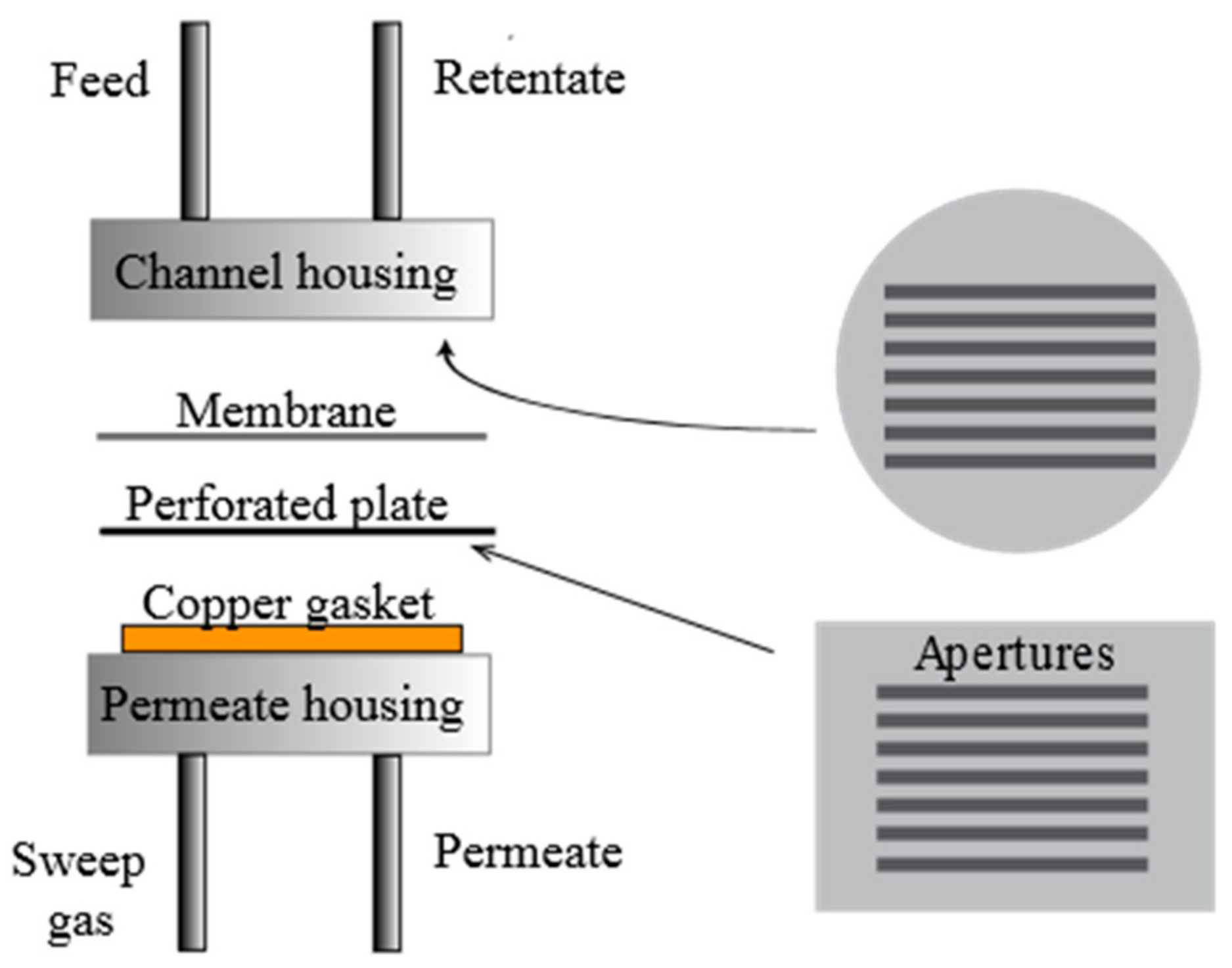
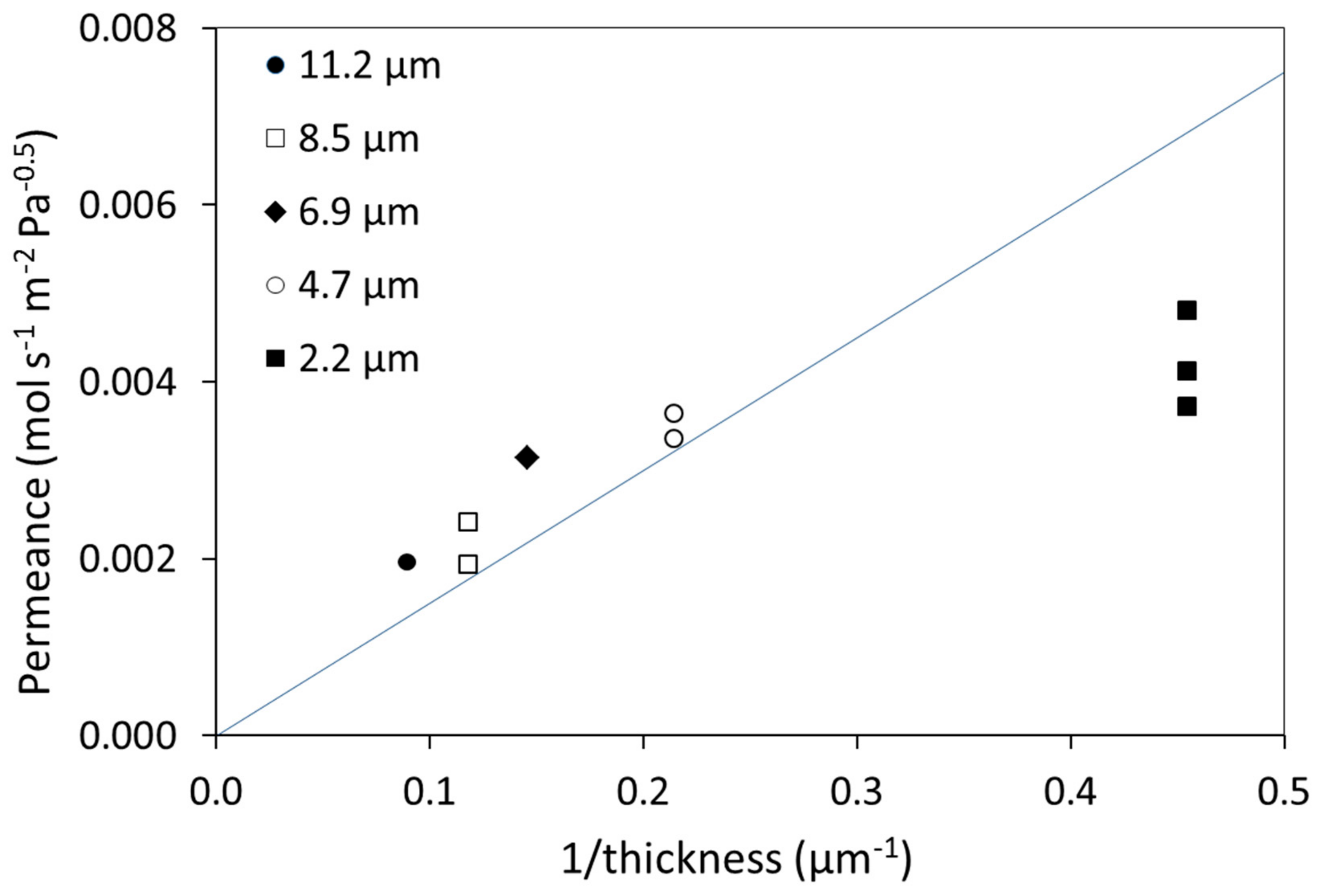
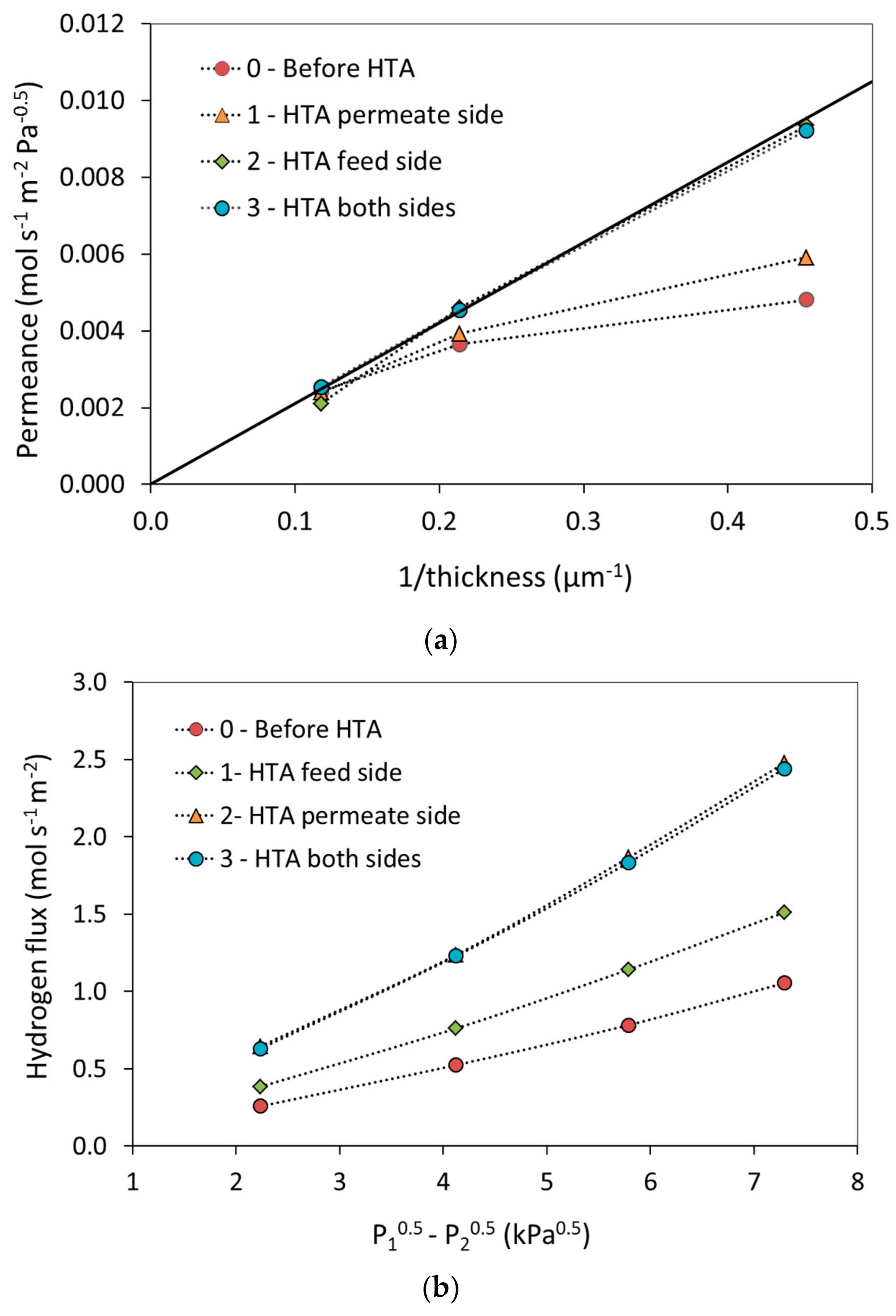
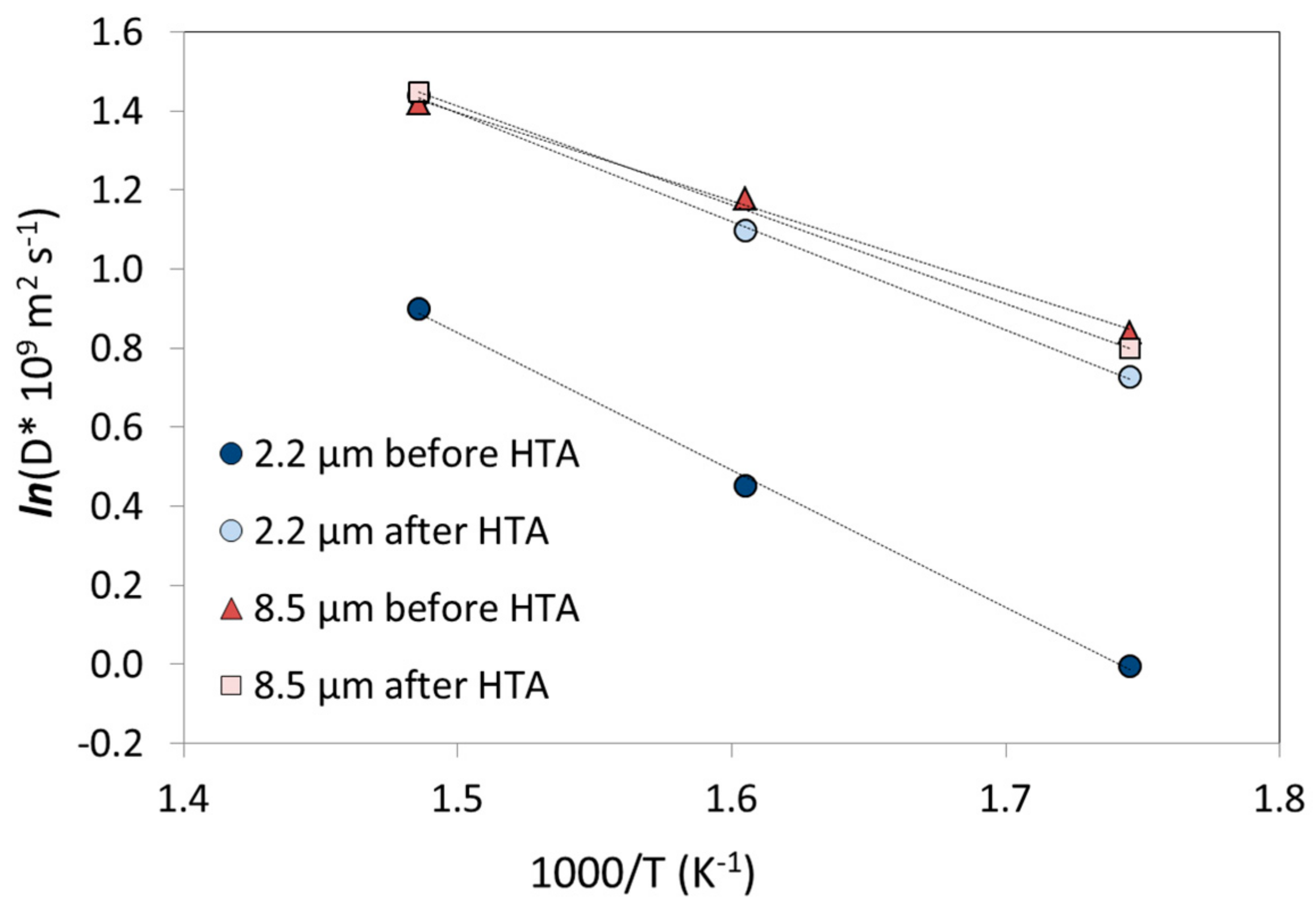
| T (°C) | Permeability 108 (mol·m·m−2·s−1·Pa−0.5) | |||||||
|---|---|---|---|---|---|---|---|---|
| Main HTA Sequence | HTA2 Sequence | |||||||
| Before | Feed | Perm | Both | Before | Feed | Perm | Both | |
| 300 | 1.1 | 1.3 | 2.1 | 2.0 | 0.9 | 1.3 | 2.2 | 2.1 |
| 350 | 1.2 | 1.5 | 2.3 | 2.3 | 1.1 | 1.6 | 2.4 | 2.3 |
| 400 | 1.5 | 1.8 | 2.5 | 2.5 | 1.5 | 2.1 | 2.7 | 2.7 |
| Sample Thickness (µm) | Temperature (°C) | Sieverts’ Constant (µmol/g·Pa0.5) | |
|---|---|---|---|
| Before HTA | After HTA | ||
| 8.5 | 300 | 0.77 | 0.79 |
| 350 | 0.53 | 0.63 | |
| 400 | 0.44 | 0.45 | |
| 2.2 | 300 | 0.82 | 0.81 |
| 350 | 0.57 | 0.60 | |
| 400 | 0.47 | 0.47 | |
| Sample Thickness (µm) | Before HTA | After HTA | ||
|---|---|---|---|---|
| D0 (m2/s) | Ea (kJ/mol) | D0 (m2/s) | Ea (kJ/mol) | |
| 2.2 | 4.9 × 10−7 | 29 | 3.6 × 10−7 | 24 |
| 8.5 | 1.5 × 10−7 | 20 | 1.7 × 10−7 | 20 |
| Membrane Thickness (µm) | Roughness (nm) | |||||
|---|---|---|---|---|---|---|
| As-Grown | Hydrogen Stabilization | HTA | ||||
| Growth/Feed | Substrate/Permeate | Growth/Feed | Substrate/Permeate | Growth/Feed | Substrate/Permeate | |
| 2.2 | 8.4 ± 0.3 | 0.29 ± 0.02 | 13.8 ± 0.8 | 12.3 ± 0.6 | 20.3 ± 1.3 | 11.6 ± 0.6 |
| 4.7 | 10.7 ± 0.6 | 0.19 ± 0.01 | 18.2 ± 0.7 | 5.0 ± 0.4 | 24.6 ± 1.6 | 9.0 ± 0.2 |
| 6.9 | 11.8 ± 1.6 | 0.38 ± 0.04 | 12.0 ± 0.7 | 3.8 ± 0.6 | - | - |
| 8.5 | 10.2 ± 0.6 | 0.40 ± 0.03 | 24.0 ± 1.2 | 1.0 ± 0.07 | 26.9 ± 2.8 | 14.3 ± 0.6 |
| 11.2 | 13.2 ± 2.3 | 0.21 ± 0.01 | 20.2 ± 1.5 | 1.5 ± 0.3 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicinanza, N.; Svenum, I.-H.; Peters, T.; Bredesen, R.; Venvik, H. New Insight to the Effects of Heat Treatment in Air on the Permeation Properties of Thin Pd77%Ag23% Membranes. Membranes 2018, 8, 92. https://doi.org/10.3390/membranes8040092
Vicinanza N, Svenum I-H, Peters T, Bredesen R, Venvik H. New Insight to the Effects of Heat Treatment in Air on the Permeation Properties of Thin Pd77%Ag23% Membranes. Membranes. 2018; 8(4):92. https://doi.org/10.3390/membranes8040092
Chicago/Turabian StyleVicinanza, Nicla, Ingeborg-Helene Svenum, Thijs Peters, Rune Bredesen, and Hilde Venvik. 2018. "New Insight to the Effects of Heat Treatment in Air on the Permeation Properties of Thin Pd77%Ag23% Membranes" Membranes 8, no. 4: 92. https://doi.org/10.3390/membranes8040092
APA StyleVicinanza, N., Svenum, I.-H., Peters, T., Bredesen, R., & Venvik, H. (2018). New Insight to the Effects of Heat Treatment in Air on the Permeation Properties of Thin Pd77%Ag23% Membranes. Membranes, 8(4), 92. https://doi.org/10.3390/membranes8040092






