Low Dispersity and High Conductivity Poly(4-styrenesulfonic acid) Membranes Obtained by Inexpensive Free Radical Polymerization of Sodium 4-styrenesulfonate
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Polymerization
2.3. Polymeric Fiber Precipitation and Protonation
2.4. Polymeric Membranes and Conductivity Measurements
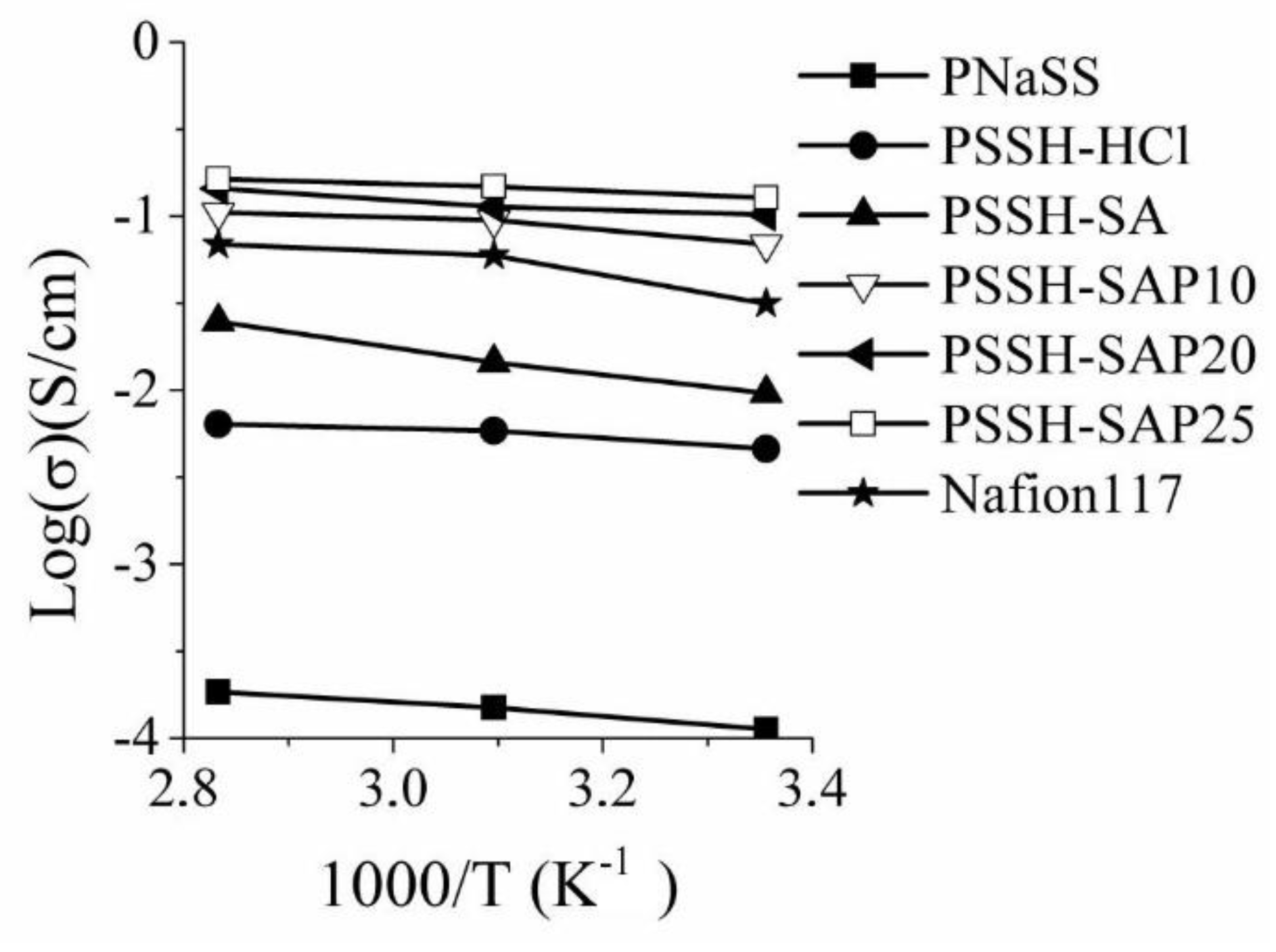
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitsukami, Y.; Donovan, M.S.; Lowe, A.B.; McCormick, C.L. Water-Soluble Polymers. 81. Direct Synthesis of Hydrophilic Styrenic-Based Homopolymers and Block Copolymers in Aqueous Solution via RAFT. Macromolecules 2001, 34, 2248–2256. [Google Scholar] [CrossRef]
- Park, Y.; Müller-Meskamp, L.; Vandewal, K.; Leo, K. PEDOT:PSS with embedded TiO2 nanoparticles as light trapping electrode for organic photovoltaics. Appl. Phys. Lett. 2016, 108, 253302. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Collins, B.A.; Sessolo, M.; Stavrinidou, E.; Strakosas, X.; Tassone, C.; Delongchamp, D.M.; Malliaras, G.G. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 2016, 7, 11287. [Google Scholar] [CrossRef] [PubMed]
- Higgins, T.M.; Park, S.H.; King, P.J.; Zhang, C.; McEvoy, N.; Berner, N.C.; Daly, D.; Shmeliov, A.; Khan, U.; Duesberg, G.; et al. A commercial Conducting Polymer as Both Binder and Conductive Additive for Silicon Nanoparticle-Based Lithium-Ion Battery Negative Electrodes. ACS Nano 2016, 10, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Yoon, S.H.; Ryu, K.-S.; Park, Y.J. PEDOT: PSS as multi-functional composite material for enhanced Li-air-battery air electrodes. Sci. Rep. 2016, 6, 19962. [Google Scholar] [CrossRef] [PubMed]
- Lica, C.G.; Segărceanu, M.; Pleşca, M.; Rikabi, A.A.; Nechifor, G. Synthesis of a New Polymer Poly ( Styrene Sulfonic Acid-Co-4-Vinylpyridine ) for Proton Exchange Membrane for Fuel Cell. UPB Sci. Bull. Ser. B 2014, 76, 151–158. [Google Scholar]
- Gadim, T.D.O.; Figueiredo, A.G.; Rosero-Navarro, N.C.; Vilela, C.; Gamelas, J.A.; Barros-Timmons, A.; Neto, C.P.; Silvestre, A.J.; Freire, C.S.; Figueiredo, F.M. Nanostructured bacterial cellulose-poly(4-styrene sulfonic acid) composite membranes with high storage modulus and protonic conductivity. ACS Appl. Mater. Interfaces 2014, 6, 7864–7875. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.-Q.; Woo, J.-J.; Seo, S.-J.; Lee, J.-S.; Moon, S.-H. Sulfonated polystyrene/polyvinyl chloride composite membranes for PEMFC applications. J. Membr. Sci. 2008, 309, 156–164. [Google Scholar] [CrossRef]
- Holboke, A.E.; Plnnell, R.P. Sulfonation of Polystyrene: Preparation and Characterization of an Ion Exchange Resin in the Organic Laboratory. J. Chem. Educ. 1989, 66, 613–614. [Google Scholar] [CrossRef]
- Idibie, C.A.; Abdulkareem, S.A.; Pienaar, C.H.; Iyuke, S.E.; VanDyk, L. Mechanism and Kinetics of Sulfonation of Polystyrene—Butadiene Rubber with Chlorosulfonic Acid. Ind. Eng. Chem. Res. 2010, 49, 1600–1604. [Google Scholar] [CrossRef]
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612. [Google Scholar] [CrossRef] [PubMed]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Iddon, P.D.; Robinson, K.L.; Armes, S.P. Polymerization of sodium 4-styrenesulfonate via atom transfer radical polymerization in protic media. Polymer 2004, 45, 759–768. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Pintauer, T.; Matyjaszewski, K. Deactivation Efficiency and Degree of Control over Polymerization in ATRP in Protic Solvents. Macromolecules 2004, 37, 9768–9778. [Google Scholar] [CrossRef]
- Mannan, M.A.; Fukuda, K.; Miura, Y. Living Radical Polymerization of Sodium 4-Styrenesulfonate Mediated by New Water-Soluble Nitroxides. Polym. J. 2007, 39, 500–501. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Homogeneous controlled free radical polymerization in aqueous media. Aust. J. Chem. 2002, 55, 367–379. [Google Scholar] [CrossRef]
- Barsbay, M.; Güven, O.; Davis, T.P.; Barner-Kowollik, C.; Barner, L. RAFT-mediated polymerization and grafting of sodium 4-styrenesulfonate from cellulose initiated via γ-radiation. Polymer 2009, 50, 973–982. [Google Scholar] [CrossRef]
- Wiley, R.H.; Reed, S.F.J. Sulfostyrenes. Polymers and copolymers of potassium p-vinylbenzenesulfonate. J. Am. Chem. Soc. 1956, 78, 2171–2173. [Google Scholar] [CrossRef]
- Wiley, R.H.; Smith, N.R.; Ketterer, C.C. Sulfostyrenes. Preparation and Polymerization of Potassium p-Vinylbenzenesulfonate. J. Am. Chem. Soc. 1954, 76, 720–723. [Google Scholar] [CrossRef]
- Safonova, L.P.; Kiselev, M.G.; Fedorova, I.V. Complexes of sulfuric acid with N,N-dimethylformamide: An ab initio investigation. Pure Appl. Chem. 2012, 85, 225–236. [Google Scholar] [CrossRef]
- Kong, X. Characterization of Proton Exchange Membrane Materials for Fuel Cells by Solid State Nuclear Magnetic Resonance; Iowa State University: Ames, IA, USA, 2010. [Google Scholar]
- Kim, H.-M. Benzoyl Peroxide; SIDnitial Assessment Report; SIAM: Boston, MA, USA, 22–25 October 2002. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; Haynes, W.M., Lide, D.R., Bruno, T.J., Eds.; CRC press: Boca Raton, FL, USA, 2014; ISBN 9781482208689. [Google Scholar]
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781439802465. [Google Scholar]
- Carroll, W.R.; Eisenberg, H. Narrow molecular weight distribution poly(styrenesulfonic acid). Part I. Preparation, solution properties, and phase separation. J. Polym. Sci. Part A-2 Polym. Chem. 1966, 4, 599–610. [Google Scholar] [CrossRef]
- Stepto, R.F.T.; Gilbert, R.G.; Hess, M.; Jenkins, A.D.; Jones, R.G.; Kratochbil, P. Dispersity in polymer science (iupac recommendation 2009) international union of pure and applied chemistry, polymer division, sub-committee on polymer terminology. Polym. Int. 2010, 59, 23–24. [Google Scholar] [CrossRef]
- Keoshkerian, B.; Georges, M.K.; Boils-boissier, D. Living Free-Radical Aqueous Polymerization. Macromolecules 1995, 28, 6381–6382. [Google Scholar] [CrossRef]
- Choi, C.; Kim, Y. Atom Transfer Radical Polymerization of Styrenesulfonic Acid Sodium Salts ( SSNa ) in Aqueous Phase. Polym. Bull. 2003, 49, 433–439. [Google Scholar] [CrossRef]
- Ma, S.; Siroma, Z.; Tanaka, H. Anisotropic Conductivity Over In-Plane and Thickness Directions in Nafion-117. J. Electrochem. Soc. 2006, 153, A2274–A2281. [Google Scholar] [CrossRef]
- Silva, R.F.; Francesco, M.; De Pozio, A. Tangential and normal conductivities of Nafion® membranes used in polymer electrolyte fuel cells. J. Power Sources 2004, 134, 18–26. [Google Scholar] [CrossRef]
- Yadav, R.; Fedkiw, P.S. Analysis of EIS Technique and Nafion 117 Conductivity as a Function of Temperature and Relative Humidity. J. Electrochem. Soc. 2012, 159, B340–B346. [Google Scholar] [CrossRef]

| System | W/DMF | tpa (h) | Recovery (%) b (1st; 2nd; 3rd) | Mw (kDa) (1st) | ÐMc (1st) |
|---|---|---|---|---|---|
| PNaSS91 | 9/1 | 17 | 93; 90; 88 | 680 | 1.85 |
| PNaSS82 | 8/2 | 16 | 98; 96; 95 | 748 | 1.85 |
| PNaSS73 | 7/3 | 14 | 96; 94; 90 | 450 | 1.52 |
| PNaSS64 | 6/4 | 13 | 96; 93; 90 | 500 | 1.54 |
| PNaSS46 | 4/6 | 9 | 92; 90; 84 | 376 | 1.40 |
| PNaSS37 | 3/7 | 9 | 85; 83; 80 | 355 | 1.74 |
| PNaSS28 | 2/8 | 6 | 70; 72; 78 | 364 | 1.66 |
| PNaSS19 | 1/9 | 6 | 60; 58; 53 | 195 | 1.15 |
| Sample | IEC a meq/g | Water b (%) | T c (°C) | σ⊥ (mS/cm) |
|---|---|---|---|---|
| PNaSS | 0 | 9 | 25 | 0.11 |
| 50 | 0.15 | |||
| 80 | 0.18 | |||
| PSSH82-HCl | 1.3 | 10 | 25 | 4.62 |
| 50 | 5.85 | |||
| 80 | 6.40 | |||
| PSSH82-SA | 3.0 | 16 | 25 | 9.63 |
| 50 | 14.4 | |||
| 80 | 24.7 | |||
| PSSH82-SAP10 | 4.8 | 10 | 25 | 68.9 |
| 50 | 95.2 | |||
| 80 | 105.2 | |||
| PSSH82-SAP22 | 4.8 | 22 | 25 | 102.4 |
| 50 | 113.5 | |||
| 80 | 144.8 | |||
| PSSH82-SAP28 | 4.8 | 28 | 25 | 127.8 |
| 50 | 148.4 | |||
| 80 | 164.3 | |||
| Nafion117 | 0.9 | 6 | 25 | 31.5 |
| 50 | 59.7 | |||
| 80 | 68.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepulveda, V.R.; Sierra, L.; López, B.L. Low Dispersity and High Conductivity Poly(4-styrenesulfonic acid) Membranes Obtained by Inexpensive Free Radical Polymerization of Sodium 4-styrenesulfonate. Membranes 2018, 8, 58. https://doi.org/10.3390/membranes8030058
Sepulveda VR, Sierra L, López BL. Low Dispersity and High Conductivity Poly(4-styrenesulfonic acid) Membranes Obtained by Inexpensive Free Radical Polymerization of Sodium 4-styrenesulfonate. Membranes. 2018; 8(3):58. https://doi.org/10.3390/membranes8030058
Chicago/Turabian StyleSepulveda, Victor Raul, Ligia Sierra, and Betty Lucy López. 2018. "Low Dispersity and High Conductivity Poly(4-styrenesulfonic acid) Membranes Obtained by Inexpensive Free Radical Polymerization of Sodium 4-styrenesulfonate" Membranes 8, no. 3: 58. https://doi.org/10.3390/membranes8030058
APA StyleSepulveda, V. R., Sierra, L., & López, B. L. (2018). Low Dispersity and High Conductivity Poly(4-styrenesulfonic acid) Membranes Obtained by Inexpensive Free Radical Polymerization of Sodium 4-styrenesulfonate. Membranes, 8(3), 58. https://doi.org/10.3390/membranes8030058





