Intensified LOHC-Dehydrogenation Using Multi-Stage Microstructures and Pd-Based Membranes
Abstract
:1. Introduction
2. Materials and Methods
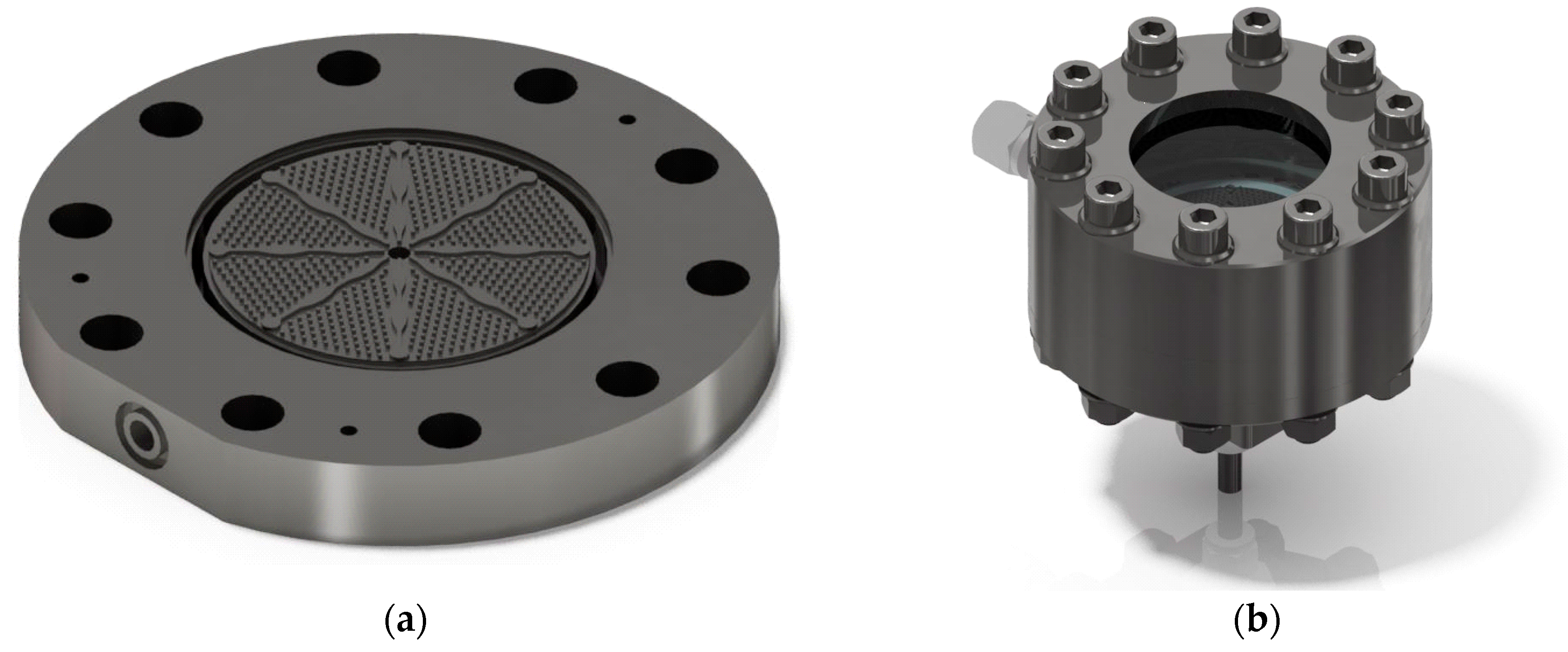
2.1. Radial Flow Reactor
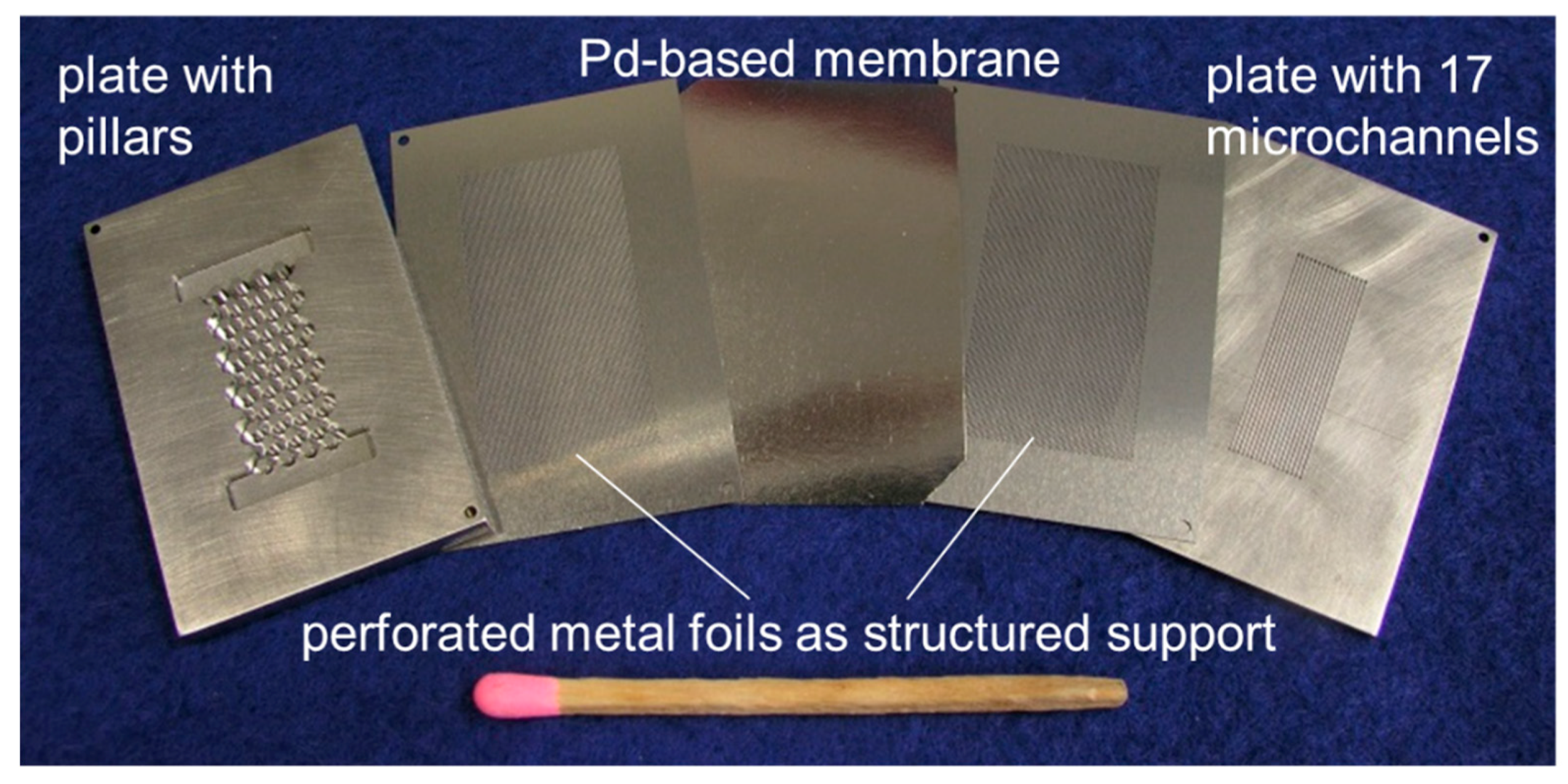
2.2. Membrane Apparatus
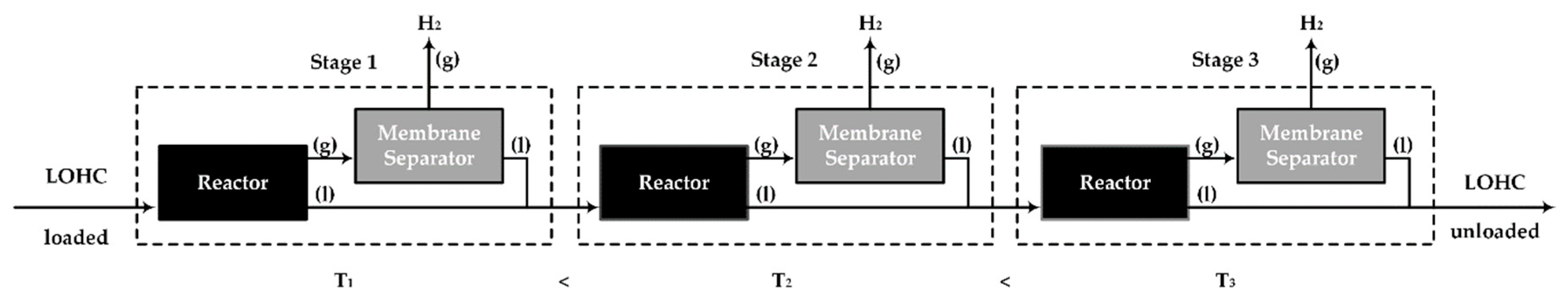
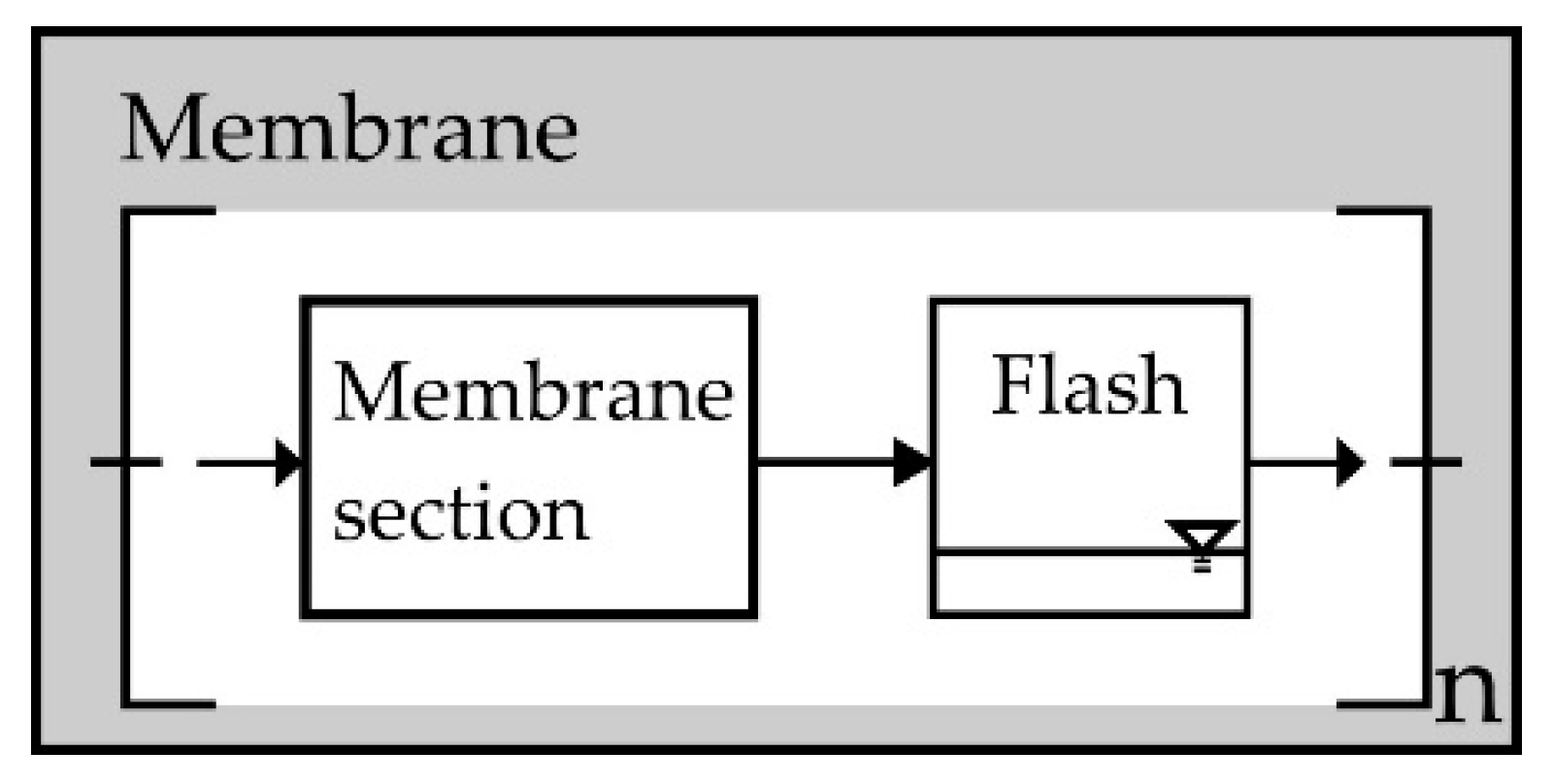
2.3. Multi-Stage Reactor Concept with Intermediate Hydrogen Separation
3. Results and Discussion
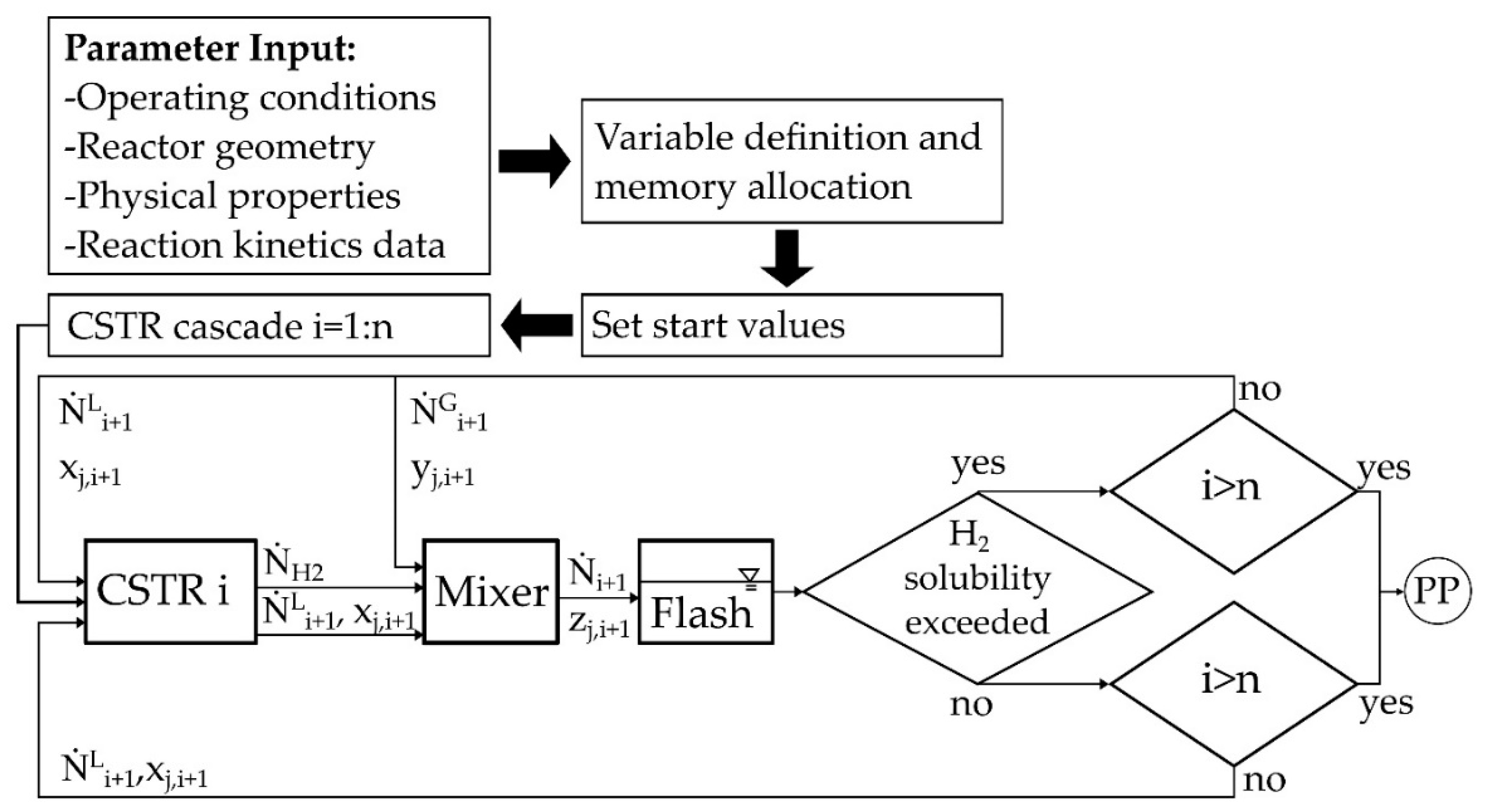
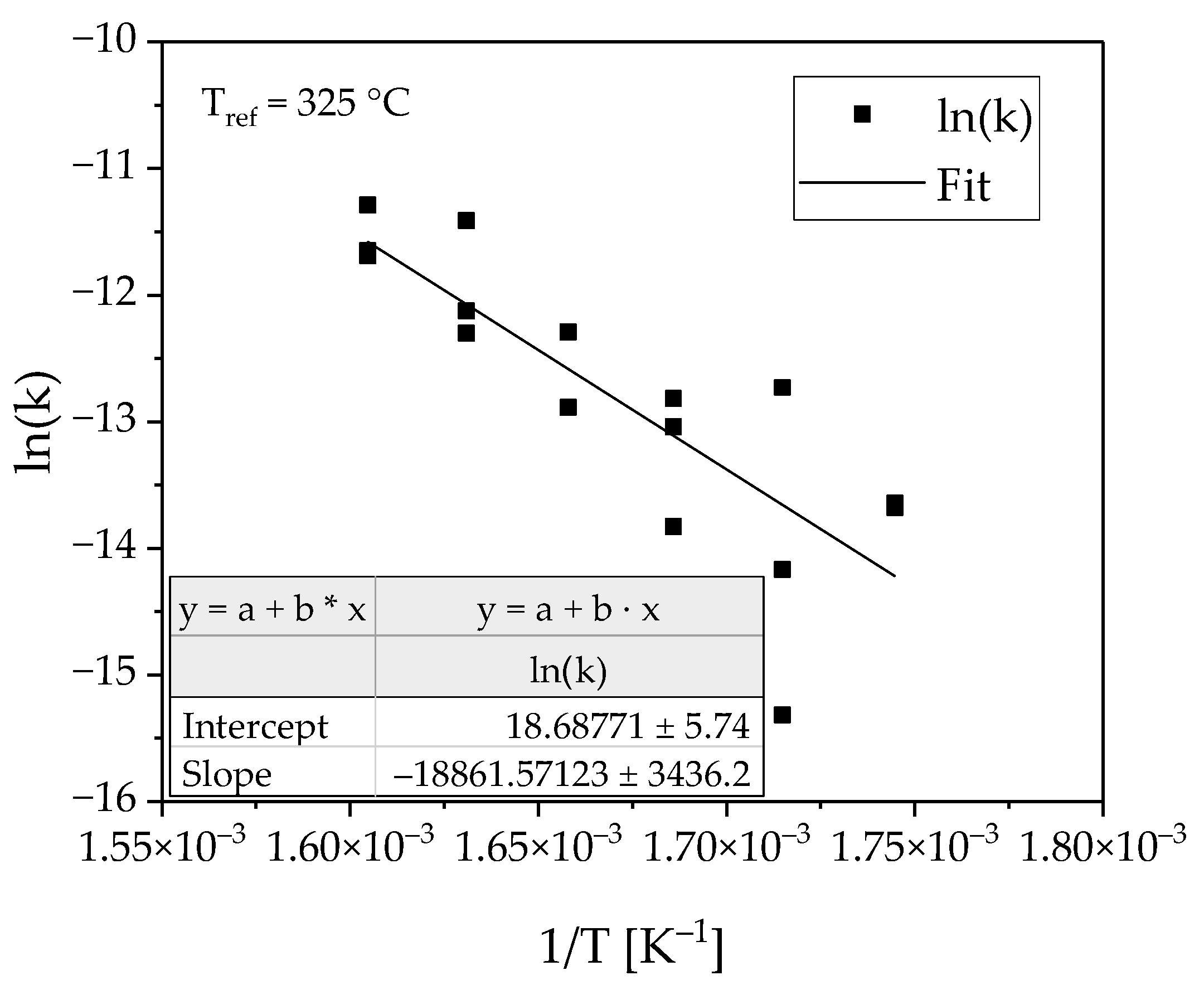
3.1. Determination of the Reaction Kinetics
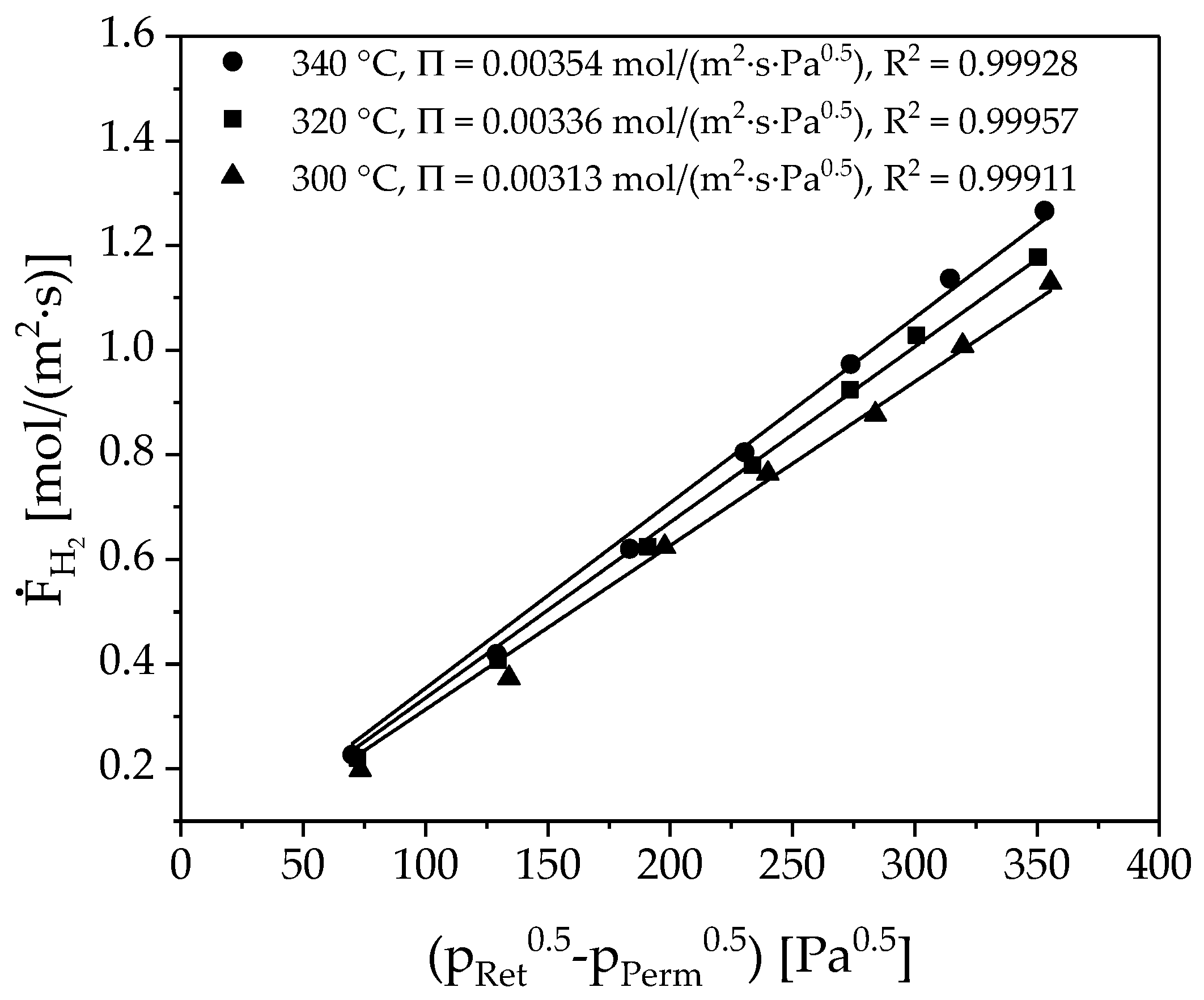
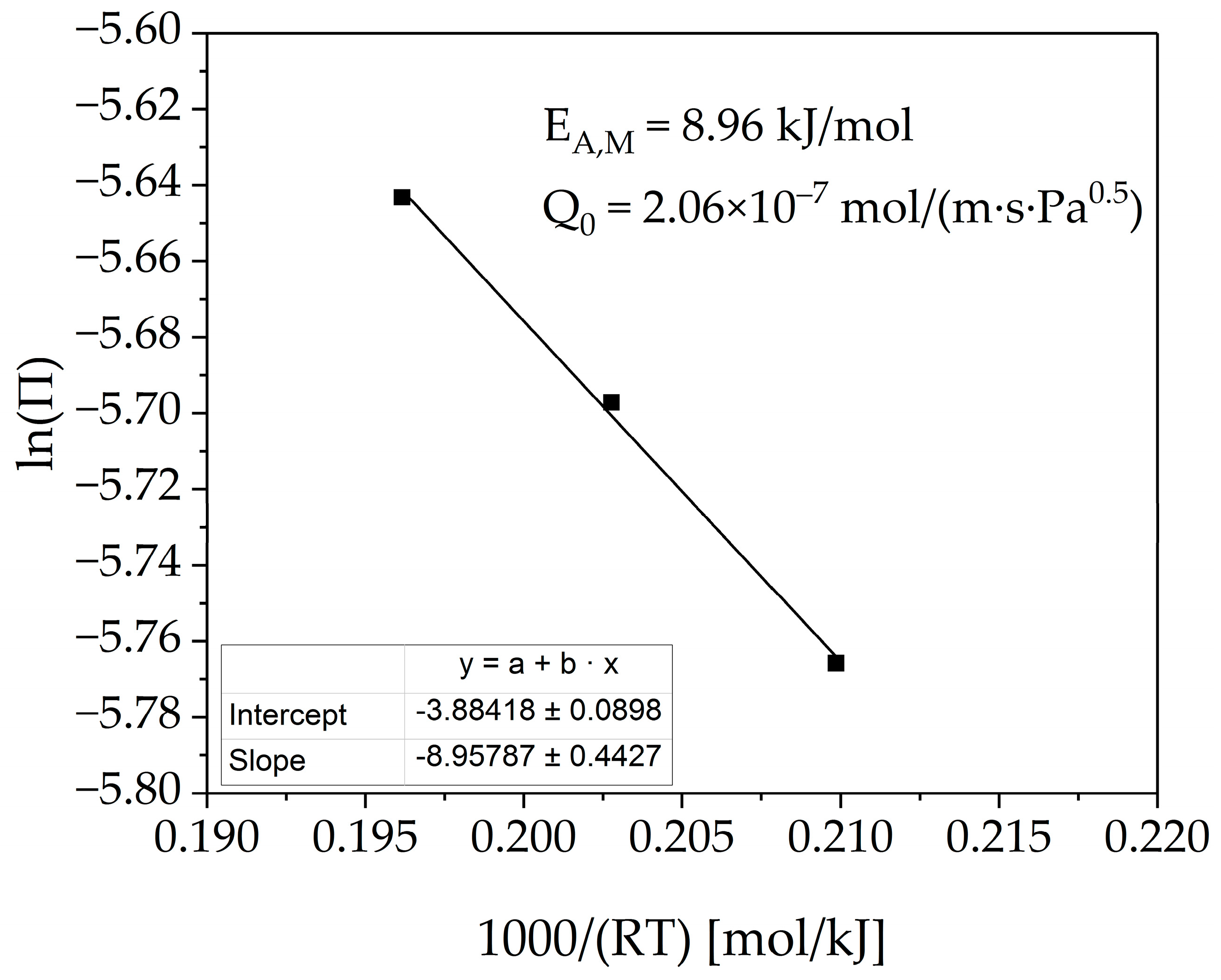
3.2. Membrane Characterization
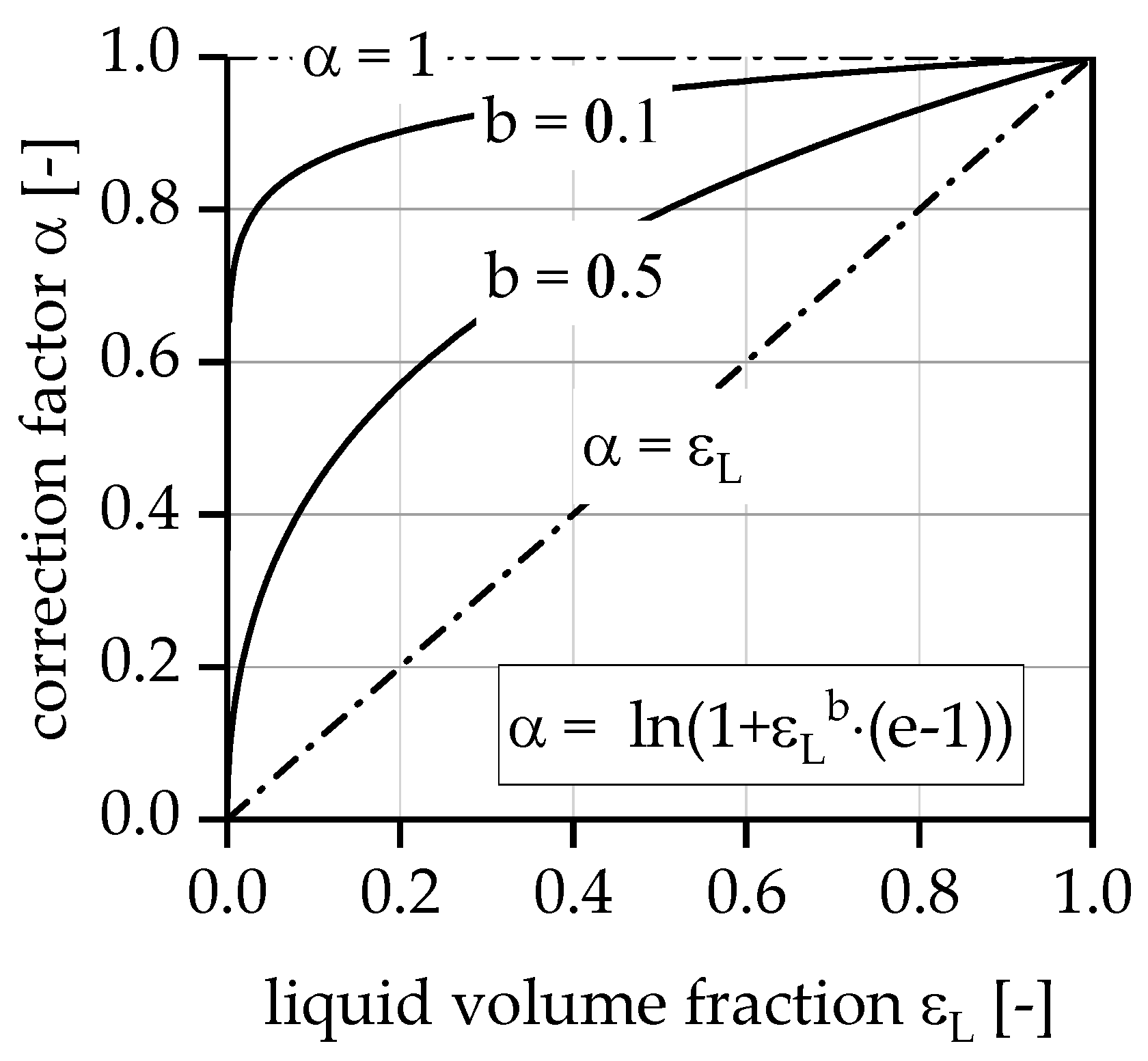
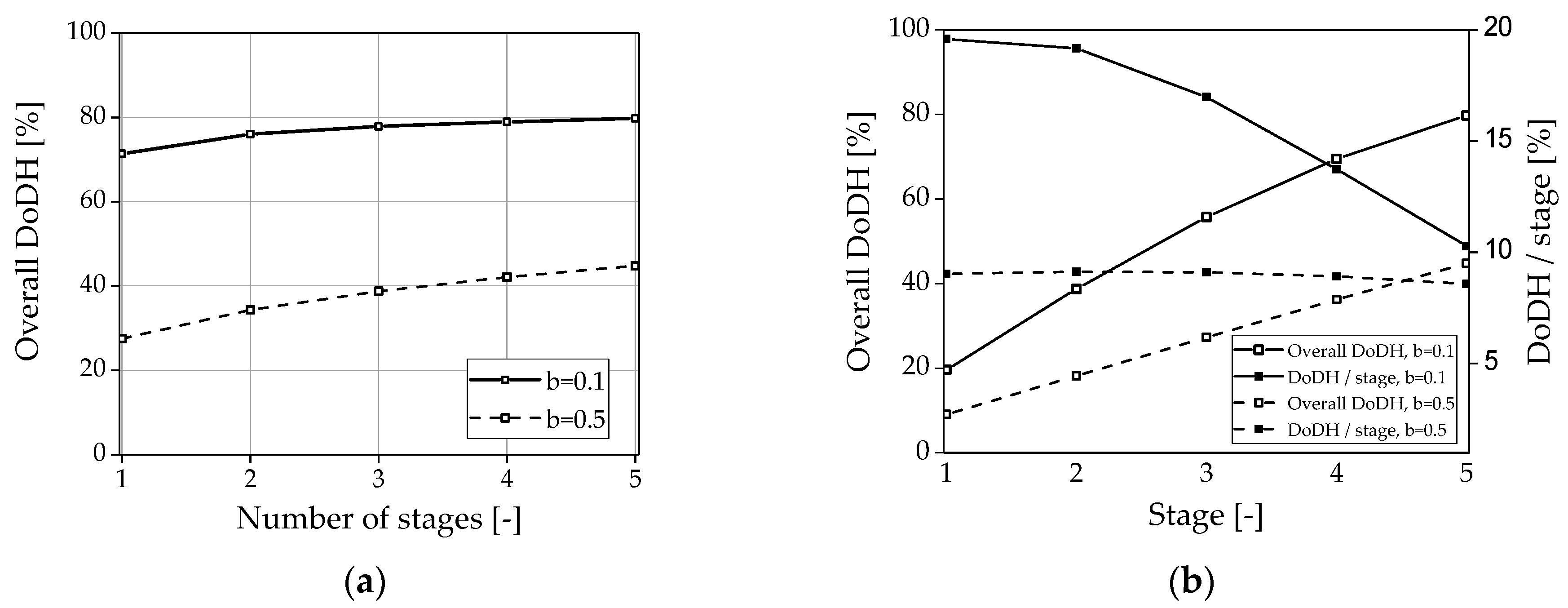
3.3. Evaluation of the Multi-Stage Reactor Approach with Intermediate Hydrogen Separation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teichmann, D.; Arlt, W.; Wasserscheid, P.; Freymann, R. A future energy supply based on Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2011, 4, 2767–2773. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P. Liquid Organic Hydrogen Carriers as an efficient vector for the transport and storage of renewable energy. Int. J. Hydrog. Energy 2012, 37, 18118–18132. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Preuster, P.; Alekseev, A.; Wasserscheid, P. Hydrogen storage technologies for future energy systems. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 445–471. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, N.; Obesser, K.; Bosmann, A.; Teichmann, D.; Arlt, W.; Dungs, J.; Wasserscheid, P. Evaluation of industrially applied heat-transfer fluids as Liquid Organic Hydrogen Carrier systems. ChemSusChem 2014, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, M.; Zhang, Y.Q.; Bosmann, A.; Bruckner, N.; Thoming, J.; Wasserscheid, P.; Stolte, S. Environmental and health impact assessment of Liquid Organic Hydrogen Carrier (LOHC) systems—Challenges and preliminary results. Energy Environ. Sci. 2015, 8, 1035–1045. [Google Scholar] [CrossRef]
- IFA-Institut-für-Arbeitsschutz-der-Deutschen-Gesetzlichen-Unfallversicherung. Material Safety Data Sheet (MSDS)—Dibenzyltoluene, Isomers: GESTIS-Stoffdatenbank; IFA: Berlin, Germany, 2018. [Google Scholar]
- Hydrogenious-Technologies-GmbH. Material Safety Data Sheet (MSDS)—Verordnung (EG) Nr. 1907/2006 Version 1.1; Hydrogenious-Technologies-GmbH: Erlangen, Germany, 2017. [Google Scholar]
- Boeltken, T.; Belimov, M.; Pfeifer, P.; Peters, T.A.; Bredesen, R.; Dittmeyer, R. Fabrication and testing of a planar microstructured concept module with integrated palladium membranes. Chem. Eng. Process. 2013, 67, 136–147. [Google Scholar] [CrossRef]
- Preuster, P. Entwicklung eines Reaktors zur Dehydrierung Chemischer Wasserstoffträger als Bestandteil eines Dezentralen, Stationären Energiespeichers Dr.-Ing. Ph.D. Thesis, Friedrich-Alexander Universität Erlangen-Nürnberg, Erlangen, Germany, 2017. [Google Scholar]
- Boeltken, T.; Wunsch, A.; Gietzelt, T.; Pfeifer, P.; Dittmeyer, R. Ultra-compact microstructured methane steam reformer with integrated Palladium membrane for on-site production of pure hydrogen: Experimental demonstration. Int. J. Hydrog. Energy 2014, 39, 18058–18068. [Google Scholar] [CrossRef]
- Kreuder, H.; Muller, C.; Meier, J.; Gerhards, U.; Dittmeyer, R.; Pfeifer, P. Catalyst development for the dehydrogenation of MCH in a microstructured membrane reactor-For heat storage by a Liquid Organic Reaction Cycle. Catal. Today 2015, 242, 211–220. [Google Scholar] [CrossRef]
- Kreuder, H.; Boeltken, T.; Cholewa, M.; Meier, J.; Pfeifer, P.; Dittmeyer, R. Heat storage by the dehydrogenation of methylcyclohexane—Experimental studies for the design of a microstructured membrane reactor. Int. J. Hydrog. Energy 2016, 41, 12082–12092. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Boeltken, T.; Piermartini, P.; Selinsek, M.; Loewert, M.; Dallmann, F.; Kreuder, H.; Cholewa, M.; Wunsch, A.; Belimov, M.; et al. Micro and micro membrane reactors for advanced applications in chemical energy conversion. Curr. Opin. Chem. Eng. 2017, 17, 108–125. [Google Scholar] [CrossRef]
- Cholewa, M.; Durrschnabel, R.; Boukis, N.; Pfeifer, P. High pressure membrane separator for hydrogen purification of gas from hydrothermal treatment of biomass. Int. J. Hydrog. Energy 2018, 43, 13294–13304. [Google Scholar] [CrossRef]
- Cholewa, M.; Zehner, B.; Kreuder, H.; Pfeifer, P. Optimization of membrane area to catalyst mass in a microstructured membrane reactor for dehydrogenation of methylcyclohexane. Chem. Eng. Process. 2018, 125, 325–333. [Google Scholar] [CrossRef]
- Aslam, R.; Muller, K.; Ant, W. Experimental study of solubility of water in Liquid Organic Hydrogen Carriers. J. Chem. Eng. Data 2015, 60, 1997–2002. [Google Scholar] [CrossRef]
- Aslam, R.; Muller, K.; Muller, M.; Koch, M.; Wasserscheid, P.; Arlt, W. Measurement of hydrogen solubility in potential Liquid Organic Hydrogen Carriers. J. Chem. Eng. Data 2016, 61, 643–649. [Google Scholar] [CrossRef]
- Aslam, R.; Muller, K. Adsorption isotherm of dibenzyl toluene and its partially hydrogenated forms over phenyl hexyl silica. Mod. Chem. Appl. 2017, 5. [Google Scholar] [CrossRef]
- Muller, K.; Stark, K.; Emel’yanenko, V.N.; Varfolomeev, M.A.; Zaitsau, D.H.; Shoifet, E.; Schick, C.; Verevkin, S.P.; Arlt, W. Liquid Organic Hydrogen Carriers: Thermophysical and thermochemical studies of benzyl- and dibenzyl-toluene derivatives. Ind. Eng. Chem. Res. 2015, 54, 7967–7976. [Google Scholar] [CrossRef]
- Do, G.; Preuster, P.; Aslam, R.; Bosmann, A.; Muller, K.; Arlt, W.; Wasserscheid, P. Hydrogenation of the liquid organic hydrogen carrier compound dibenzyltoluene—Reaction pathway determination by H-1 NMR spectroscopy. React. Chem. Eng. 2016, 1, 313–320. [Google Scholar] [CrossRef]
- Peters, T.A.; Stange, M.; Bredesen, R. 2—Fabrication of palladium-based membranes by magnetron sputtering. In Palladium Membrane Technology for Hydrogen Production, Carbon Capture and Other Applications; Doukelis, A., Panopoulos, K., Koumanakos, A., Kakaras, E., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 25–41. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wunsch, A.; Mohr, M.; Pfeifer, P. Intensified LOHC-Dehydrogenation Using Multi-Stage Microstructures and Pd-Based Membranes. Membranes 2018, 8, 112. https://doi.org/10.3390/membranes8040112
Wunsch A, Mohr M, Pfeifer P. Intensified LOHC-Dehydrogenation Using Multi-Stage Microstructures and Pd-Based Membranes. Membranes. 2018; 8(4):112. https://doi.org/10.3390/membranes8040112
Chicago/Turabian StyleWunsch, Alexander, Marijan Mohr, and Peter Pfeifer. 2018. "Intensified LOHC-Dehydrogenation Using Multi-Stage Microstructures and Pd-Based Membranes" Membranes 8, no. 4: 112. https://doi.org/10.3390/membranes8040112
APA StyleWunsch, A., Mohr, M., & Pfeifer, P. (2018). Intensified LOHC-Dehydrogenation Using Multi-Stage Microstructures and Pd-Based Membranes. Membranes, 8(4), 112. https://doi.org/10.3390/membranes8040112





