The Role of Ion Exchange Membranes in Membrane Capacitive Deionisation
Abstract
:1. Introduction
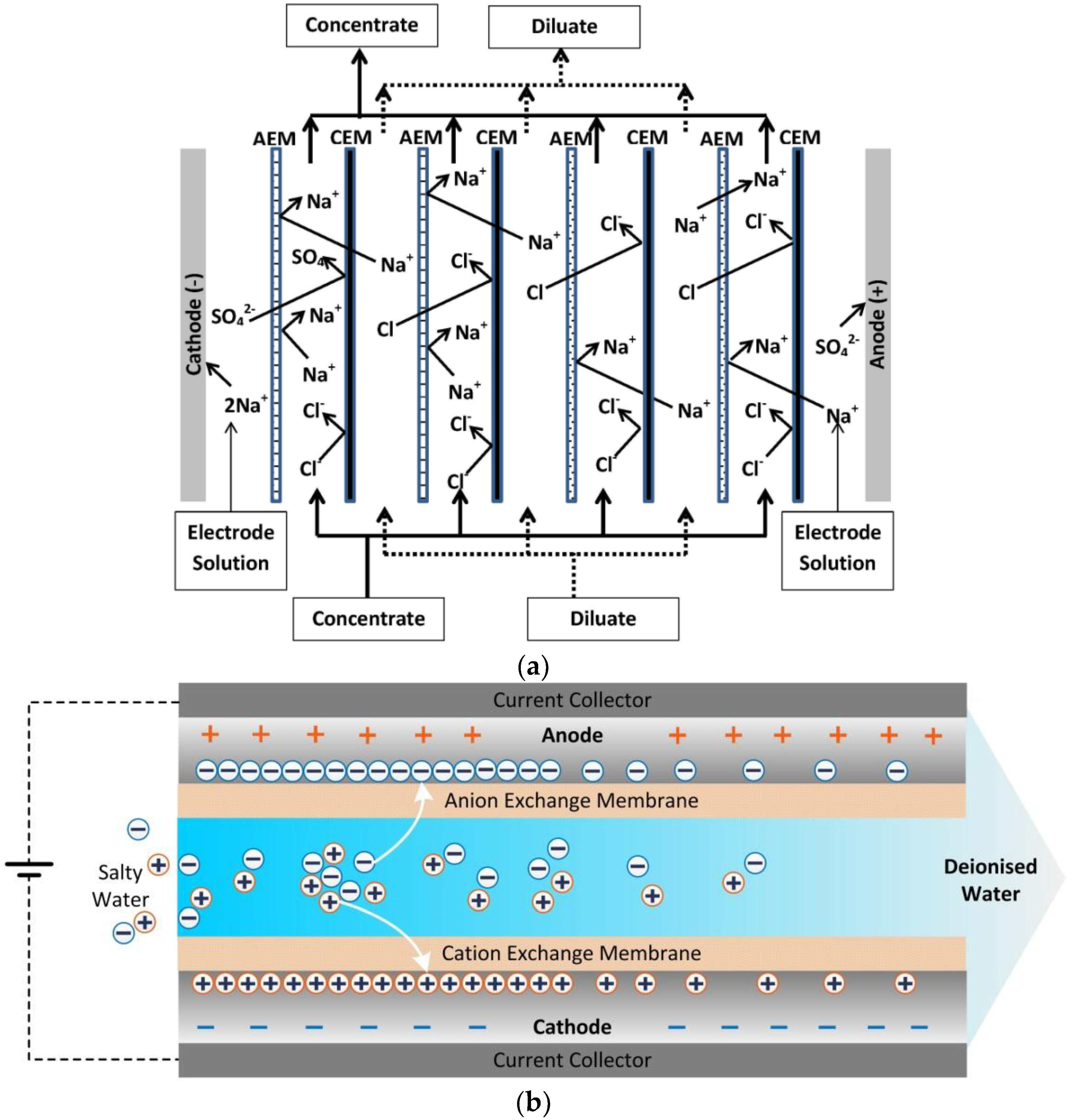
2. Ion Exchange Membranes for Membrane Capacitive Deionisation (MCDI) Applications
2.1. Homogeneous Ion Exchange Membranes
2.2. Composite Electrodes for MCDI
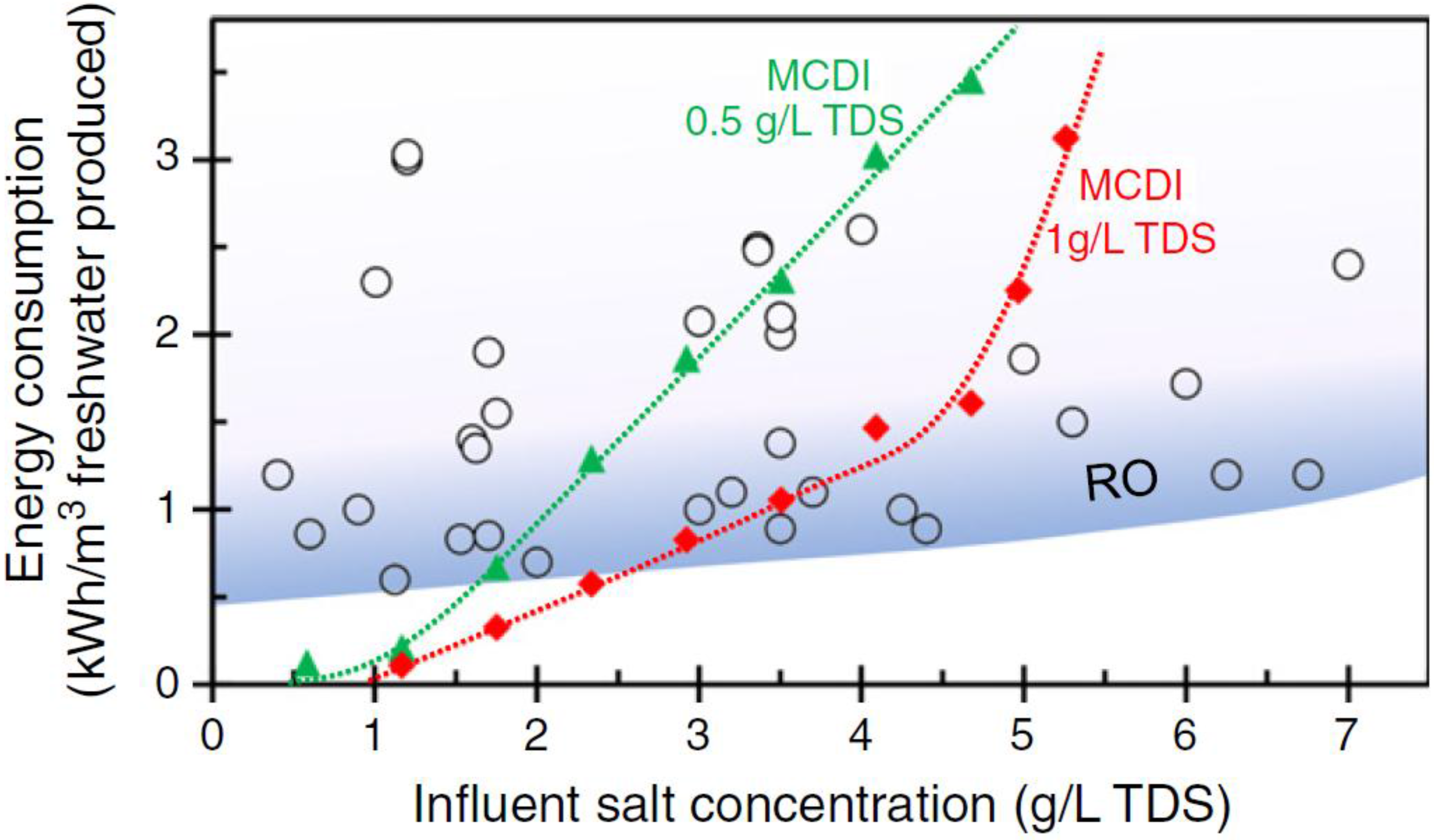
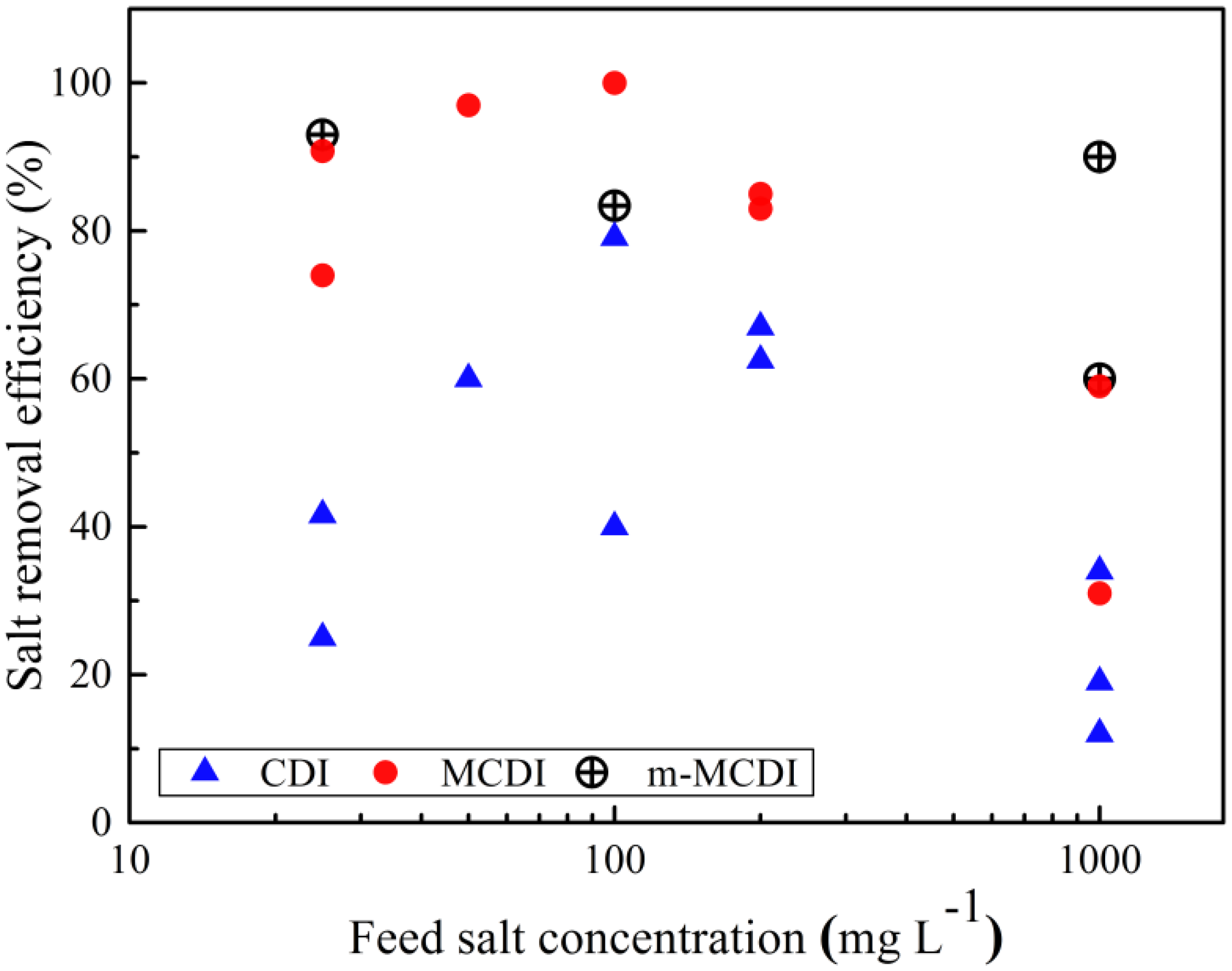
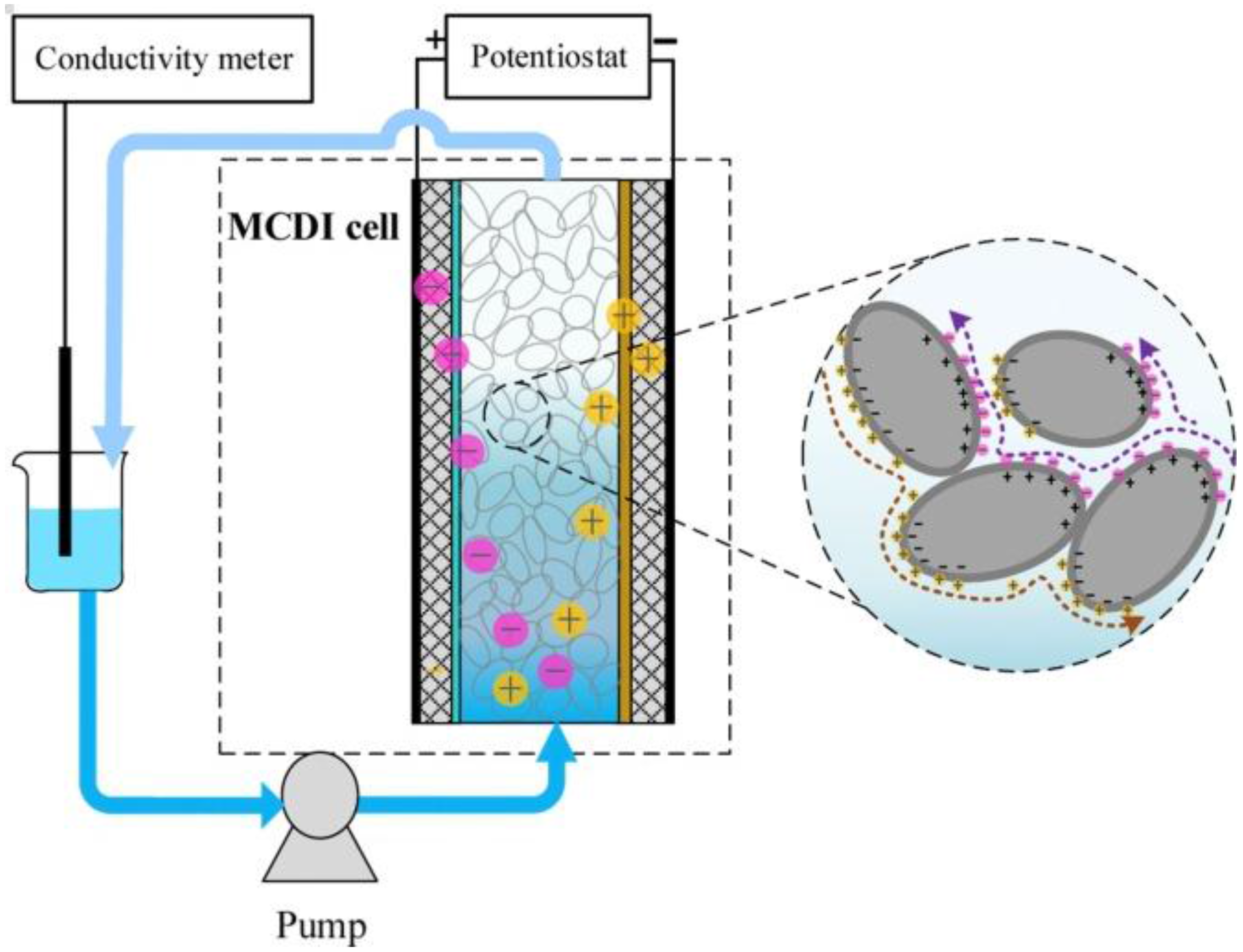
3. MCDI Performance Parameters
4. Fouling
4.1. Fouling in Capacitive Deionisation
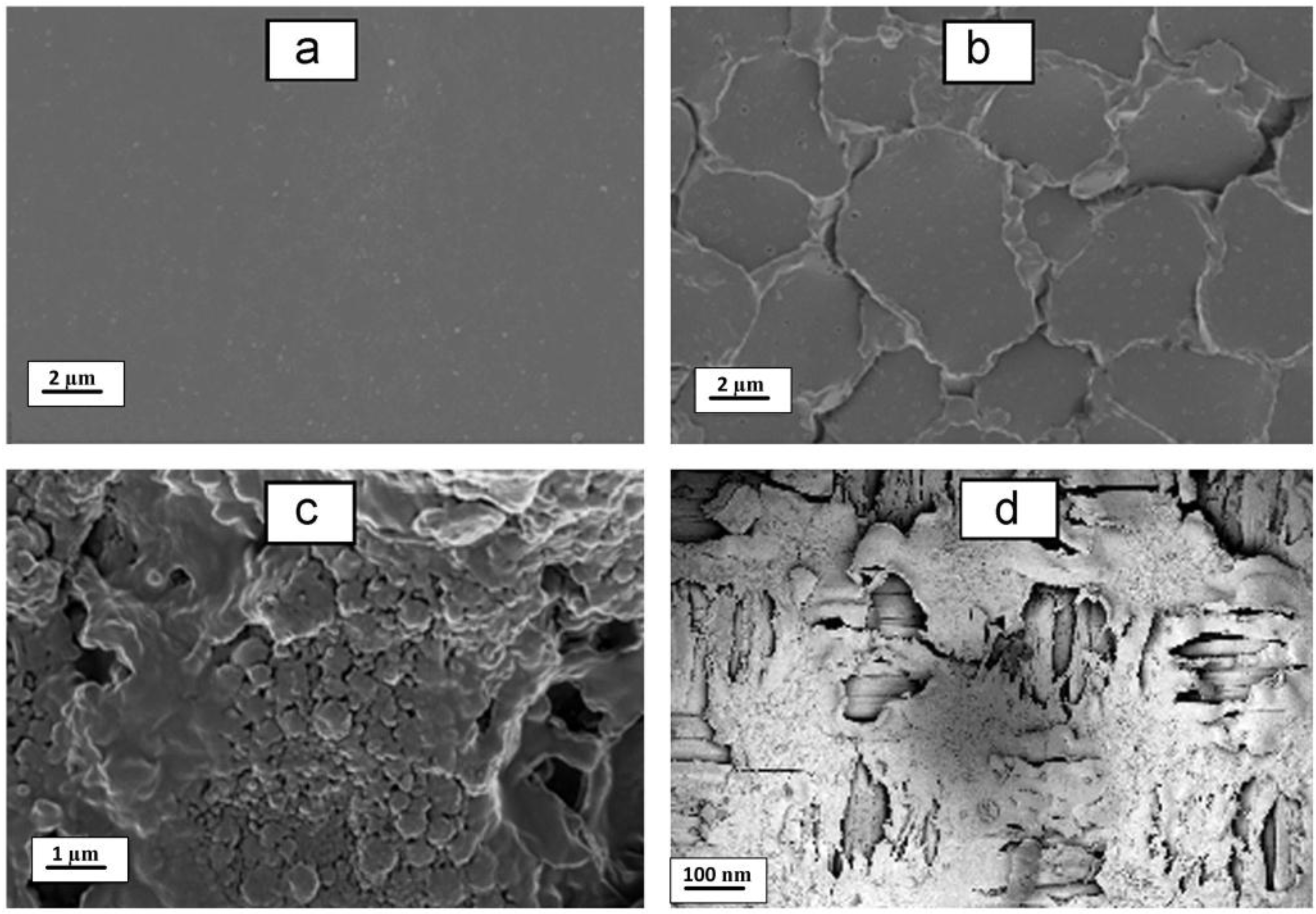
4.2. Fouling of Ion Exchange Membranes
4.3. Alterations to Membrane Chemistry
4.4. Cleaning Solutions
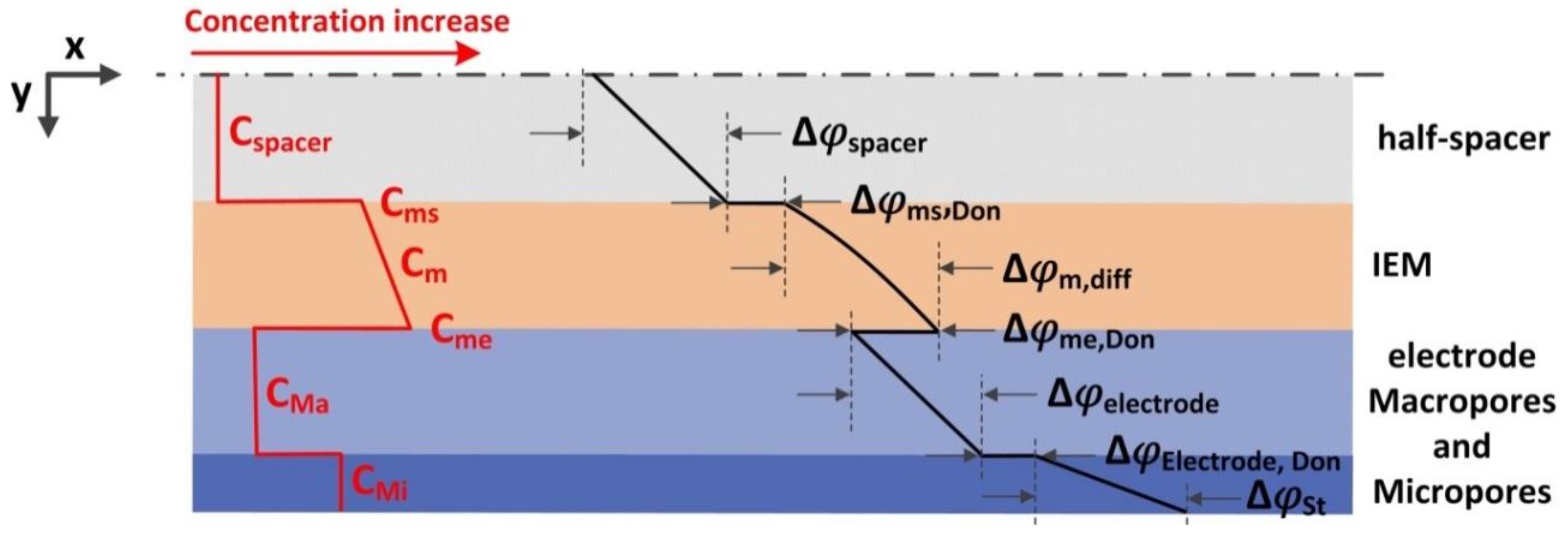
5. Modelling MCDI Behaviour
6. MCDI Novel Stack Developments and Outlook
6.1. Radial Deionisation (RDI)
6.2. Flow Chamber Modification
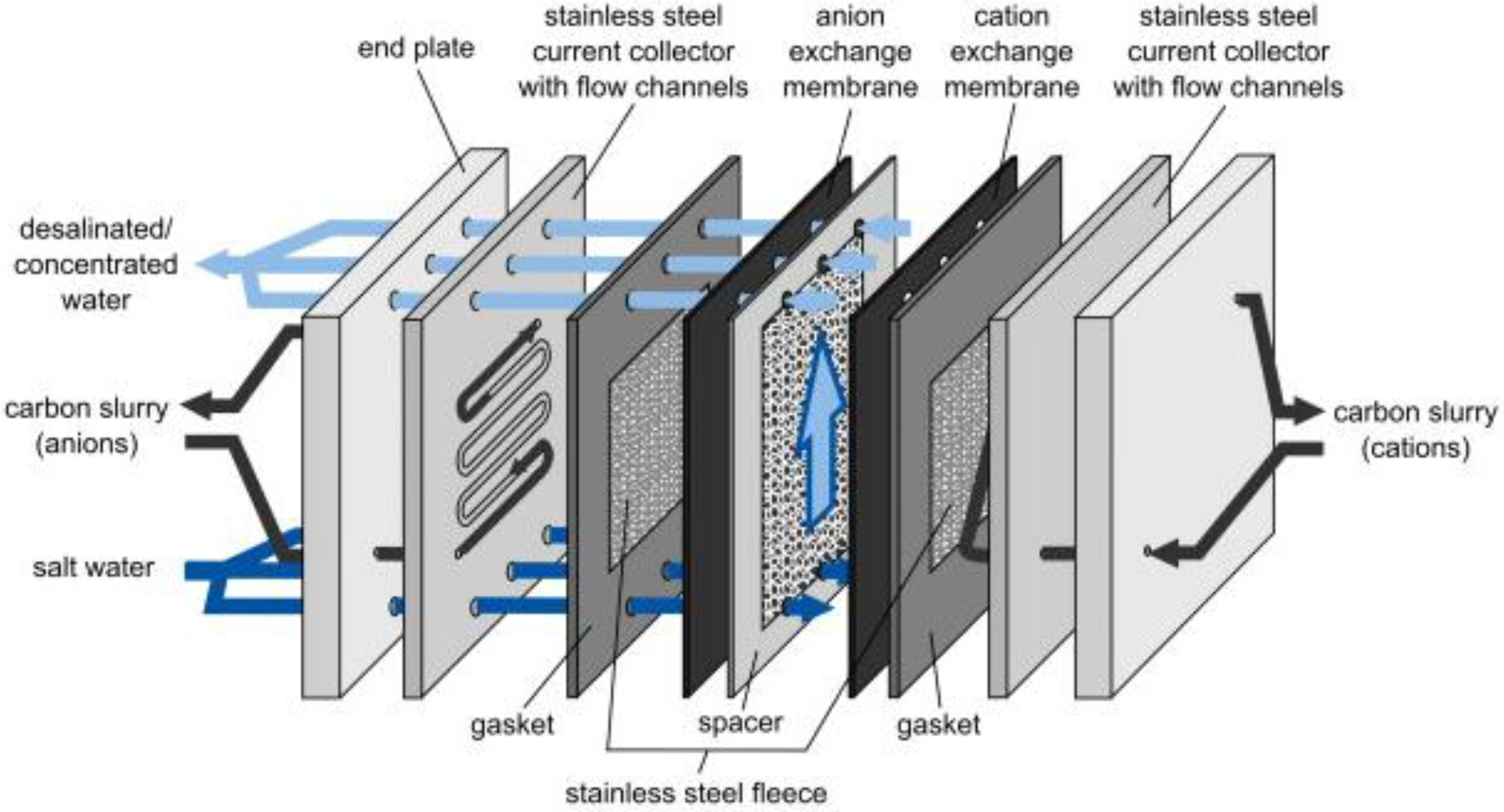
6.3. Flow-Electrodes Capacitive Deionisation
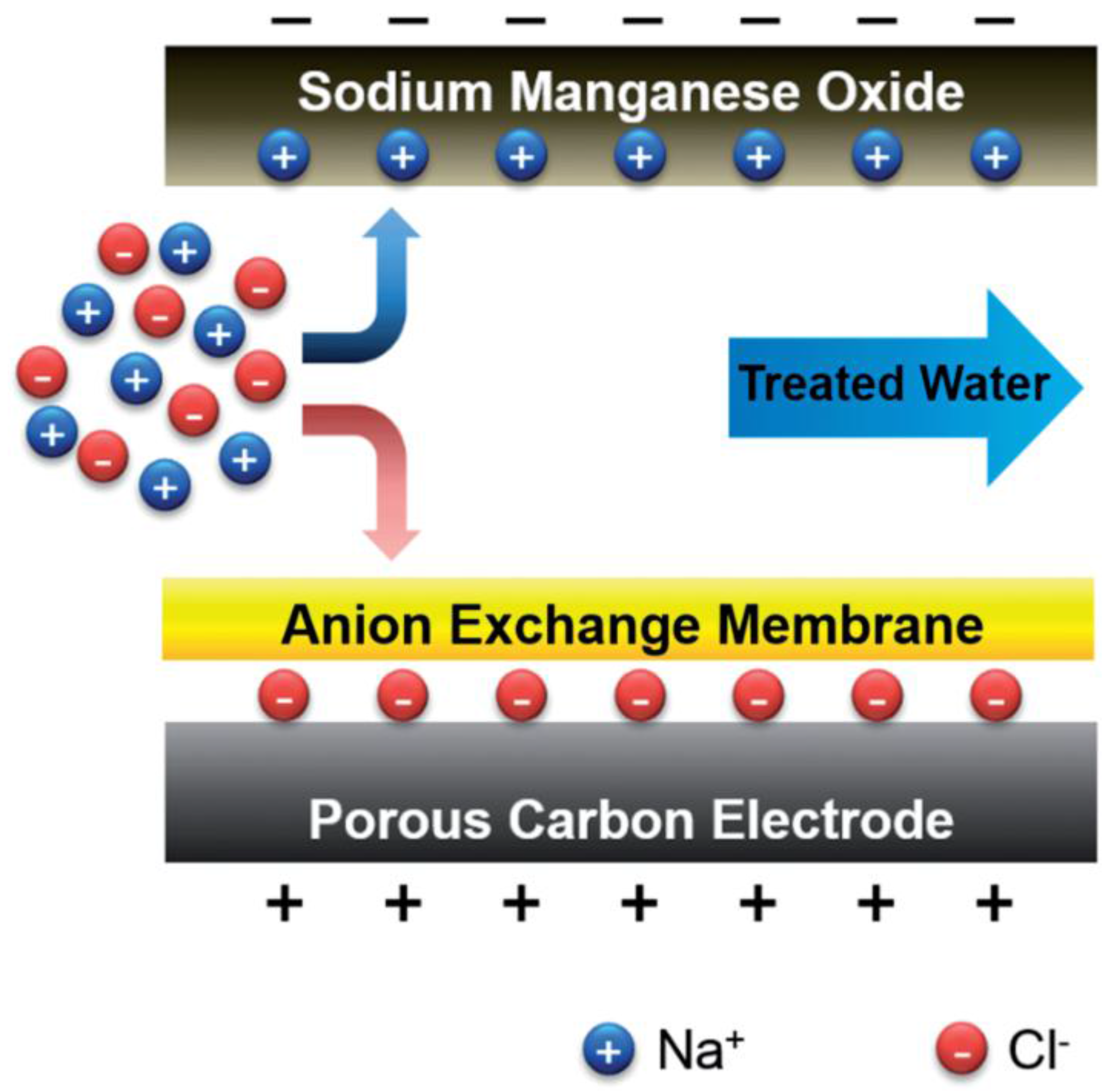
6.4. Hybrid Capacitive Deionisation (CDI)
7. Conclusions and Future Research Directions
Acknowledgments
Conflicts of Interest
References
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Sata, T. Ion Exchange Membranes: Preparation, Characterization, Modification and Application; Royal Society of Chemistry: London, UK, 2004. [Google Scholar]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-Exchange Membrane Separation Processes; Elsevier: Amsterdam, The Netherlands, 2004; Volume 9. [Google Scholar]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K. Recent developments on ion-exchange membranes and electro-membrane processes. Adv. Colloid Interface Sci. 2006, 119, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis and its application in the chemical process industry. Sep. Purif. Methods 1985, 14, 41–66. [Google Scholar] [CrossRef]
- Bazinet, L. Electrodialytic phenomena and their applications in the dairy industry: A review. Crit. Rev. Food Sci. Nutr. 2005, 45, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.E.; Kloester, E.; Stevens, G.W.; Scholes, C.A.; Dumée, L.F. Electrodialysis in aqueous-organic mixtures. Sep. Purif. Rev. 2015, 44, 269–282. [Google Scholar] [CrossRef]
- Demirer, O.N.; Naylor, R.M.; Rios Perez, C.A.; Wilkes, E.; Hidrovo, C. Energetic performance optimization of a capacitive deionization system operating with transient cycles and brackish water. Desalination 2013, 314, 130–138. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Jiang, Y.; Wang, T.-J.; Wang, H. Effects of the hydration ratio on the electrosorption selectivity of ions during capacitive deionization. Desalination 2016, 399, 171–177. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionisation. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef]
- Han, L.; Karthikeyan, K.G.; Anderson, M.A.; Wouters, J.J.; Gregory, K.B. Mechanistic insights into the use of oxide nanoparticles coated asymmetric electrodes for capacitive deionisation. Electrochim. Acta 2013, 90, 573–581. [Google Scholar] [CrossRef]
- Lee, J.-B.; Park, K.-K.; Eum, H.-M.; Lee, C.-W. Desalination of a thermal power plant wastewater by membrane capacitive deionization. Desalination 2006, 196, 125–134. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Zhao, R.; Porada, S.; van der Wal, A. Theory of membrane capacitive deionisation including the effect of the electrode pore space. J. Colloid Interface Sci. 2011, 360, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Porada, S.; Biesheuvel, P.M.; van der Wal, A. Energy consumption in membrane capacitive deionisation for different water recoveries and flow rates, and comparison with reverse osmosis. Desalination 2013, 330, 35–41. [Google Scholar] [CrossRef]
- McRae, W.A. Electroseparations, Electrodialysis. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- He, S.; Liu, L.; Wang, X.; Zhang, S.; Guiver, M.D.; Li, N. Azide-assisted self-crosslinking of highly ion conductive anion exchange membranes. J. Membr. Sci. 2016, 509, 48–56. [Google Scholar] [CrossRef]
- Kariduraganavar, M.Y.; Nagarale, R.K.; Kittur, A.A.; Kulkarni, S.S. Ion-exchange membranes: Preparative methods for electrodialysis and fuel cell applications. Desalination 2006, 197, 225–246. [Google Scholar] [CrossRef]
- Kwak, N.-S.; Koo, J.S.; Hwang, T.S.; Choi, E.M. Synthesis and electrical properties of NaSS–MAA–MMA cation exchange membranes for membrane capacitive deionisation (MCDI). Desalination 2012, 285, 138–146. [Google Scholar] [CrossRef]
- Kang, K.W.; Hwang, C.W.; Hwang, T.S. Synthesis and properties of sodium vinylbenzene sulfonate-grafted poly(vinylidene fluoride) cation exchange membranes for membrane capacitive deionisation process. Macromol. Res. 2015, 23, 1126–1133. [Google Scholar] [CrossRef]
- Jeong, K.S.; Hwang, W.C.; Hwang, T.S. Synthesis of an aminated poly(vinylidene fluride-g-4-vinyl benzyl chloride) anion exchange membrane for membrane capacitive deionisation(MCDI). J. Membr. Sci. 2015, 495, 316–321. [Google Scholar] [CrossRef]
- Qiu, Q.; Cha, J.-H.; Choi, Y.-W.; Choi, J.-H.; Shin, J.; Lee, Y.-S. Preparation of stable polyethylene membranes filled with crosslinked sulfonated polystyrene for membrane capacitive deionisation by γ-irradiation. Macromol. Res. 2017, 25, 92–95. [Google Scholar] [CrossRef]
- Lee, J.-B.; Park, K.-K.; Yoon, S.-W.; Park, P.-Y.; Park, K.-I.; Lee, C.-W. Desalination performance of a carbon-based composite electrode. Desalination 2009, 237, 155–161. [Google Scholar] [CrossRef]
- Hou, C.-H.; Liu, N.-L.; Hsu, H.-L.; Den, W. Development of multi-walled carbon nanotube/poly(vinyl alcohol) composite as electrode for capacitive deionisation. Sep. Purif. Technol. 2014, 130, 7–14. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, L.; Xu, X.; Lu, T.; Sun, Z.; Chua, D.H. Enhanced desalination efficiency in modified membrane capacitive deionisation by introducing ion-exchange polymers in carbon nanotubes electrodes. Electrochim. Acta 2014, 130, 619–624. [Google Scholar] [CrossRef]
- Kim, J.-S.; Choi, J.-H. Fabrication and characterization of a carbon electrode coated with cation-exchange polymer for the membrane capacitive deionisation applications. J. Membr. Sci. 2010, 355, 85–90. [Google Scholar] [CrossRef]
- Qian, B.; Wang, G.; Ling, Z.; Dong, Q.; Wu, T.; Zhang, X.; Qiu, J. Sulfonated Graphene as Cation-Selective Coating: A New Strategy for High-Performance Membrane Capacitive Deionization. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Choi, J.-H. Improvement of desalination efficiency in capacitive deionization using a carbon electrode coated with an ion-exchange polymer. Water Res. 2010, 44, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.; Shin, H.; Rhim, J. Application of synthesized anion and cation exchange polymers to membrane capacitive deionization (MCDI). Macromol. Res. 2015, 23, 360–366. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Seo, S.-J.; Yun, S.-H.; Moon, S.-H. Preparation of ion exchanger layered electrodes for advanced membrane capacitive deionization (MCDI). Water Res. 2011, 45, 5375–5380. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, H.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Grafting sulfonic and amine functional groups on 3D graphene for improved capacitive deionization. J. Mater. Chem. A 2016, 4, 5303–5313. [Google Scholar] [CrossRef]
- Kim, J.S.; Jeon, Y.S.; Rhim, J.W. Application of poly(vinyl alcohol) and polysulfone based ionic exchange polymers to membrane capacitive deionization for the removal of mono- and divalent salts. Sep. Purif. Technol. 2016, 157, 45–52. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, H.S.; Cho, M.D.; Kang, H.R. Regeneration Methods of Capacitive Deionization Electrodes in Water Purification. U.S. Patent 20,160,289,097 A1, 6 October 2016. [Google Scholar]
- Kim, Y.-J.; Choi, J.-H. Selective removal of nitrate ion using a novel composite carbon electrode in capacitive deionization. Water Res. 2012, 46, 6033–6039. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.-H.; Choi, J.-H. Enhancement of nitrate removal from a solution of mixed nitrate, chloride and sulfate ions using a nitrate-selective carbon electrode. Desalination 2013, 320, 10–16. [Google Scholar] [CrossRef]
- Tian, G.; Liu, L.; Meng, Q.; Cao, B. Preparation and characterization of cross-linked quaternised polyvinyl alcohol membrane/activated carbon composite electrode for membrane capacitive deionisation. Desalination 2014, 354, 107–115. [Google Scholar] [CrossRef]
- Gu, X.; Deng, Y.; Wang, C. Fabrication of anion-exchange polymer layered graphene-melamine electrodes for membrane capacitive deionisation. ACS Sustain. Chem. Eng. 2017, 5, 325–333. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Pan, L.; Zhang, Y.; Chen, Y.; Sun, Z. Electrosorptive desalination by carbon nanotubes and nanofibres electrodes and ion-exchange membranes. Water Res. 2008, 42, 4923–4928. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Choi, J.-H. Enhanced desalination efficiency in capacitive deionisation with an ion-selective membrane. Sep. Purif. Technol. 2010, 71, 70–75. [Google Scholar] [CrossRef]
- Li, H.; Zou, L. Ion-exchange membrane capacitive deionisation: A new strategy for brackish water desalination. Desalination 2011, 275, 62–66. [Google Scholar] [CrossRef]
- Liang, P.; Yuan, L.; Yang, X.; Zhou, S.; Huang, X. Coupling ion-exchangers with inexpensive activated carbon fiber electrodes to enhance the performance of capacitive deionisation cells for domestic wastewater desalination. Water Res. 2013, 47, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Omosebi, A.; Gao, X.; Landon, J.; Liu, K. Asymmetric electrode configuration for enhanced membrane capacitive deionisation. ACS Appl. Mater. Interfaces 2014, 6, 12640–12649. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Wang, R.; Wu, Y.; Xu, S.; Wang, J. Performance comparison and energy consumption analysis of capacitive deionization and membrane capacitive deionization processes. Desalination 2013, 324, 127–133. [Google Scholar] [CrossRef]
- Ding, M.; Shi, W.; Guo, L.; Leong, Z.Y.; Baji, A.; Yang, H.Y. Bimetallic metal–organic framework derived porous carbon nanostructures for high performance membrane capacitive desalination. J. Mat. Chem. A 2017, 5, 6113–6121. [Google Scholar] [CrossRef]
- Duan, F.; Li, Y.; Cao, H.; Wang, Y.; Zhang, Y.; Crittenden, J.C. Activated carbon electrodes: Electrochemical oxidation coupled with desalination for wastewater treatment. Chemosphere 2015, 125, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Biesheuvel, P.M.; Miedema, H.; Bruning, H.; van der Wal, A. Charge efficiency: A functional tool to probe the double-layer structure inside of porous electrodes and application in the modeling of capacitive deionisation. J. Phys. Chem. Lett. 2010, 1, 205–210. [Google Scholar] [CrossRef]
- Porada, S.; Bryjak, M.; van der Wal, A.; Biesheuvel, P.M. Effect of electrode thickness variation on operation of capacitive deionisation. Electrochim. Acta 2012, 75, 148–156. [Google Scholar] [CrossRef]
- Huyskens, C.; Helsen, J.; de Haan, A.B. Capacitive deionisation for water treatment: Screening of key performance parameters and comparison of performance for different ions. Desalination 2013, 328, 8–16. [Google Scholar] [CrossRef]
- Choi, J.-H. Comparison of constant voltage (CV) and constant current (CC) operation in the membrane capacitive deionisation process. Desalination Water Treat. 2014, 56, 921–928. [Google Scholar] [CrossRef]
- Qu, Y.; Campbell, P.G.; Gu, L.; Knipe, J.M.; Dzenitis, E.; Santiago, J.G.; Stadermann, M. Energy consumption analysis of constant voltage and constant current operations in capacitive deionisation. Desalination 2016, 400, 18–24. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Hur, J.; Bae, W.; Choi, J.-H. Desalination of brackish water containing oil compound by capacitive deionisation process. Desalination 2010, 253, 119–123. [Google Scholar] [CrossRef]
- Li, H.; Nie, C.; Pan, L.; Sun, Z. The study of membrane capacitive deionisation from charge efficiency. Desalin. Water Treat. 2012, 42, 210–215. [Google Scholar] [CrossRef]
- Wimalasiri, Y.; Mossad, M.; Zou, L. Thermodynamics and kinetics of adsorption of ammonium ions by graphene laminate electrodes in capacitive deionisation. Desalination 2015, 357, 178–188. [Google Scholar] [CrossRef]
- Mossad, M.; Zou, L. A study of the capacitive deionisation performance under various operational conditions. J. Hazard. Mater. 2012, 213–214, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Dlugolecki, P.; van der Wal, A. Energy recovery in membrane capacitive deionisation. Environ. Sci. Technol. 2013, 47, 4904–4910. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Kim, J.-H.; Choi, J.-H. Selective removal of nitrate ions by controlling the applied current in membrane capacitive deionisation (MCDI). J. Membr. Sci. 2013, 429, 52–57. [Google Scholar] [CrossRef]
- Ryu, T.; Lee, D.-H.; Ryu, J.C.; Shin, J.; Chung, K.-S.; Kim, Y.H. Lithium recovery system using electrostatic field assistance. Hydrometallurgy 2015, 151, 78–83. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.; Kim, S.-R.; Kang, J.; Kim, S.; Kim, C.; Yoon, J. Capacitive deionisation with Ca-alginate coated-carbon electrode for hardness control. Desalination 2016, 392, 46–53. [Google Scholar] [CrossRef]
- Choi, J.; Lee, H.; Hong, S. Capacitive deionisation (CDI) integrated with monovalent cation selective membrane for producing divalent cation-rich solution. Desalination 2016, 400, 38–46. [Google Scholar] [CrossRef]
- Rice, G.; Barber, A.R.; O’Connor, A.J.; Stevens, G.W.; Kentish, S.E. Rejection of dairy salts by a nanofiltration membrane. Sep. Purif. Technol. 2011, 79, 92–102. [Google Scholar] [CrossRef]
- Garcia-Aleman, J.; Dickson, J.M. Permeation of mixed-salt solutions with commercial and pore-filled nanofiltration membranes: Membrane charge inversion phenomena. J. Membr. Sci. 2004, 239, 163–172. [Google Scholar] [CrossRef]
- Labbez, C.; Fievet, P.; Szymczyk, A.; Vidonne, A.; Foissy, A.; Pagetti, J. Retention of mineral salts by a polyamide nanofiltration membrane. Sep. Purif. Technol. 2003, 30, 47–55. [Google Scholar] [CrossRef]
- Schaep, J.; Vandecasteele, C.; Mohammad, A.W.; Bowen, W.R. Analysis of the salt retention of nanofiltration membranes using the Donnan-steric partitioning pore model. Sep. Sci. Technol. 1999, 34, 3009–3030. [Google Scholar] [CrossRef]
- Van Limpt, B.; van der Wal, A. Water and chemical savings in cooling towers by using membrane capacitive deionisation. Desalination 2014, 342, 148–155. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Goh, P.S.; Lau, W.J.; Misdan, N.; Ismail, A.F. Nanomaterials for biofouling and scaling mitigation of thin film composite membrane: A review. Desalination 2016, 393, 2–15. [Google Scholar] [CrossRef]
- Piyadasa, C.; Ridgway, H.F.; Yeager, T.R.; Stewart, M.B.; Pelekani, C.; Gray, S.R.; Orbell, J.D. The application of electromagnetic fields to the control of the scaling and biofouling of reverse osmosis membranes—A review. Desalination 2017, 418, 19–34. [Google Scholar] [CrossRef]
- Mossad, M.; Zou, L. Study of fouling and scaling in capacitive deionisation by using dissolved organic and inorganic salts. J. Hazard. Mater. 2013, 244–245, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mossad, M.; Zou, L. A study of the long-term operation of capacitive deionisation in inland brackish water desalination. Desalination 2013, 320, 80–85. [Google Scholar] [CrossRef]
- Wang, C.; Song, H.; Zhang, Q.; Wang, B.; Li, A. Parameter optimization based on capacitive deionisation for highly efficient desalination of domestic wastewater biotreated effluent and the fouled electrode regeneration. Desalination 2015, 365, 407–415. [Google Scholar] [CrossRef]
- Korngold, E.; De Korosy, F.; Rahav, R.; Taboch, M.F. Fouling of anion-selective membranes in electrodialysis. Desalination 1970, 8, 195–220. [Google Scholar] [CrossRef]
- Lee, H.-J.; Moon, S.-H.; Tsai, S.-P. Effects of pulsed electric fields on membrane fouling in electrodialysis of NaCl solution containing humate. Sep. Purif. Technol. 2002, 27, 89–95. [Google Scholar] [CrossRef]
- Langevin, M.-E.; Bazinet, L. Ion-exchange membrane fouling by peptides: A phenomenon governed by electrostatic interactions. J. Membr. Sci. 2011, 369, 359–366. [Google Scholar] [CrossRef]
- Fidaleo, M.; Moresi, M. Electrodialysis applications in the food industry. Adv. Food Nutr. Res. 2006, 51, 265–360. [Google Scholar] [PubMed]
- Lee, H.-J.; Hong, M.-K.; Han, S.-D.; Cho, S.-H.; Moon, S.-H. Fouling of an anion exchange membrane in the electrodialysis desalination process in the presence of organic foulants. Desalination 2009, 238, 60–69. [Google Scholar] [CrossRef]
- Lindstrand, V.; Sundström, G.; Jönsson, A.-S. Fouling of electrodialysis membranes by organic substances. Desalination 2000, 128, 91–102. [Google Scholar] [CrossRef]
- Chen, G.Q.; Eschbach, F.I.I.; Weeks, M.; Gras, S.L.; Kentish, S.E. Removal of lactic acid from acid whey using electrodialysis. Sep. Purif. Technol. 2016, 158, 230–237. [Google Scholar] [CrossRef]
- Diblíková, L.; Čurda, L.; Kinčl, J. The effect of dry matter and salt addition on cheese whey demineralisation. Int. Dairy J. 2013, 31, 29–33. [Google Scholar] [CrossRef]
- Sienkiewicz, T.; Riedel, C.-L. Whey and Whey Utilization; VEB Fachbuchverlag: Leipzig, Germany, 1986. [Google Scholar]
- Ayala-Bribiesca, E.; Pourcelly, G.; Bazinet, L. Nature identification and morphology characterization of cation-exchange membrane fouling during conventional electrodialysis. J. Colloid Interface Sci. 2006, 300, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Bribiesca, E.; Pourcelly, G.; Bazinet, L. Nature identification and morphology characterization of anion-exchange membrane fouling during conventional electrodialysis. J. Colloid Interface Sci. 2007, 308, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, L.; Montpetit, D.; Ippersiel, D.; Mahdavi, B.; Amiot, J.; Lamarche, F. Neutralization of hydroxide generated during skim milk electroacidification and its effect on bipolar and cationic membrane integrity. J. Membr. Sci. 2003, 216, 229–239. [Google Scholar] [CrossRef]
- Ayala-Bribiesca, E.; Araya-Farias, M.; Pourcelly, G.; Bazinet, L. Effect of concentrate solution pH and mineral composition of a whey protein diluate solution on membrane fouling formation during conventional electrodialysis. J. Membr. Sci. 2006, 280, 790–801. [Google Scholar] [CrossRef]
- Bleha, M.; Tishchenko, G.; Šumberová, V.; Kůdela, V. Characteristic of the critical state of membranes in ED-desalination of milk whey. Desalination 1992, 86, 173–186. [Google Scholar] [CrossRef]
- Bazinet, L.; Araya-Farias, M. Effect of calcium and carbonate concentrations on cationic membrane fouling during electrodialysis. J. Colloid Interface Sci. 2005, 281, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Casademont, C.; Farias, M.A.; Pourcelly, G.; Bazinet, L. Impact of electrodialytic parameters on cation migration kinetics and fouling nature of ion-exchange membranes during treatment of solutions with different magnesium/calcium ratios. J. Membr. Sci. 2008, 325, 570–579. [Google Scholar] [CrossRef]
- Trägårdh, G. Membrane cleaning. Desalination 1989, 71, 325–335. [Google Scholar] [CrossRef]
- Katz, W.E. The electrodialysis reversal (EDR) process. Desalination 1979, 28, 31–40. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Kunteng, D.; Veerman, J.; Saakes, M.; Nijmeijer, K. Periodic feedwater reversal and air sparging as antifouling strategies in reverse electrodialysis. Environ. Sci. Technol. 2014, 48, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Shee, F.L.T.; Angers, P.; Bazinet, L. Microscopic approach for the identification of cationic membrane fouling during cheddar cheese whey electroacidification. J. Colloid Interface Sci. 2008, 322, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Sata, T.; Tsujimoto, M.; Yamaguchi, T.; Matsusaki, K. Change of anion exchange membranes in an aqueous sodium hydroxide solution at high temperature. J. Membr. Sci. 1996, 112, 161–170. [Google Scholar] [CrossRef]
- Komkova, E.N.; Stamatialis, D.F.; Strathmann, H.; Wessling, M. Anion-exchange membranes containing diamines: Preparation and stability in alkaline solution. J. Membr. Sci. 2004, 244, 25–34. [Google Scholar] [CrossRef]
- Vega, J.A.; Chartier, C.; Mustain, W.E. Effect of hydroxide and carbonate alkaline media on anion exchange membranes. J. Power Sources 2010, 195, 7176–7180. [Google Scholar] [CrossRef]
- Garcia-Vasquez, W.; Ghalloussi, R.; Dammak, L.; Larchet, C.; Nikonenko, V.; Grande, D. Structure and properties of heterogeneous and homogeneous ion-exchange membranes subjected to ageing in sodium hypochlorite. J. Membr. Sci. 2014, 452, 104–116. [Google Scholar] [CrossRef]
- Ghalloussi, R.; Garcia-Vasquez, W.; Chaabane, L.; Dammak, L.; Larchet, C.; Deabate, S.V.; Nevakshenova, E.; Nikonenko, V.; Grande, D. Ageing of ion-exchange membranes in electrodialysis: A structural and physicochemical investigation. J. Membr. Sci. 2013, 436, 68–78. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, P.; Cong, W. Cation-exchange membrane fouling and cleaning in bipolar membrane electrodialysis of industrial glutamate production wastewater. Sep. Purif. Technol. 2011, 79, 103–113. [Google Scholar] [CrossRef]
- Haddad, M.; Mikhaylin, S.; Bazinet, L.; Savadogo, O.; Paris, J. Electrochemical acidification of kraft black liquor: Effect of fouling and chemical cleaning on ion exchange membrane integrity. ACS Sustain. Chem. Eng. 2016, 5, 168–178. [Google Scholar] [CrossRef]
- Association, A.W.W. Electrodialysis and Electrodialysis Reversal: M38; American Water Works Association: Washington, DC, USA, 1995; Volume 38. [Google Scholar]
- Guo, H.; You, F.; Yu, S.; Li, L.; Zhao, D. Mechanisms of chemical cleaning of ion exchange membranes: A case study of plant-scale electrodialysis for oily wastewater treatment. J. Membr. Sci. 2015, 496, 310–317. [Google Scholar] [CrossRef]
- Garcia-Vasquez, W.; Dammak, L.; Larchet, C.; Nikonenko, V.; Grande, D. Effects of acid-base cleaning procedure on structure and properties of anion-exchange membranes used in electrodialysis. J. Membr. Sci. 2016, 507, 12–23. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Porada, S.; Levi, M.; Bazant, M.Z. Attractive forces in microporous carbon electrodes for capacitive deionisation. J. Solid State Electrochem. 2014, 18, 1365–1376. [Google Scholar] [CrossRef]
- Hassanvand, A.; Chen, G.Q.; Webley, P.A.; Kentish, S.E. Improvement of MCDI operation and design through experiment and modelling: Regeneration with brine and optimum residence time. Desalination 2017, 417, 36–51. [Google Scholar] [CrossRef]
- Xie, W.; Cook, J.; Park, H.B.; Freeman, B.D.; Lee, C.H.; McGrath, J.E. Fundamental salt and water transport properties in directly copolymerized disulfonated poly(arylene ether sulfone) random copolymers. Polymer 2011, 52, 2032–2043. [Google Scholar] [CrossRef]
- Geise, G.M.; Freeman, B.D.; Paul, D.R. Characterization of a sulfonated pentablock copolymer for desalination applications. Polymer 2010, 51, 5815–5822. [Google Scholar] [CrossRef]
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Fundamental water and salt transport properties of polymeric materials. Prog. Polym. Sci. 2014, 39, 1–42. [Google Scholar] [CrossRef]
- Sata, T.; Sata, T.; Yang, W. Studies on cation-exchange membranes having permselectivity between cations in electrodialysis. J. Membr. Sci. 2002, 206, 31–60. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Koninckx, A.; Vandecasteele, C. Separation of monovalent and divalent ions from aqueous solution by electrodialysis and nanofiltration. Water Res. 2004, 38, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Galama, A.H.; Daubaras, G.; Burheim, O.S.; Rijnaarts, H.H.M.; Post, J.W. Seawater electrodialysis with preferential removal of divalent ions. J. Membr. Sci. 2014, 452, 219–228. [Google Scholar] [CrossRef]
- Ju, H.; Sagle, A.C.; Freeman, B.D.; Mardel, J.I.; Hill, A.J. Characterization of sodium chloride and water transport in crosslinked poly(ethylene oxide) hydrogels. J. Membr. Sci. 2010, 358, 131–141. [Google Scholar] [CrossRef]
- Crank, J.; Park, G.S. Diffusion in Polymers; Academic Press: Cambridge, MA, USA, 1968. [Google Scholar]
- Pintauro, P.N.; Bennion, D.N. Mass transport of electrolytes in membranes. 2. Determination of sodium chloride equilibrium and transport parameters for Nafion. Ind. Eng. Chem. Fundam. 1984, 23, 234–243. [Google Scholar] [CrossRef]
- Helfferich, F.G. Ion Exchange; Dover Publications: New York, NY, USA, 1995. [Google Scholar]
- Mackie, J.; Meares, P. The diffusion of electrolytes in a cation-exchange resin membrane I. Theoretical. In Proceedings of the Royal Society of London A: Mathematical, Physical and Engineering Sciences; The Royal Society: London, UK, 1955. [Google Scholar]
- Kamcev, J.; Paul, D.R.; Freeman, B.D. Ion activity coefficients in ion exchange polymers: Applicability of Manning’s counterion condensation theory. Macromolecules 2015, 48, 8011–8024. [Google Scholar] [CrossRef]
- Kamcev, J.; Galizia, M.; Benedetti, F.M.; Jang, E.-S.; Paul, D.R.; Freeman, B.; Manning, G.S. Partitioning of Mobile Ions Between Ion Exchange Polymers and Aqueous Salt Solutions: Importance of Counter-ion Condensation. Phys. Chem. Chem. Phys. 2016, 18, 6021–6031. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.; Meares, P. The electrical conductivity and electro-osmotic permeability of a cation-exchange resin. Trans. Faraday Soc. 1959, 55, 1221–1238. [Google Scholar] [CrossRef]
- Kamo, N.; Toyoshima, Y.; Kobatake, Y. Fixed charge density effective to membrane phenomena. Colloid Polym. Sci. 1971, 249, 1069–1071. [Google Scholar]
- Ueda, T.; Kamo, N.; Ishida, N.; Kobatake, Y. Effective fixed charge density governing membrane phenomena. IV. Further study of activity coefficients and mobilities of small ions in charged membranes. J. Phys. Chem. 1972, 76, 2447–2452. [Google Scholar] [CrossRef]
- Manning, G.S. Limiting laws and counterion condensation in polyelectrolyte solutions II. Self-Diffusion of the small ions. J. Phys. Chem. 1969, 51, 934–938. [Google Scholar] [CrossRef]
- Kamcev, J.; Paul, D.R.; Manning, G.S.; Freeman, B.D. Predicting salt permeability coefficients in highly swollen, highly charged ion exchange membranes. ACS Appl. Mater. Interfaces 2017, 9, 4044–4056. [Google Scholar] [CrossRef] [PubMed]
- Curran, P.M. Concentric Layer Electric Double Layer Capacitor Cylinder, System, and Method of Use. U.S. Patent 9,193,612, 24 November 2015. [Google Scholar]
- RDI™ Desalination System. 2017. Available online: http://www.atlantis-water.com/rdi-desalination-system-2/ (accessed on 7 July 2017).
- Bian, Y.; Yang, X.; Liang, P.; Jiang, Y.; Zhang, C.; Huang, X. Enhanced desalination performance of membrane capacitive deionisation cells by packing the flow chamber with granular activated carbon. Water Res. 2015, 85, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-I.; Park, H.-R.; Yeo, J.-G.; Yang, S.C.; Cho, C.H.; Han, M.H.; Kim, D.K. Desalination via a new membrane capacitive deionisation process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. [Google Scholar] [CrossRef]
- Gendel, Y.; Rommerskirchen, A.K.E.; David, O.; Wessling, M. Batch mode and continuous desalination of water using flowing carbon deionisation (FCDI) technology. Electrochem. Commun. 2014, 46, 152–156. [Google Scholar] [CrossRef]
- Porada, S.; Weingarth, D.; Hamelers, H.V.; Bryjak, M.; Presser, V.; Biesheuvel, P. Carbon flow electrodes for continuous operation of capacitive deionisation and capacitive mixing energy generation. J. Mater. Chem. A 2014, 2, 9313–9321. [Google Scholar] [CrossRef]
- Yang, S.; Jeon, S.-I.; Kim, H.; Choi, J.; Yeo, J.-G.; Park, H.-R.; Kim, D.K. Stack design and operation for scaling up the capacity of flow-electrode capacitive deionisation technology. ACS Sustain. Chem. Eng. 2016, 4, 4174–4180. [Google Scholar] [CrossRef]
- Rommerskirchen, A.; Gendel, Y.; Wessling, M. Single module flow-electrode capacitive deionisation for continuous water desalination. Electrochem. Commun. 2015, 60, 34–37. [Google Scholar] [CrossRef]
- Yang, S.-C.; Choi, J.; Yeo, J.-G.; Jeon, S.-I.; Park, H.-R.; Kim, D.K. Flow-electrode capacitive deionisation using an aqueous electrolyte with a high salt concentration. Environ. Sci. Technol. 2016, 50, 5892–5899. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; He, D.; Tang, W.; Kovalsky, P.; He, C.; Zhang, C.; Waite, T.D. Development of redox-active flow electrodes for high-performance capacitive deionisation. Environ. Sci. Technol. 2016, 50, 13495–13501. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, N.C.; Savinell, R.F.; Wainright, J.S. Modeling of flowable slurry electrodes with combined Faradaic and nonfaradaic currents. Chem. Eng. Sci. 2016, 144, 288–297. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kim, C.; Yoon, J. Hybrid capacitive deionisation to enhance the desalination performance of capacitive techniques. Energy Environ. Sci. 2014, 7, 3683–3689. [Google Scholar] [CrossRef]
- Suss, M.; Porada, S.; Sun, X.; Biesheuvel, P.; Yoon, J.; Presser, V. Water desalination via capacitive deionisation: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef]



| Materials | Polymer or Polymer Coating | Water Uptake (wt %) | IEC (meq g−1) | Electrical Resistance (Ω cm2) | Thickness of Polymer or Coating (μm) | Specific Capacitance (F g−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Commercial Membranes | Fumasep FKS | 12–15 | 0.8–1.2 | 2.0–4.5 | 120 | - | Supplier |
| Neosepta CMX | 25–30 | 1.5–1.8 | 3.0 | 170 | - | [9] | |
| Selemion CMV | 25 | 2.4 | - | 120 | - | [9] | |
| Dupont Nafion | 16 | 0.9 | 1.5 | 117 | - | [9] | |
| IEMs for MCDI | NaSS-MAA-MMA | 121 | 0.99 | 0.7 | 90–140 | - | [20] |
| PVDF-g-PSVBS | 61 | 1.14 | 2 | - | - | [21] | |
| Crosslinked Sulfonated polystyrene | 30 | 0.9 | 0.37 | 25 | - | [23] | |
| Composite Electrodes | Polyethyleneimine (PEI) | - | - | - | - | 52 | [26] |
| PVA/SSA-coated | - | - | 0.67–1.17 | - | 97–116 | [27] | |
| SG-CNFs a | - | - | - | <1 | 117 | [28] | |
| PVA/SSA-coated | - | - | 0.64 | 10 | 0.74 F cm−2 | [29] | |
| Sulfonated PPO b | - | 0.93 | - | 4.9 | - | [30] | |
| Sulfonated BPPO c coated | - | - | - | - | - | [31] | |
| Sulfonated graphene | - | - | - | - | 108 | [32] | |
| PVA/SSA/SSA-MA | 44 | 2.8 | - | 6.4 | - | [33] |
| Materials | Polymer or Polymer Coating | Water Uptake (wt %) | IEC (meq g−1) | Electrical Resistance (Ω cm2) | Thickness of Polymer or Coating (μm) | Specific Capacitance (F g−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Commercial Membranes | Fumasep FAS | 15–3013–23 | 1.6–2.01.0–1.3 | 0.3–0.61.7–3.0 | 30135 | - | Supplier |
| Neosepta AMX | 25–30 | 1.4–1.7 | 2.4 | 140 | - | [9] | |
| Selemion AMV | 19 | 1.9 | 2.8 | 120 | - | [9] | |
| IEMs for MCDI | Aminated PVDF-g-VBC | 25 | 1.0 | 4.8 | - | - | [34] |
| Composite Electrodes | Purolite | - | - | - | - | 19 | [24] |
| dimethyldiallyl ammonium chloride | - | - | - | - | 53 | [26] | |
| Anion exchange resin | - | 1.20 | - | - | - | [35] | |
| - | 2.9 | - | ~40 | - | [36] | ||
| Aminated PVA | - | 1.76 | - | 20–40 | 180 | [37] | |
| Aminated PVA | - | - | - | - | 184 | [38] | |
| Aminated PSF a | - | 1.0 | 5.2 | - | - | [30] | |
| Aminated BPPO | - | - | - | - | - | [31] | |
| Aminated Graphene | 91 | [32] | |||||
| Aminated PSF | 37 | 2.1 | - | 10.6 | - | [33] |
| Feed Salt Concentration | Adsorption Capacity (mg g−1) | |||
|---|---|---|---|---|
| (mg L−1) | m-MCDI | MCDI | CDI | Reference |
| 50 | - | 2.04 | 1.05 | [41] |
| 100 | 2.09 | - | 2.0 | [31] |
| 200 | - | 5.3 | 3.7 | [40] |
| 200 | - | 10.2 | 8.1 | [29] |
| 300 | - | 3.5 | 1 | [43] |
| 400 | - | 4.3 | 3 | [44] |
| 400 | 9.5 | - | 5.0 | [28] |
| 500 | 9.3 | 6.5 | - | [26] |
| 750 | - | 45.6 | 30.3 | [45] |
| 1000 | 9 | 6 | 1.9 | [46] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanvand, A.; Wei, K.; Talebi, S.; Chen, G.Q.; Kentish, S.E. The Role of Ion Exchange Membranes in Membrane Capacitive Deionisation. Membranes 2017, 7, 54. https://doi.org/10.3390/membranes7030054
Hassanvand A, Wei K, Talebi S, Chen GQ, Kentish SE. The Role of Ion Exchange Membranes in Membrane Capacitive Deionisation. Membranes. 2017; 7(3):54. https://doi.org/10.3390/membranes7030054
Chicago/Turabian StyleHassanvand, Armineh, Kajia Wei, Sahar Talebi, George Q. Chen, and Sandra E. Kentish. 2017. "The Role of Ion Exchange Membranes in Membrane Capacitive Deionisation" Membranes 7, no. 3: 54. https://doi.org/10.3390/membranes7030054
APA StyleHassanvand, A., Wei, K., Talebi, S., Chen, G. Q., & Kentish, S. E. (2017). The Role of Ion Exchange Membranes in Membrane Capacitive Deionisation. Membranes, 7(3), 54. https://doi.org/10.3390/membranes7030054






