A Contribution for the Valorisation of Sheep and Goat Cheese Whey through Nanofiltration
Abstract
1. Introduction
- (1)
- Evaluate the performance of nanofiltration (applied to the permeates of ultrafiltration from sheep and goat cheese whey) for recovering lactose and reducing their final organic load;
- (2)
- Apply mass transfer models to the experimental results for identifying the main causes affecting the performance of the process.
2. Mass Transfer Models for Nanofiltration of the Permeates of Ultrafiltration
3. Materials and Methods
3.1. Sampling and Storage of Samples
3.2. Pretreatment of the Samples
3.2.1. Samples of Sheep Cheese Whey
3.2.2. Samples of Goat Cheese Whey
3.3. Physicochemical Characterisation of Samples
3.4. Nanofiltration Experiments
3.4.1. Membrane Characterisation
3.4.2. Nanofiltration Experiments in Total Recirculation Mode
3.4.3. Nanofiltration Experiments in Concentration Mode
3.4.4. Cleaning and Disinfection Cycle
4. Results and Discussion
4.1. Physicochemical Characterisation of Sheep and Goat Cheese Whey
4.2. Characterisation of Nanofiltration Membranes
4.3. Total Recirculation Mode Experiments
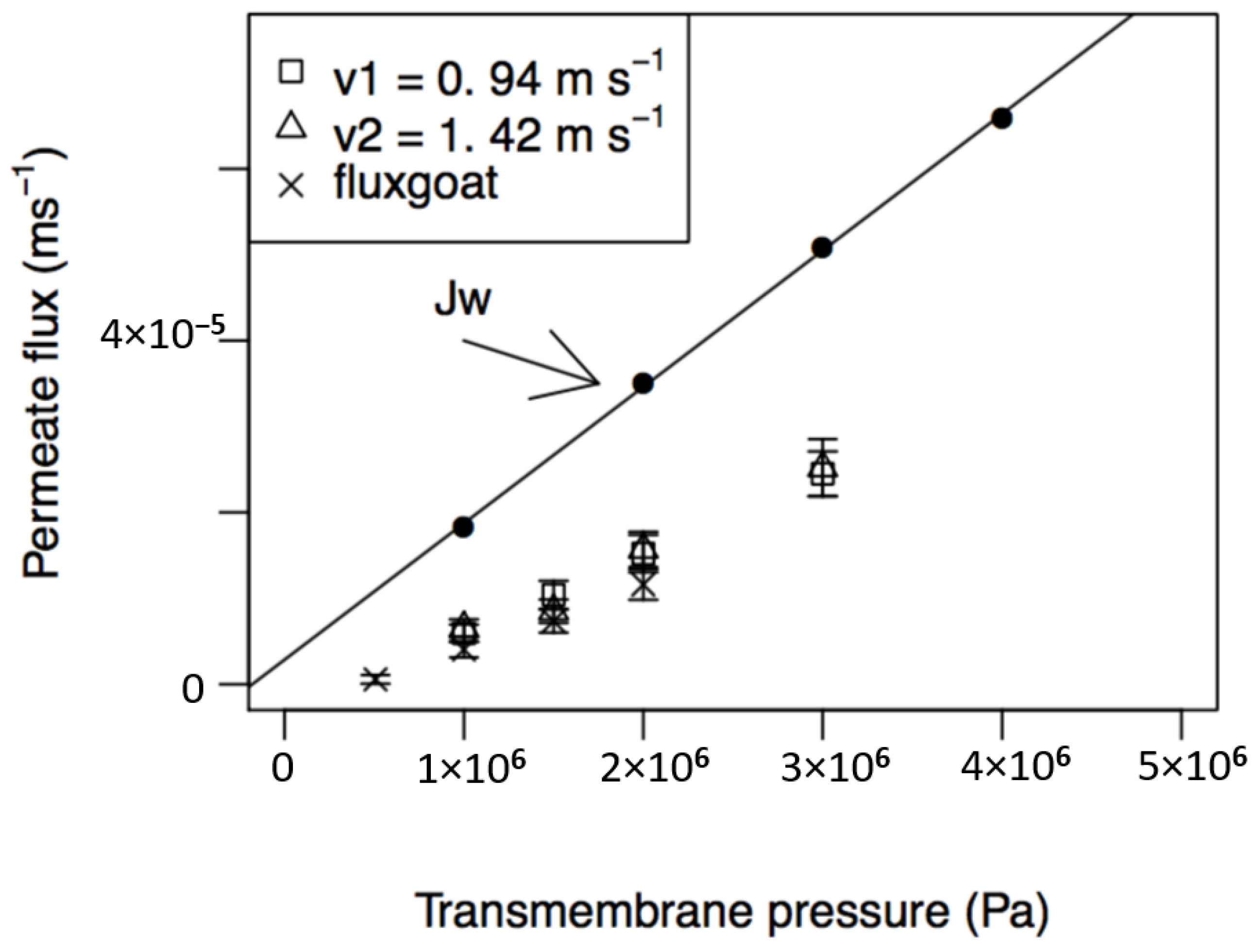
4.3.1. Influence of Transmembrane Pressure and Feed Circulation Velocity on Permeate Fluxes
4.3.2. Influence of Transmembrane Pressure and Feed Circulation Velocity on Apparent Rejection Coefficients
4.4. Concentration Mode Experiments
4.4.1. Nanofiltration of Permeates from Sheep Cheese Whey
4.4.2. Nanofiltration of Permeates from Goat Cheese Whey
4.5. Application of a Mass Transfer Model to the Permeates of Nanofiltration of PUF-S
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mollea, C.; Marmo, L.; Bosco, F. Valorisation of cheese whey, a by-product from the dairy industry. In Food Industry; InTech: London, UK, 2013; pp. 549–588. [Google Scholar]
- Guimarães, P.M.R.; Teixeira, J.A.; Domingues, L. Fermentation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorisation of cheese whey. Biotechnol. Adv. 2010, 28, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Baldasso, C.; Barros, T.C.; Tessaro, I.C. Concentration and purification of whey proteins by ultrafiltration. Desalination 2011, 278, 381–386. [Google Scholar] [CrossRef]
- Bordenave-Juchereau, S.; Almeida, B.; Piot, J.-M.; Sannier, F. Effect of protein concentration, pH, lactose content and pasteurization on thermal gelation of acid caprine whey protein concentrates. J. Dairy Res. 2005, 72, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Pulina, G.; Milàn Sendra, M.J.; Lavin, M.P.; Theodoridis, A.; Morin, E.; Capote, J. New opportunities for dairy sheep and goats (oral presentation). In Small Ruminant Symposium, Proceedings of the American Dairy Science Association (ADSA) Animal Meeting, Pitsburgh, PA, USA, 25–28 June 2017; University Autonoma of Barcelona: Bellaterra, Spain, 2017. [Google Scholar]
- Hernández-Ledesma, B.; Ramos, M.; Gómez-Ruiz, J.Á. Bioactive components of ovine and caprine cheese whey. Small Rumin. Res. 2011, 101, 196–204. [Google Scholar] [CrossRef]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Alexander, D.D.; Schmitt, D.F.; Tran, N.L.; Barraj, L.M.; Cushing, C.A. Partially hydrolyzed 100% whey protein infant formula and atopic dermatitis risk reduction: A systematic review of the literature. Nutr. Rev. 2010, 68, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A. Application of membrane technologies in the valuation of by-products resulting from the manufacture of Serpa cheese. In Final Progress Report of the Agro Project No. 327. (Program Agro, Ministry of Agriculture and Fisheries of Portugal); Instituto Politécnico de Beja: Beja, Portugal, 2005; 45p. [Google Scholar]
- Pereira, C.D.; Díaz, O.; Cobos, A. Valorization of by-products from ovine cheese manufacture: Clarification by thermocalcic precipitation/microfiltration before ultrafiltration. Int. Dairy J. 2002, 12, 773–783. [Google Scholar] [CrossRef]
- Maduko, C.; Park, Y. Production of infant formula analogs by membrane fractionation of caprine milk: Effect of temperature treatment on membrane performance. J. Food Nutr. Sci. 2011, 2, 1097–1104. [Google Scholar] [CrossRef]
- Rice, G.S.; Kentish, S.E.; O’Connor, A.J.; Barber, A.R.; Pihljamaki, A.; Nystrom, M.; Stevens, G.W. Analysis of separation and fouling behaviour during nanofiltration of dairy ultrafiltration permeates. Desalination 2009, 236, 23–29. [Google Scholar] [CrossRef]
- Garem, A.; Jeantet, R. Fouling occurring in nanofiltration of dairy products. In Fouling and Cleaning in Pressure Driven Membrane Processes; Special Issue 9504; International Dairy Federation: Brussels, Belgian, 1995; pp. 71–77. ISBN 92 9098 020 3. [Google Scholar]
- Walstra, P.; Geurts, T.J.; Noomen, A.; Jellema, A.; van Bokel, M.A.J.S. Ciencia de la Leche y Tecnologia de los Produtos Lácteos; Editorial Acribia, S.A.: Zaragoza, Spain, 2001; ISBN 84-200-0961-X. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Dordrecht, the Netherlands, 1996; pp. 418–420. ISBN 0-7923-4248-8. [Google Scholar]
- Pouliot, Y.; Jelen, P. Pretreatments of dairy fluids to minimize long-term membrane fouling. In Fouling and Cleaning in Pressure Driven Membrane Processes; Special Issue 9504; International Dairy Federation: Brussels, Belgian, 1995; pp. 80–92. ISBN 92-9098-020-3. [Google Scholar]
- Macedo, A.; Duarte, E.; Pinho, M. The role of concentration polarization in ultrafiltration of ovine cheese whey. J. Membr. Sci. 2011, 381, 34–40. [Google Scholar] [CrossRef]
- Portuguese Standard NP 675. Milk and dry milk. In Determination of Lactose Concentration; Diário da República III série, no. 123; 1986.
- AOAC Official Methods of Analysis. Solids (Total) in milk, IDF-ISO-AOAC method I. In Official Methods of Analysis of AOAC International; Cunliffe, P., Ed.; AOAC Official Methods of Analysis: Gaithersburg, MD, USA, 1990; p. 807. [Google Scholar]
- Portuguese Standard NP 1986. Milk. In Determination of Crude Protein; Diário da República, III série, no. 142; 1991.
- Barbano, D.M.; Lynch, J.M.; Fleming, J.R. Direct and indirect determination of true protein content of milk by Kjeldahl analysis: Collaborative study. J. Assoc. Off. Anal. Chem. 1991, 73, 281–288. [Google Scholar]
- APHA. Suspended solids. In Standard Methods for Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Clesceri, L.S., Baird, R.B., Eaton, A.D., Eds.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- APHA. Determination of oil and grease. In Standard Methods for Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Clesceri, L.S., Baird, R.B., Eaton, A.D., Eds.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Gonçalves, M.L. Determination of sodium and potassium in milk. In Análise de Soluções; Fundação Calouste Gulbenkian: Lisboa, Portugal, 1983. [Google Scholar]
- Gonçalves, M.L. Determination of calcium and magnesium in milk. In Análise de Soluções; Fundação Calouste Gulbenkian: Lisboa, Portugal, 1983. [Google Scholar]
- Portuguese Standard NP 471. Milk. In Determination of Chloride in Milk; Diário da República, III série, no. 236; 1983.
- APHA. Phosphate. In Standard Methods for Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Clesceri, L.S., Baird, R.B., Eaton, A.D., Eds.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- APHA. Chemical Oxygen Demand–Section 5220. In Standard Methods for Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Clesceri, L.S., Baird, R.B., Eaton, A.D., Eds.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- APHA. Oxygen Demand (biological)–Section 5210. In Standard Methods for Examination of Water and Wastewater, 22nd ed.; Rice, E.W., Clesceri, L.S., Baird, R.B., Eaton, A.D., Eds.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 85–96. [Google Scholar]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Enginneers; John Wiley & Sons, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Marshall, D.; Dauphin, G. Physicochemical aspects of membrane fouling by dairy fluids. In Fouling and Cleaning in Pressure Driven Membrane Processes; Special Issue 9504; International Dairy Federation: Brussels, Belgian, 1995; pp. 8–29. ISBN 92-9098-020-3. [Google Scholar]
- Božanić, R.; Barukčić, I.; Lisak, K. Possibilities of whey utilisation. J. Nutr. Food Sci. 2014, 2, 1–7. [Google Scholar]
- Pereira, C.D.; Diaz, O.; Cobos, A. Valorization of by-products from ovine cheese manufacture: Clarification by thermocalcic precipitation/microfiltration before ultrafiltration. Int. Dairy J. 2002, 12, 773–783. [Google Scholar] [CrossRef]
- Macedo, A. Fracionation of Ovine Whey by Membrane Technology and Study of Possible Applications of the Concentrates Produced. Ph.D. Thesis, Lisbon University-Instituto Superior de Agronomia, Lisboa, Portugal, 2010. [Google Scholar]
- Teixeira, M.; Rosa, M.; Nystrom, M. The role of membrane charge on nanofiltration performance. J. Membr. Sci. 2005, 265, 160–166. [Google Scholar] [CrossRef]
- Magueijo, V.; Minhalma, M.; Queiroz, D.; Geraldes, V.; Macedo, A.; Pinho, M.N. Reduction of wastewaters and valorisation of by-products from “Serpa” cheese manufacture using nanofiltration. Water Sci. Technol. 2005, 52, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Hiddink, J.; de Boer, R.; Nooy, P.F.C. Effect of various pretreatments on the ultrafiltration of sweet cheese whey about 55 °C. Milchwissenschaft 1981, 36, 657–663. [Google Scholar]
- Childress, A.E.; Elimelech, M. Effect of solution chemistry on the surface charge of polymeric reverse osmosis and nanofiltration membranes. J. Membr. Sci. 1996, 119, 253–268. [Google Scholar] [CrossRef]
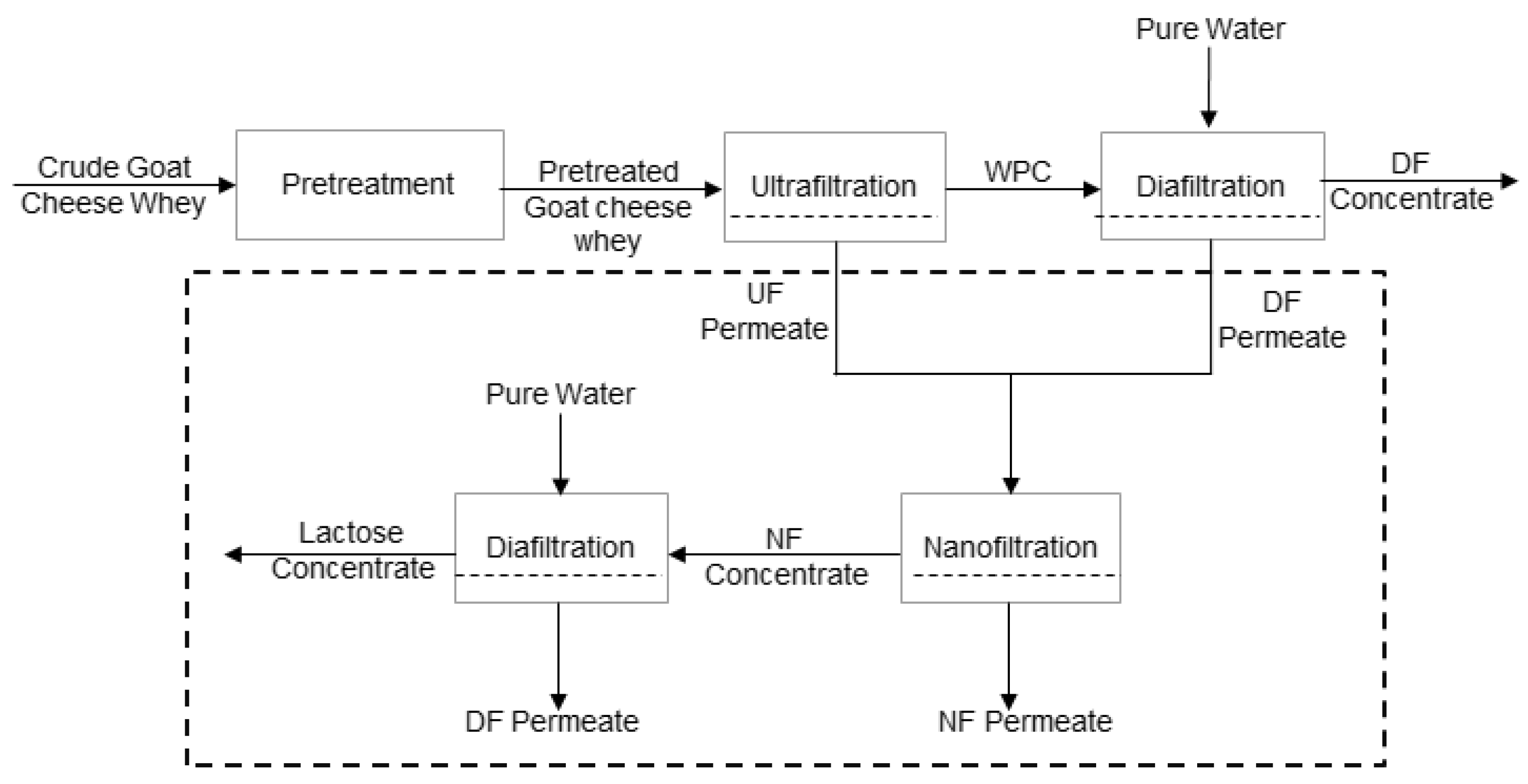

| Experimental Conditions | Short Cycle | Long Cycle | Time (min) |
|---|---|---|---|
| Operational parameters | |||
| Transmembrane pressure (Pa) | 1 × 106 | 1 × 106 | - |
| Feed circulation velocity (m·s−1) | 0.92 | 0.92 | - |
| Temperature (°C) | 25 | 25 | - |
| Cleaning | |||
| pH | 2–11 | 2–11 | - |
| NaOH (w/v %) | - | 0.05 | 15 |
| Na-EDTA (w/v %) | - | 0.20 | 15 |
| HNO3 (w/v %) | - | 0.10 | 15 |
| C6H8O7 (w/v %) | - | 0.50 | 15 |
| Disinfection | |||
| H2O2 (mg·L−1) at 25 °C | 1000 | 1000 | 30 |
| Parameter | Samples | ||||
|---|---|---|---|---|---|
| RSCW | PSCW | PUF-S | PGCW | PUF-G | |
| pH (25 °C) | 5.62 ± 0.29 | 5.58 ± 0.28 | 6.06 ± 0.04 | 5.42 ± 0.22 | 5.43 ±0.08 |
| K25 °C (S·m−1) | 2.09 ± 0.07 | 2.10 ± 0.07 | - | 2.51 ± 0.23 | 2.56 ± 0.06 |
| ST (kg·m−3) | 108.34 ± 4.74 | 87.34 ± 2.36 | 52.93 ± 0.64 | 77.94 ± 0.25 | 38.36 ± 0.01 |
| NKjeldahl (kg·m−3) | 2.777 ± 0.121 | 2.682 ± 0.109 | 0.605 ± 0.059 | 1.222 ± 0.120 | 0.251 ± 0.030 |
| Crude protein (kg·m−3) | 17.74 ± 0.77 | 17.10 ± 0.70 | 3.86 ± 0.38 | 7.80 ± 0.13 | 1.60 ± 0.30 |
| NPN (kg·m−3) | - | - | 0.490 ± 0.034 | 1.100 ± 0.020 | 0.200 ± 0.010 |
| Lactose (kg·m−3) | 52.0 ± 0.9 | 52.1 ± 1.0 | 41.6 ± 1.1 | 49.9 ± 0.60 | 38.9 ± 0.5 |
| Fat (kg·m−3) | 20.79 ± 4.12 | 0.23 ± 0.04 | 0.08 ± 0.01 | 3.90 ± 0.60 | 3.30 ± 0.02 |
| Na (kg·m−3) | 7.138 ± 0.260 | 7.142 ± 0.260 | 3.389 ± 0.141 | 4.555 ± 0.629 | 3.468 ± 0.350 |
| K (kg·m−3) | 0.993 ± 0.045 | 0.991 ± 0.042 | 1.093 ± 0.100 | 1.145 ± 0.045 | 0.849 ± 0.118 |
| Ca (kg·m−3) | 0.492 ± 0.023 | 0.474 ± 0.004 | 0.396 ± 0.019 | 0.289 ± 0.028 | 0.178 ± 0.002 |
| Mg (kg·m−3) | 0.089 ± 0.005 | 0.087 ± 0.005 | 0.102 ± 0.005 | 0.076 ± 0.002 | 0.054 ± 0.001 |
| Cl (kg·m−3) | 7.44 ± 0.44 | 7.54 ± 0.47 | 5.20 ± 0.070 | 9.82 ± 0.58 | 8.70 ± 0.467 |
| Phosphate (kg·m−3) | 1.43 ± 0.16 | 1.46 ± 0.15 | 0.43 ± 0.08 | 0.35 ± 0.12 | 0.046 ± 0.012 |
| Regression Lines (with 95% CI for the Parameters Estimated) | Hydraulic Permeability (ms−1Pa−1) | Intrinsic Hydraulic Permeability, Lp (m) |
|---|---|---|
| Jw = (·ΔP (10) R = 0.999 | 1.68 × 10−11 | 1.51 × 10−14 |
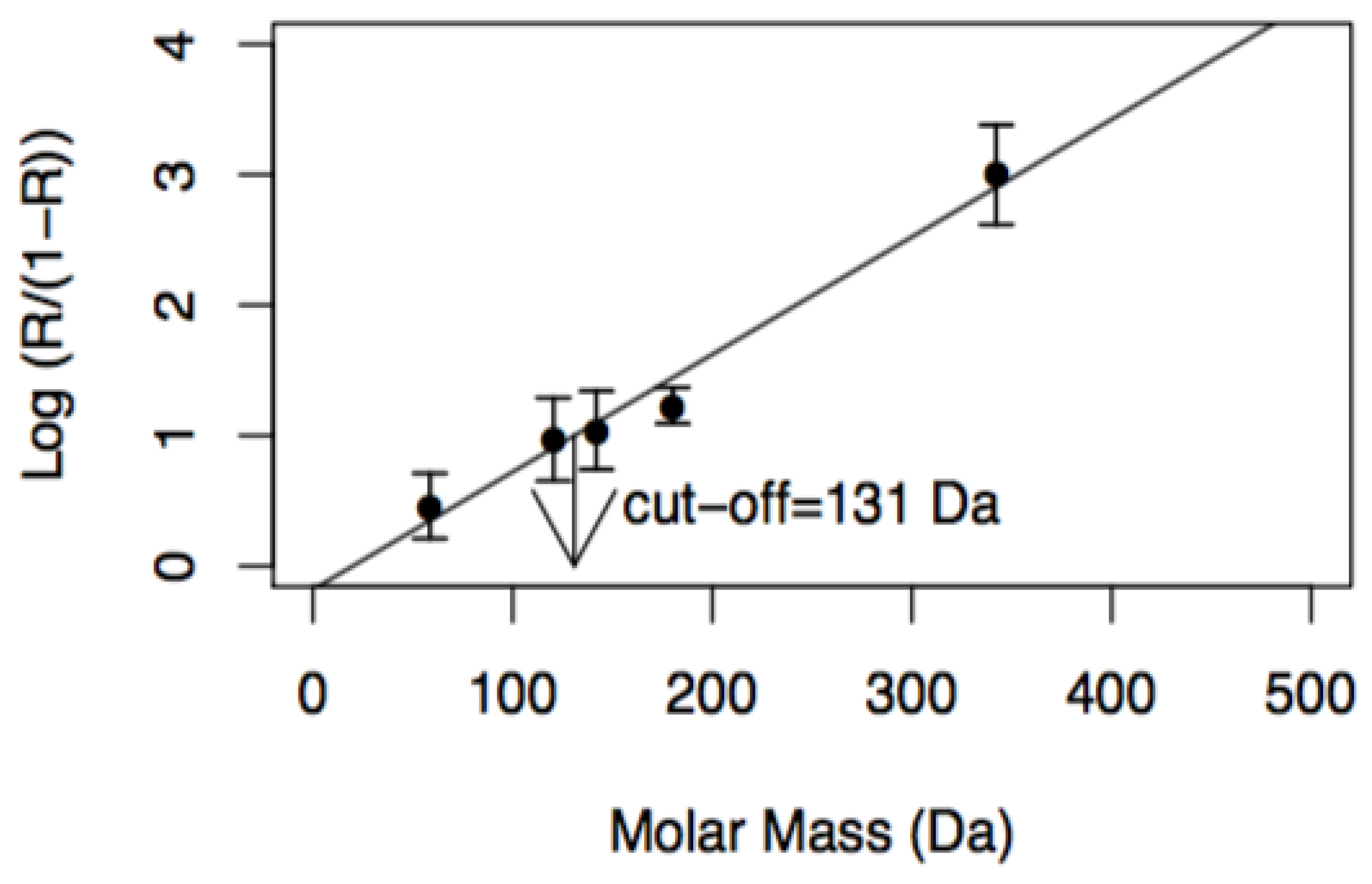
| Log (R/(1 − R)) = (± ) + (− ± )·M (11) R = 0.990 | - | - |
| ΔP (Pa) | <v> (m·s−1) | RN | Rsalts | RCOD |
|---|---|---|---|---|
| 1.00 × 106 | 0.94 | 0.576 ± 0.015 | 0.260 ± 0.015 | 0.992 ± 0.001 |
| 1.00 × 106 | 1.42 | 0.456 ± 0.012 | 0.249 ± 0.007 | 0.994 ± 0.001 |
| 1.50 × 106 | 0.94 | 0.612 ± 0.012 | 0.377 ± 0.010 | 0.994 ± 0.001 |
| 1.50 × 106 | 1.42 | 0.553 ± 0.013 | 0.362 ± 0.015 | 0.996 ± 0.001 |
| 2.00 × 106 | 0.94 | 0.632 ± 0.010 | 0.480 ± 0.004 | 0.995 ± 0.000 |
| 2.00 × 106 | 1.42 | 0.568 ± 0.013 | 0.437 ± 0.015 | 0.995 ± 0.001 |
| 3.00 × 106 | 0.94 | 0.666 ± 0.008 | 0.551 ± 0.003 | 0.996 ± 0.001 |
| 3.00 × 106 | 1.42 | 0.587 ± 0.005 | 0.528 ± 0.013 | 0.995 ± 0.001 |
| Rt (m−1) | (Rcp + Rf) (m−1) | |
|---|---|---|
| 1.08 × 1014 ± 5.93 × 10−2 | 4.16 × 1013 | 2.35 ± 0.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macedo, A.; Monteiro, J.; Duarte, E. A Contribution for the Valorisation of Sheep and Goat Cheese Whey through Nanofiltration. Membranes 2018, 8, 114. https://doi.org/10.3390/membranes8040114
Macedo A, Monteiro J, Duarte E. A Contribution for the Valorisation of Sheep and Goat Cheese Whey through Nanofiltration. Membranes. 2018; 8(4):114. https://doi.org/10.3390/membranes8040114
Chicago/Turabian StyleMacedo, Antónia, Joana Monteiro, and Elizabeth Duarte. 2018. "A Contribution for the Valorisation of Sheep and Goat Cheese Whey through Nanofiltration" Membranes 8, no. 4: 114. https://doi.org/10.3390/membranes8040114
APA StyleMacedo, A., Monteiro, J., & Duarte, E. (2018). A Contribution for the Valorisation of Sheep and Goat Cheese Whey through Nanofiltration. Membranes, 8(4), 114. https://doi.org/10.3390/membranes8040114





