Electro-Conductive Composite Gold-Polyethersulfone-Ultrafiltration-Membrane: Characterization of Membrane and Natural Organic Matter (NOM) Filtration Performance at Different In-Situ Applied Surface Potentials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Gold-Polymer-Composite Membrane
2.2. Membrane Characterization
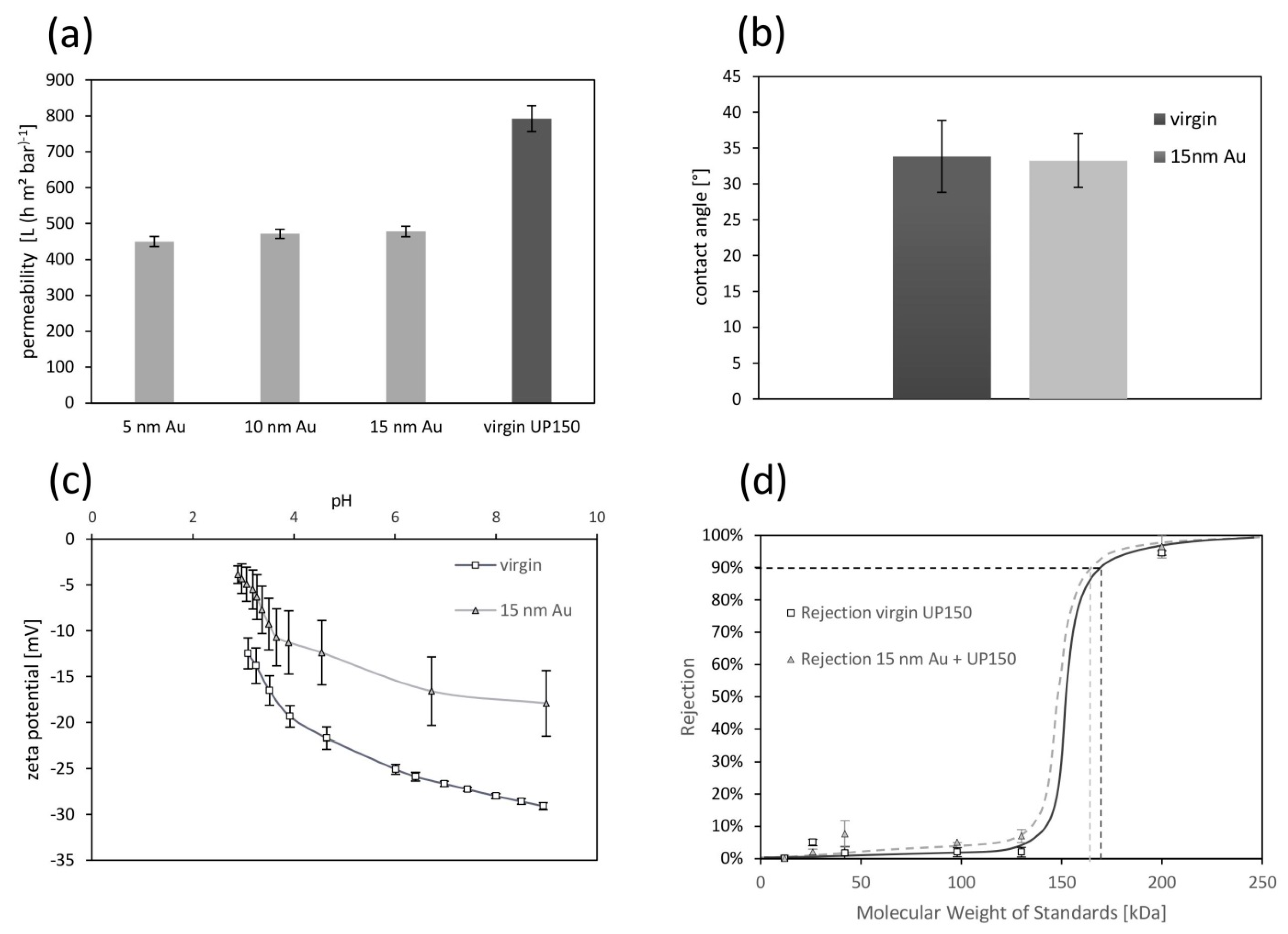
2.2.1. Pure Water Permeability
2.2.2. Hydrophobicity—Contact Angle
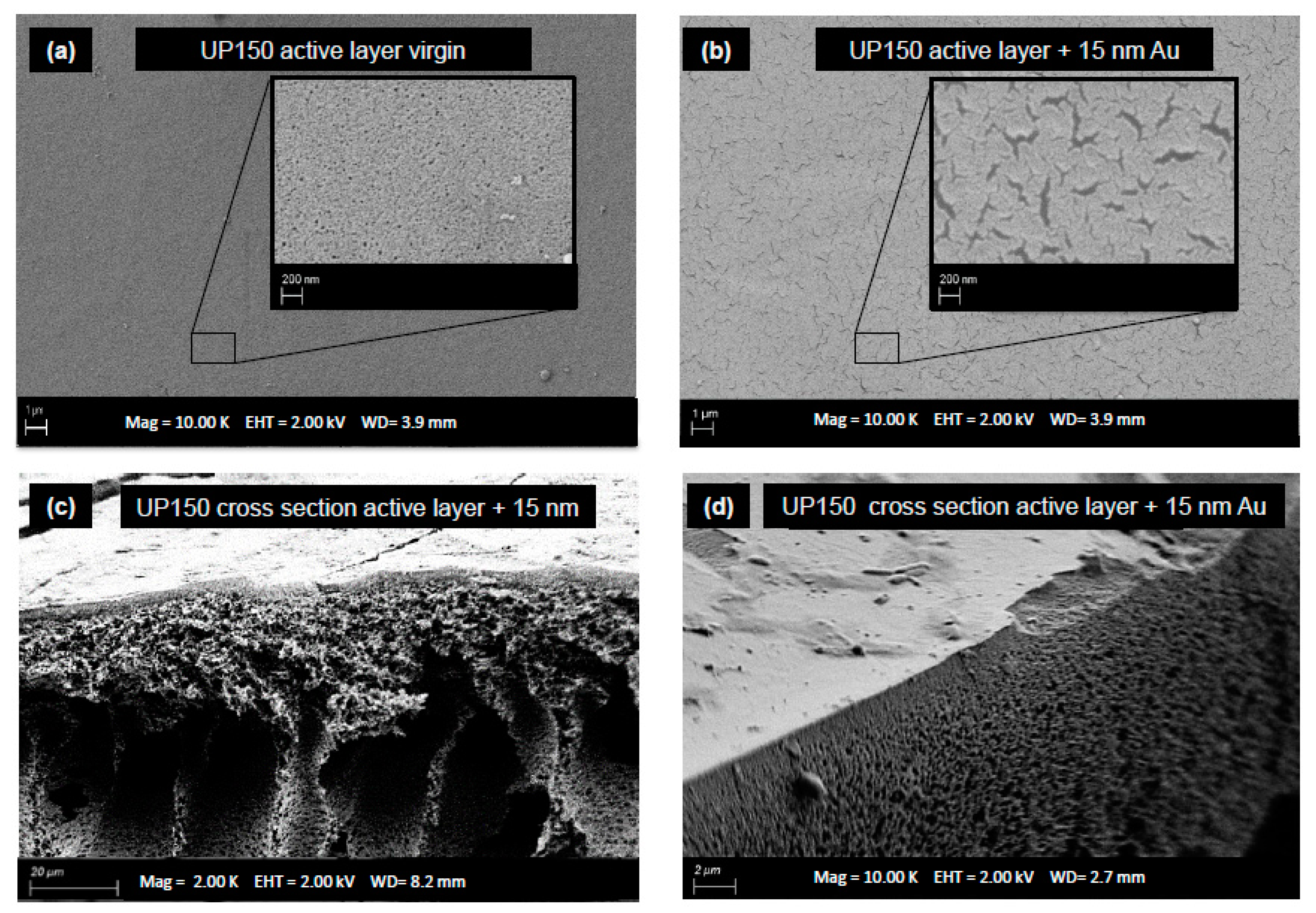
2.2.3. Scanning Electron Microscopy
2.2.4. Zeta Potential
2.2.5. Molecular Weight Cut-Off
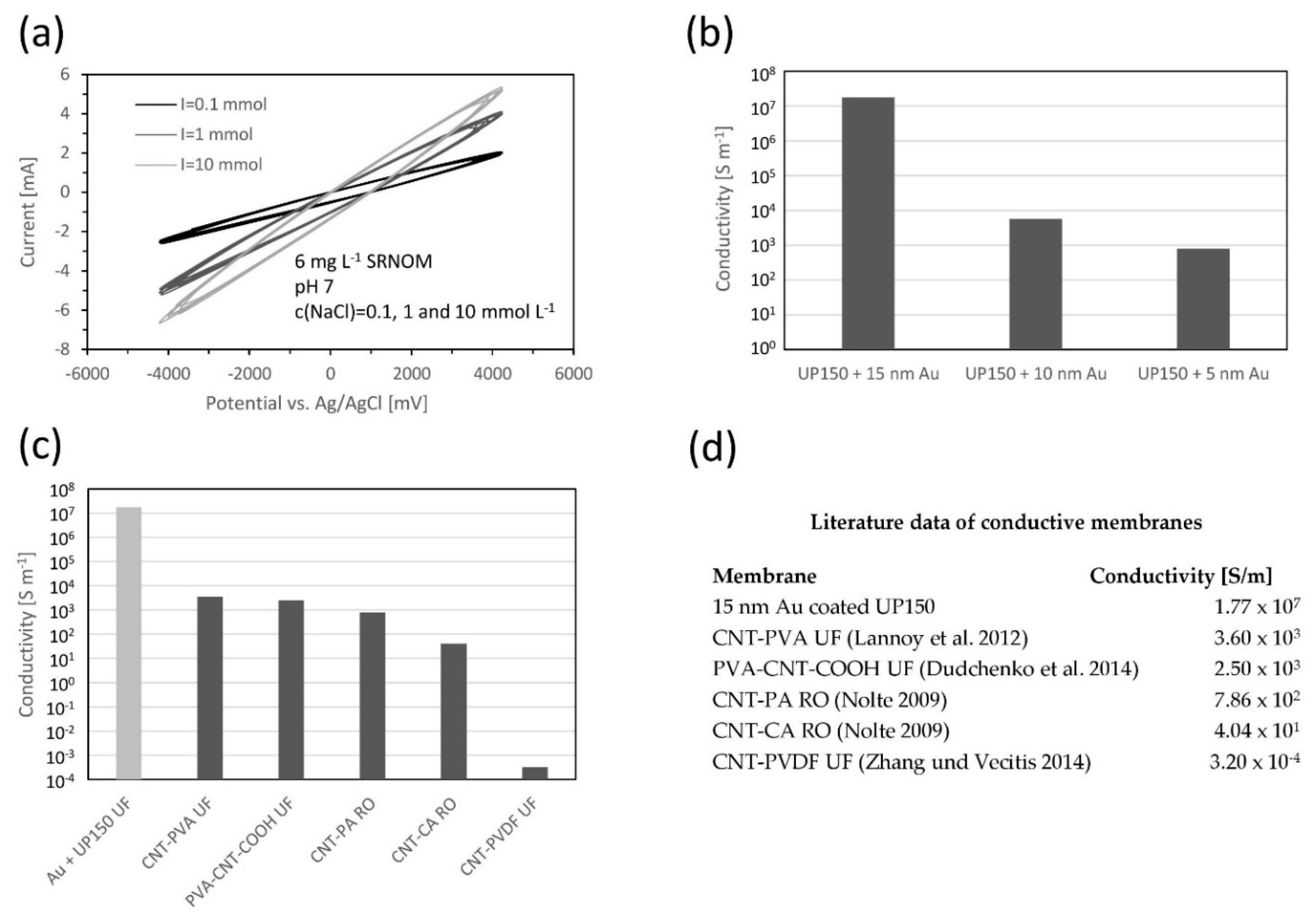
2.2.6. Electrical Properties of Conductive Membrane Electrode
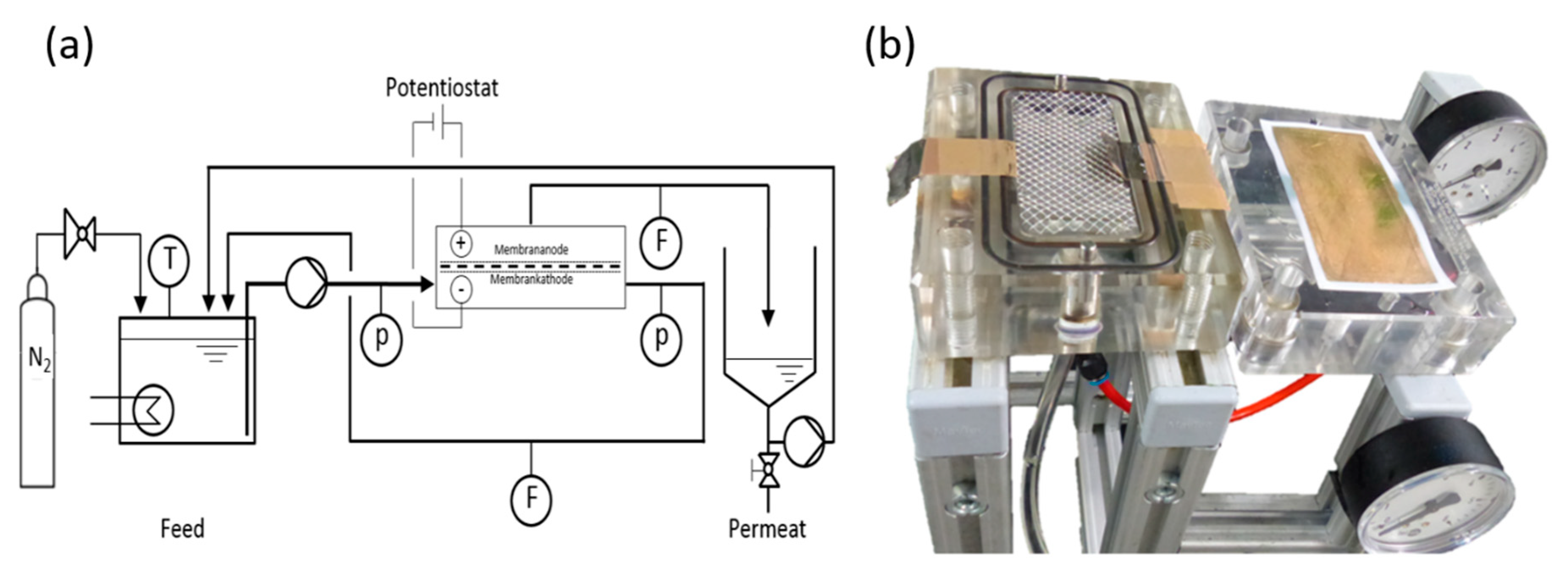
2.3. Experimental Setup and Filtration Experiments
2.3.1. Filtration Setup
2.3.2. Feed Solution
2.3.3. Filtration Experiments
3. Results
3.1. Membrane Characterization
3.2. Filtration Experiments
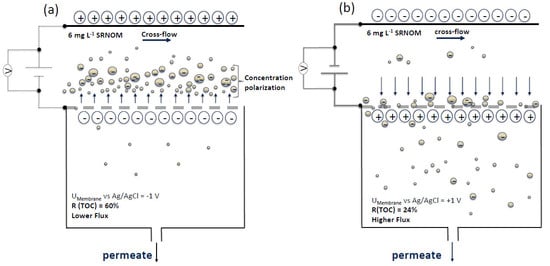
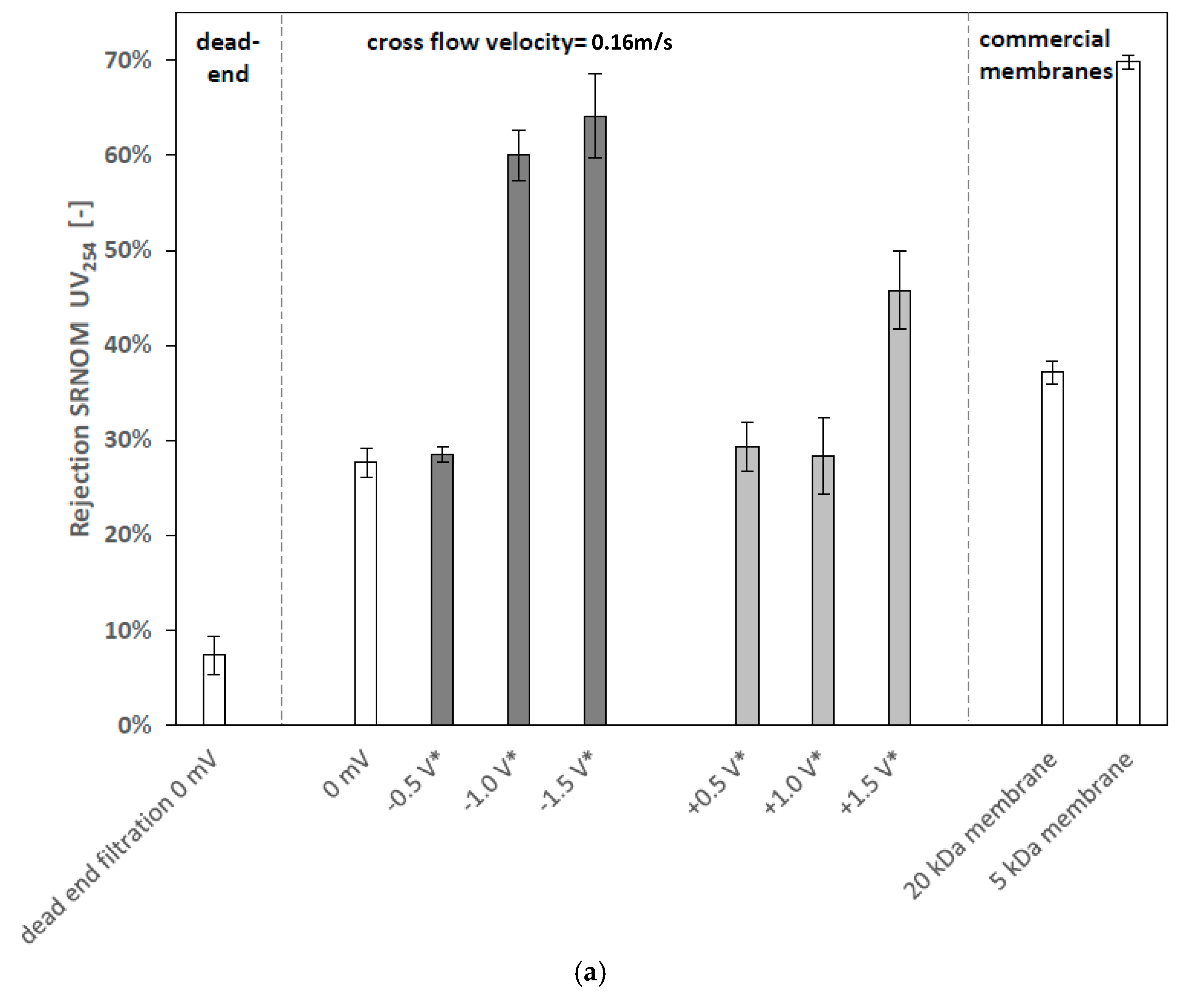
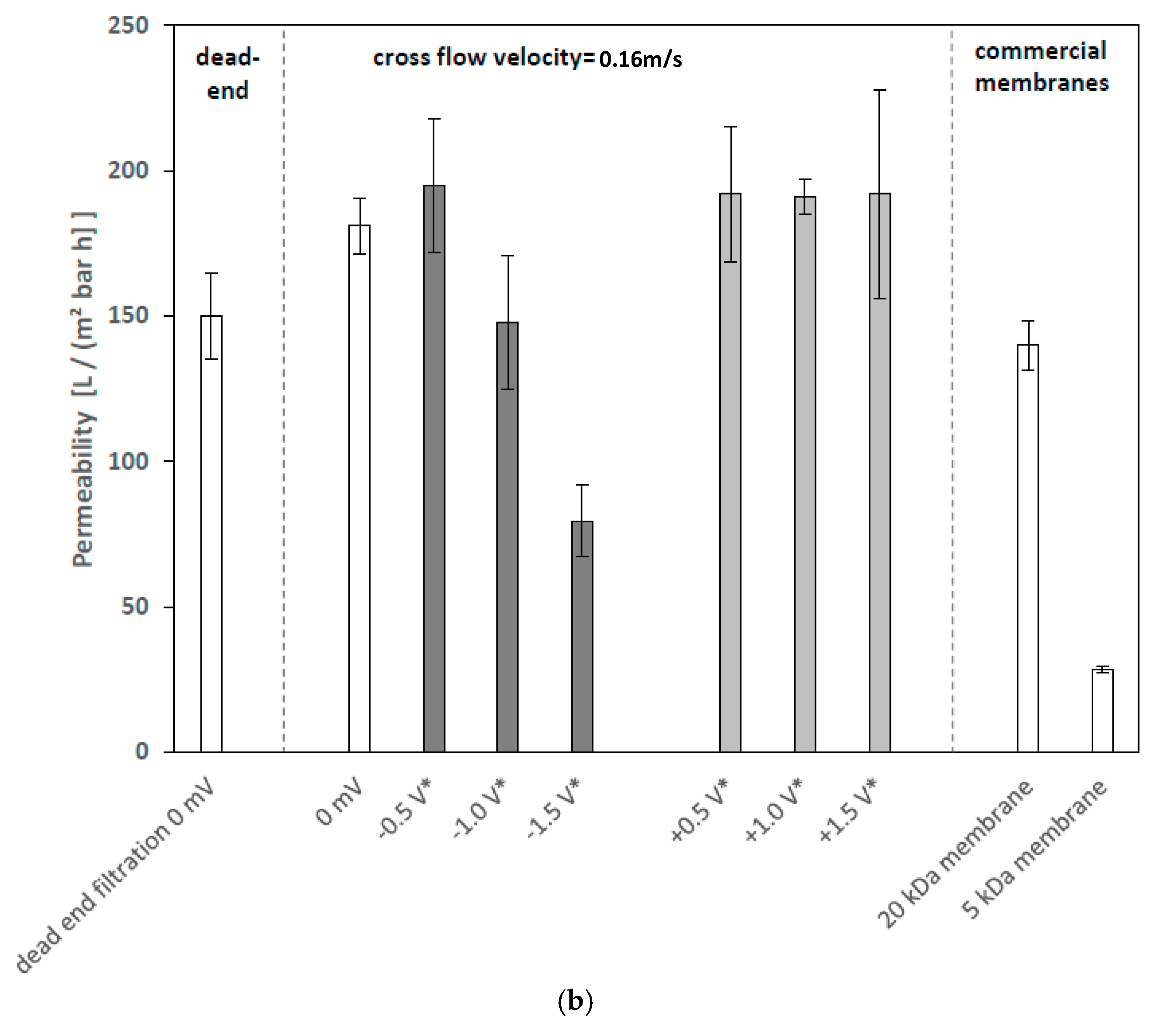
3.2.1. Effects of Cathodic and Anodic Potentials
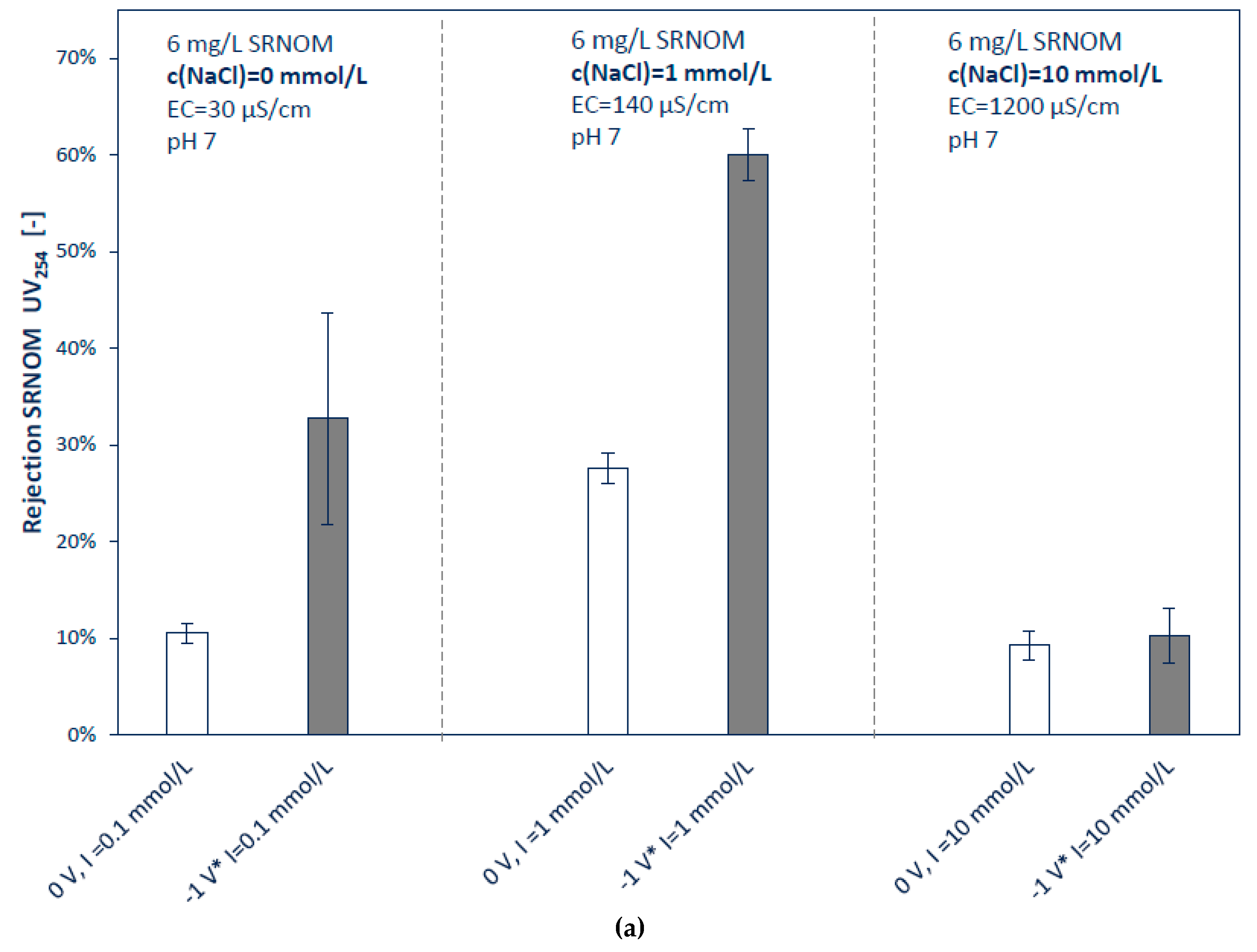
3.2.2. Influence of Ionic Strengths and pH
3.2.3. Changing Sign of Surface Potential during Filtration
4. Discussion and Conclusions
4.1. Membrane Modification with Gold Sputter Coating
4.2. Filtration Experiments with Applied Surface Potential
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schäfer, A. Natural Organics Removal Using Membranes: Principles, Performance, and Cost, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Van der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Cho, J.; Amy, G.; Pellegrino, J. Membrane filtration of natural organic matter: Factors and mechanisms affecting rejection and flux decline with charged ultrafiltration (UF) membrane. J. Membr. Sci. 2000, 164, 89–110. [Google Scholar] [CrossRef]
- Kopeć, K.K.; Dutczak, S.M.; Wessling, M.; Stamatialis, D.F. Tailoring the surface charge of an ultrafiltration hollow fiber by addition of a polyanion to the coagulation bore liquid. J. Membr. Sci. 2011, 369, 59–67. [Google Scholar] [CrossRef]
- Shao, J.; Hou, J.; Song, H. Comparison of humic acid rejection and flux decline during filtration with negatively charged and uncharged ultrafiltration membranes. Water Res. 2011, 45, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Zydney, A.L. Effect of Membrane Charge on Flow and Protein Transport during Ultrafiltration. Biotechnol. Prog. 2006, 22, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Vecitis, C.D. Conductive CNT-PVDF membrane for capacitive organic fouling reduction. J. Membr. Sci. 2014, 459, 143–156. [Google Scholar] [CrossRef]
- Ahmed, F.; Lalia, B.S.; Kochkodan, V.; Hilal, N.; Hashaikeh, R. Electrically conductive polymeric membranes for fouling prevention and detection: A review. Desalination 2016, 391, 1–15. [Google Scholar] [CrossRef]
- Formoso, P.; Pantuso, E.; De Filpo, G.; Nicoletta, F.P. Electro-Conductive Membranes for Permeation Enhancement and Fouling Mitigation: A Short Review. Membranes 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.M. Handbook of Deposition Technologies for Films and Coatings: Science, Applications and Technology, 3rd ed.; Elsevier: New York, NY, USA, 2010. [Google Scholar]
- Zhao, H.B.; Xiong, G.X.; Baron, G.V. Preparation and characterization of palladium-based composite membranes by electroless plating and magnetron sputtering. Catal. Today 2000, 56, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Gaedt, L.; Chilcott, T.C.; Chan, M.; Nantawisarakul, T.; Fane, A.G.; Coster, H.G.L. Electrical impedance spectroscopy characterisation of conducting membranes: II. Experimental. J. Membr. Sci. 2002, 195, 169–180. [Google Scholar] [CrossRef]
- Chilcott, T.C.; Chan, M.; Gaedt, L.; Nantawisarakul, T.; Fane, A.G.; Coster, H.G.L. Electrical impedance spectroscopy characterisation of conducting membranes: I. Theory. J. Membr. Sci. 2002, 195, 153–167. [Google Scholar] [CrossRef]
- Zhang, W.; Wahlgren, M.; Sivik, B. Membrane Characterization by the Contact Angle Technique. Desalination 1989, 72, 263–273. [Google Scholar] [CrossRef]
- Nyström, M.; Pihlajamäki, A.; Ehsani, N. Characterization of ultrafiltration membranes by simultaneous streaming potential and flux measurements. J. Membr. Sci. 1994, 87, 245–256. [Google Scholar] [CrossRef]
- Luxbacher, T. The Zeta Potential for Solid Surface Analysis: A Practical Guide to Streaming Potential Measurement, 1st ed.; Anton Paar GmbH: Graz, Austria, 2014. [Google Scholar]
- Lalia, B.S.; Ahmed, F.E.; Shah, T.; Hilal, N.; Hashaikeh, R. Electrically conductive membranes based on carbon nanostructures for self-cleaning of biofouling. Desalination 2015, 360, 8–12. [Google Scholar] [CrossRef]
- Liang, S.; Lu, L.; Meng, F. DOM-mediated membrane retention of fluoroquinolone as revealed by fluorescence quenching properties. Sci. Rep. 2017, 7, 5372. [Google Scholar] [CrossRef] [PubMed]
- Green, N.W.; McInnis, D.; Hertkorn, N.; Maurice, P.A.; Perdue, E.M. Perdue, Suwannee River Natural Organic Matter: Isolation of the 2R101N Reference Sample by Reverse Osmosis. Environ. Eng. Sci. 2015, 32, 38–44. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Becker, W.C.; Wattier, K.L. Surrogate Parameters for Monitoring Organic Matter and THM Precursors. J. - Am. Water Works Assoc. 1985, 77, 122–132. [Google Scholar] [CrossRef]
- Winter, J.; Uhl, W.; Bérubé, P.R. Integrated oxidation membrane filtration process—NOM rejection and membrane fouling. Water Res. 2016, 104, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Giesbers, M.; Kleijn, J.M.; Stuart, M.A.C. The electrical double layer on gold probed by electrokinetic and surface force measurements. J. Colloid Interface Sci. 2002, 248, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, A.V.; Rolf, J.; Russell, K.; Duan, W.; Jassby, D. Organic fouling inhibition on electrically conducting carbon nanotube–polyvinyl alcohol composite ultrafiltration membranes. J. Membr. Sci. 2014, 468, 1–10. [Google Scholar] [CrossRef]
- Schwartzkopf, M.; Buffet, A.; Körstgens, V.; Metwalli, E.; Schlage, K.; Benecke, G.; Perlich, J.; Rawolle, M.; Rothkirch, A.; Heidmann, B; et al. From atoms to layers: In situ gold cluster growth kinetics during sputter deposition. Nanoscale 2013, 5, 5053–5062. [Google Scholar] [CrossRef] [PubMed]
- De Lannoy, C.F.; Jassby, D.; Davis, D.D.; Wiesner, M.R. A highly electrically conductive polymer–multiwalled carbon nanotube nanocomposite membrane. J. Membr. Sci. 2012, 415, 718–724. [Google Scholar] [CrossRef]
- Nolte, M.C. Elektrisch leitfähige Umkehrosmosemembranen zur Verminderung des Biofoulings. Ph.D. Thesis, TUHH Universitätsbibliothek, Hamburg, Germany, 3 August 2009. [Google Scholar]
- Ronen, A.; Walker, S.L.; Jassby, D. Electroconductive and electroresponsive membranes for water treatment. Rev. Chem. Eng. 2016, 32, 533–550. [Google Scholar] [CrossRef]
- Duan, W.; Ronen, A.; Walker, S.; Jassby, D. Polyaniline-Coated Carbon Nanotube Ultrafiltration Membranes: Enhanced Anodic Stability for In Situ Cleaning and Electro-Oxidation Processes. ACS Appl. Mater. Interfaces 2016, 8, 22574–22584. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, S.; Jedidi, I.; Rivallin, M.; Gillot, F.; Lacour, S.; Cerneaux, S.; Cretin, M.; Amar, R.B. Elaboration and characterization of new conductive porous graphite membranes for electrochemical advanced oxidation processes. J. Membr. Sci. 2013, 446, 42–49. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, Q.; Hao, Z.; Vecitis, C.D. Carbon nanotube membrane stack for flow-through sequential regenerative electro-Fenton. Environ. Sci. Technol. 2015, 49, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Chen, G.; Chen, C.; Sanghvi, R.; Iddya, A.; Walker, S.; Liu, H.; Ronen, A.; Jassby, D. Electrochemical removal of hexavalent chromium using electrically conducting carbon nanotube/polymer composite ultrafiltration membranes. J. Membr. Sci. 2017, 531, 160–171. [Google Scholar] [CrossRef]
- Yuan, Y.; Kilduff, J.E. Hydrodynamic Modeling of NOM Transport in UF: Effects of Charge Density and Ionic Strength on Effective Size and Sieving. Environ. Sci. Technol. 2009, 43, 5449–5454. [Google Scholar] [CrossRef] [PubMed]
- Huotari, H.M.; Huisman, I.H.; Trägårdh, G. Electrically enhanced crossflow membrane filtration of oily waste water using the membrane as a cathode. J. Membr. Sci. 1999, 156, 49–60. [Google Scholar] [CrossRef]
- Van den Berg, G.B.; Smolders, C.A. Flux decline in ultrafiltration processes. Desalination 1990, 77, 101–133. [Google Scholar] [CrossRef] [Green Version]
- Gorenflo, A. Rückhalt und Fouling von natürlichen organischen Substanzen bei der Nano- und Ultrafiltration. Ph.D. Thesis, Lehrstuhl für Wasserchemie am Engler-Bunte-Inst., Karlsruhe, Germany, 3 February 2003. [Google Scholar]
- Staub, C.; Buffle, J.; Haerdi, W. Measurement of complexation properties of metal ions in natural conditions by ultrafiltration: Influence of various factors on the retention of metals and ligands by neutral and negatively charged membranes. Anal. Chem. 1984, 56, 2843–2849. [Google Scholar] [CrossRef]
- Ernst, M.; Bismarck, A.; Springer, J.; Jekel, M. Zeta-potential and rejection rates of a polyethersulfone nanofiltration membrane in single salt solutions. J. Membr. Sci. 2000, 165, 251–259. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- Barten, D.; Kleijn, J.M.; Duval, J.; Leeuwen, H.V.; Lyklema, J.; Cohen Stuart, M.A. Double Layer of a Gold Electrode Probed by AFM Force Measurements. Langmuir 2003, 19, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.R.; Amy, G.L.; Steelink, C. Molecular weight distribution, carboxylic acidity, and humic substances content of aquatic organic matter: implications for removal during water treatment. Environ. Sci. Technol. 1986, 20, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.M. Membrane Charge Characterization. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–2. [Google Scholar]
- Stumm, W. Aquatic surface chemistry: Chemical processes at the particle-water interface; Wiley: Hoboken, NJ, USA, 1987. [Google Scholar]
- Prieve, D.C. Changes in zeta potential caused by a dc electric current for thin double layers. Colloids Surf. A 2004, 250, 67–77. [Google Scholar] [CrossRef]
- Bazant, M.Z.; Kilic, M.S.; Storey, B.D.; Ajdari, A. Towards an understanding of induced-charge electrokinetics at large applied voltages in concentrated solutions. Adv. Colloid Interface Sci. 2009, 152, 48–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, J.; Lyklema, J.; Kleijn, J.M.; van Leeuwen, H.P. Amphifunctionally Electrified Interfaces: Coupling of Electronic and Ionic Surface-Charging Processes. Langmuir 2001, 17, 7573–7581. [Google Scholar] [CrossRef]
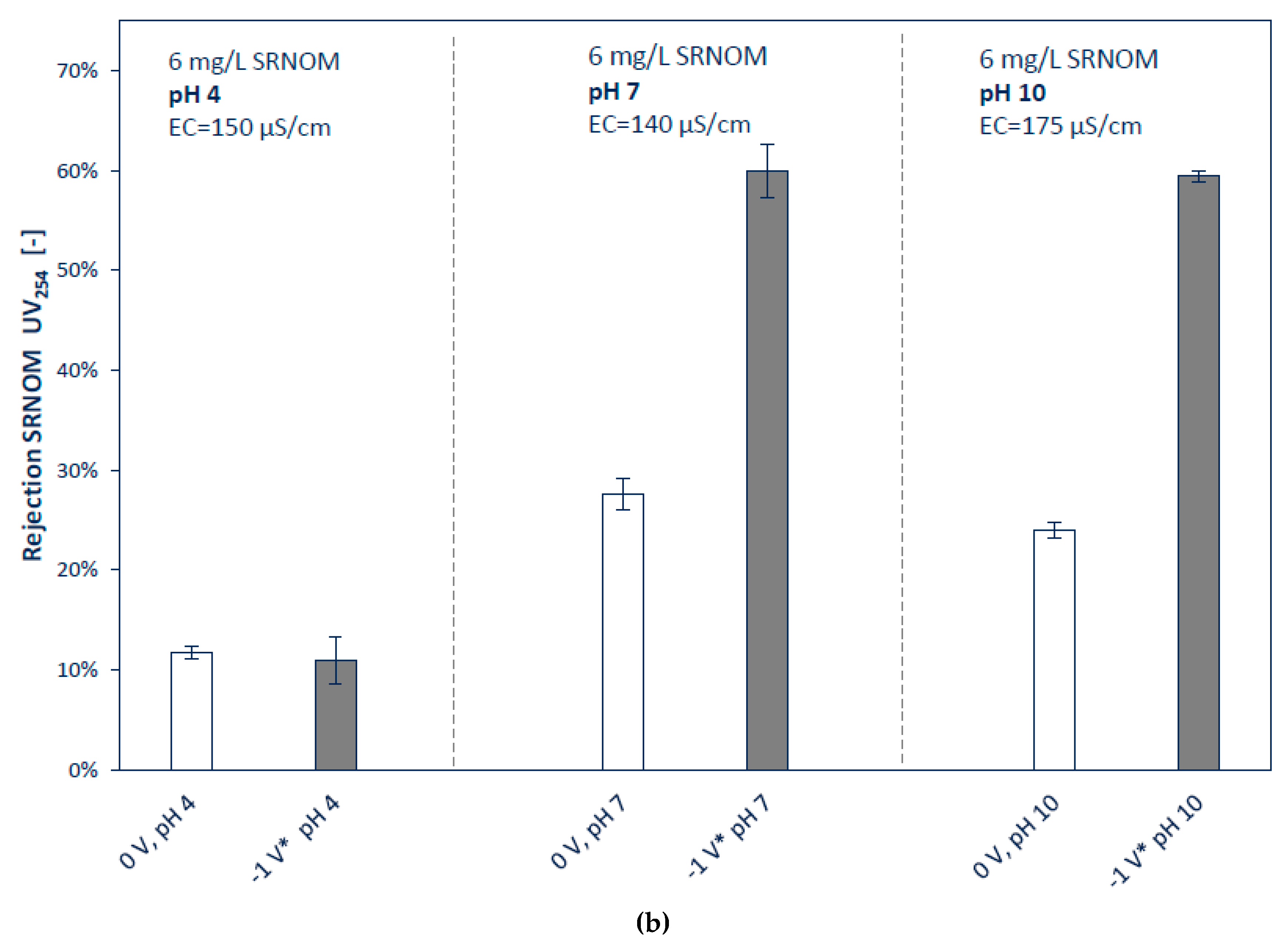
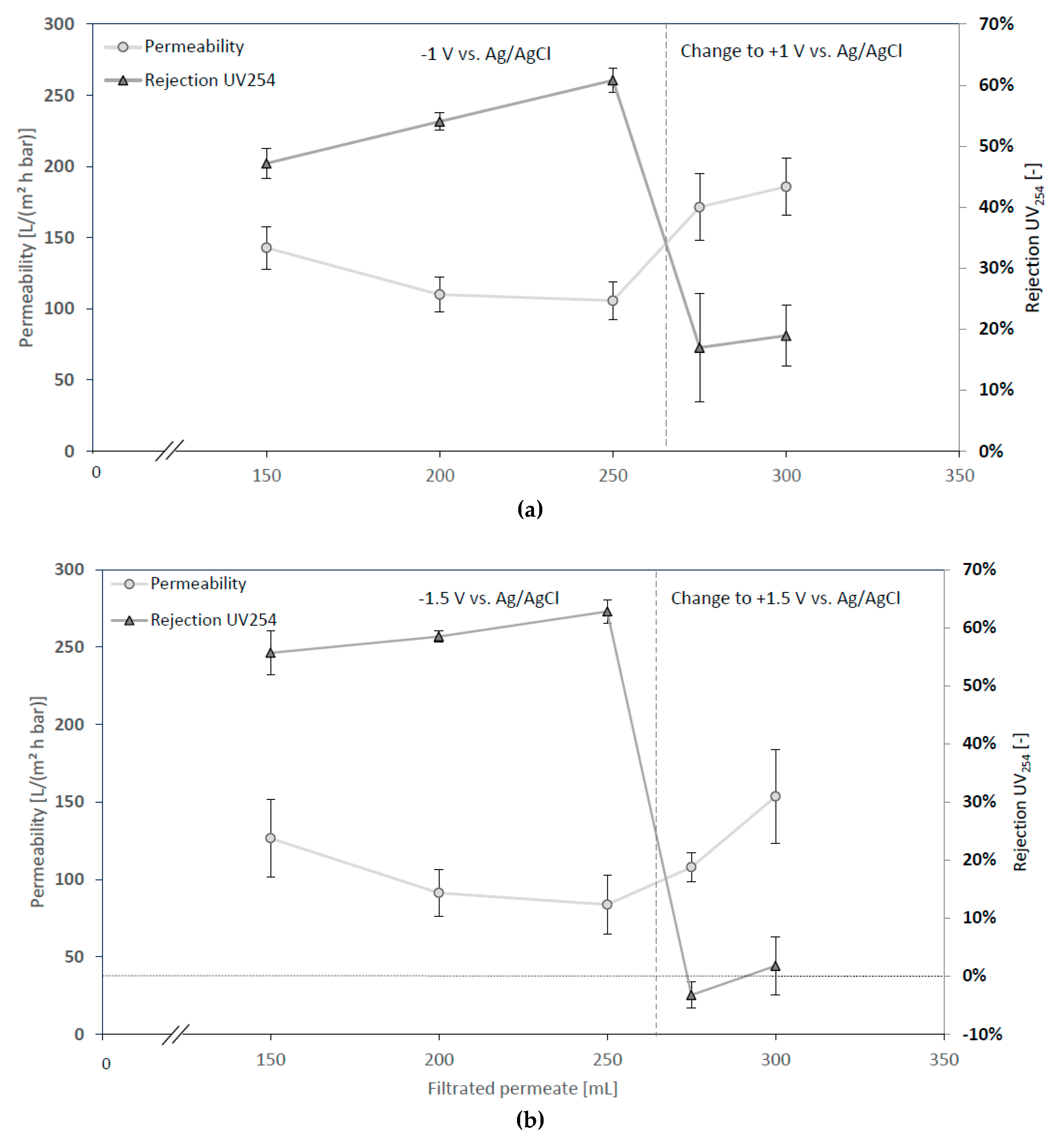
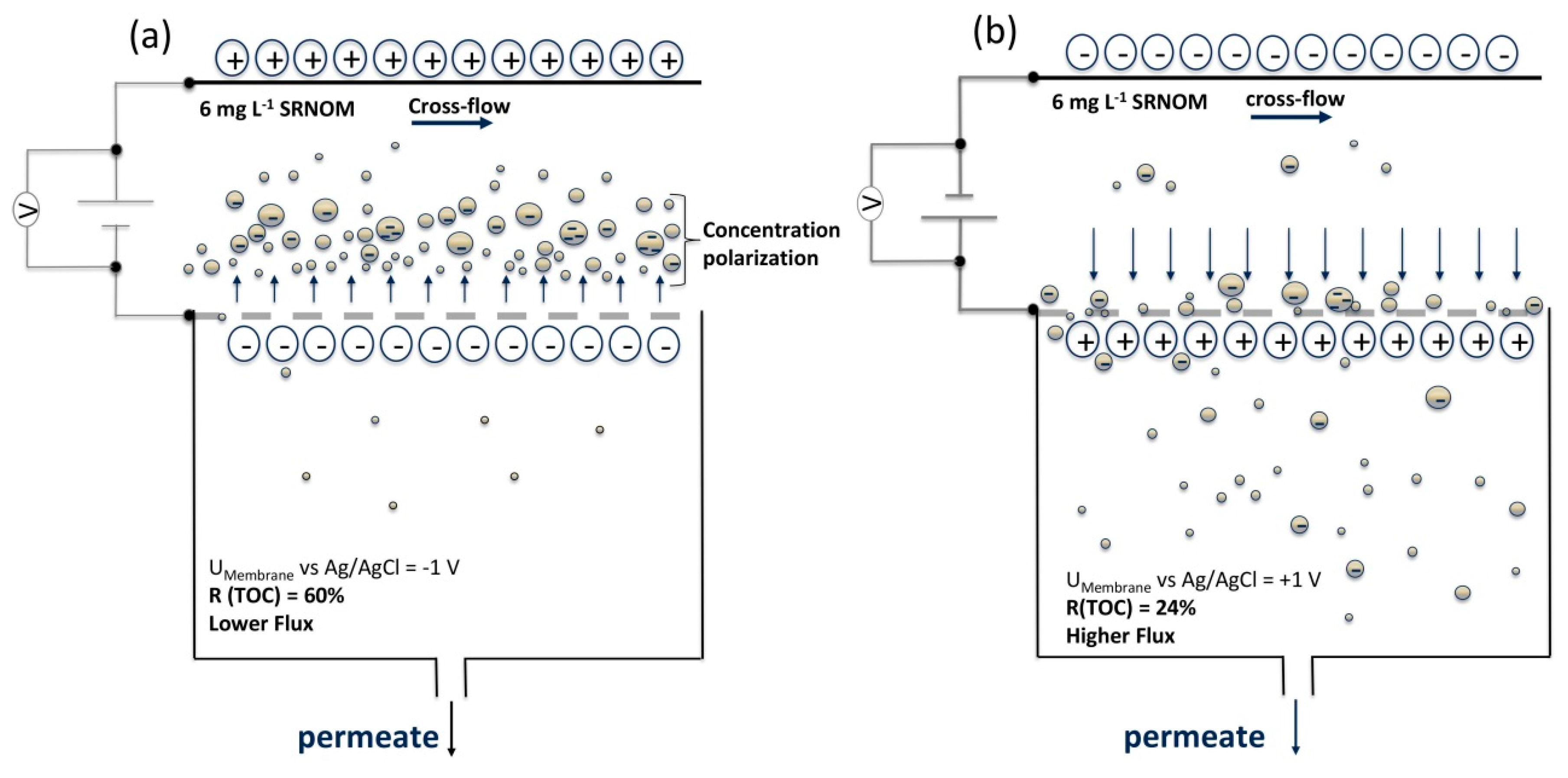
| Tested Parameter | Membrane Surface Potential | pH of Feed | Ionic Strength Feed |
|---|---|---|---|
| Membrane Surface Potential | −500, −1000, −1500, +500, +1000, +1500, 0 mV vs. Ag/AgCl | 7 | 1 mmol L−1 |
| pH of Feed | 0, −1000 mV | 4, 7, 10 | 1 mmol L−1 |
| Ionic Strength Feed | 0, −1000 mV | 7 | 0, 1, 10 mmol L−1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantel, T.; Benne, P.; Parsin, S.; Ernst, M. Electro-Conductive Composite Gold-Polyethersulfone-Ultrafiltration-Membrane: Characterization of Membrane and Natural Organic Matter (NOM) Filtration Performance at Different In-Situ Applied Surface Potentials. Membranes 2018, 8, 64. https://doi.org/10.3390/membranes8030064
Mantel T, Benne P, Parsin S, Ernst M. Electro-Conductive Composite Gold-Polyethersulfone-Ultrafiltration-Membrane: Characterization of Membrane and Natural Organic Matter (NOM) Filtration Performance at Different In-Situ Applied Surface Potentials. Membranes. 2018; 8(3):64. https://doi.org/10.3390/membranes8030064
Chicago/Turabian StyleMantel, Tomi, Paul Benne, Stanislav Parsin, and Mathias Ernst. 2018. "Electro-Conductive Composite Gold-Polyethersulfone-Ultrafiltration-Membrane: Characterization of Membrane and Natural Organic Matter (NOM) Filtration Performance at Different In-Situ Applied Surface Potentials" Membranes 8, no. 3: 64. https://doi.org/10.3390/membranes8030064
APA StyleMantel, T., Benne, P., Parsin, S., & Ernst, M. (2018). Electro-Conductive Composite Gold-Polyethersulfone-Ultrafiltration-Membrane: Characterization of Membrane and Natural Organic Matter (NOM) Filtration Performance at Different In-Situ Applied Surface Potentials. Membranes, 8(3), 64. https://doi.org/10.3390/membranes8030064