Toward the Fabrication of Advanced Nanofiltration Membranes by Controlling Morphologies and Mesochannel Orientations of Hexagonal Lyotropic Liquid Crystals
Abstract
:1. Introduction
2. Phase Transition and Retention
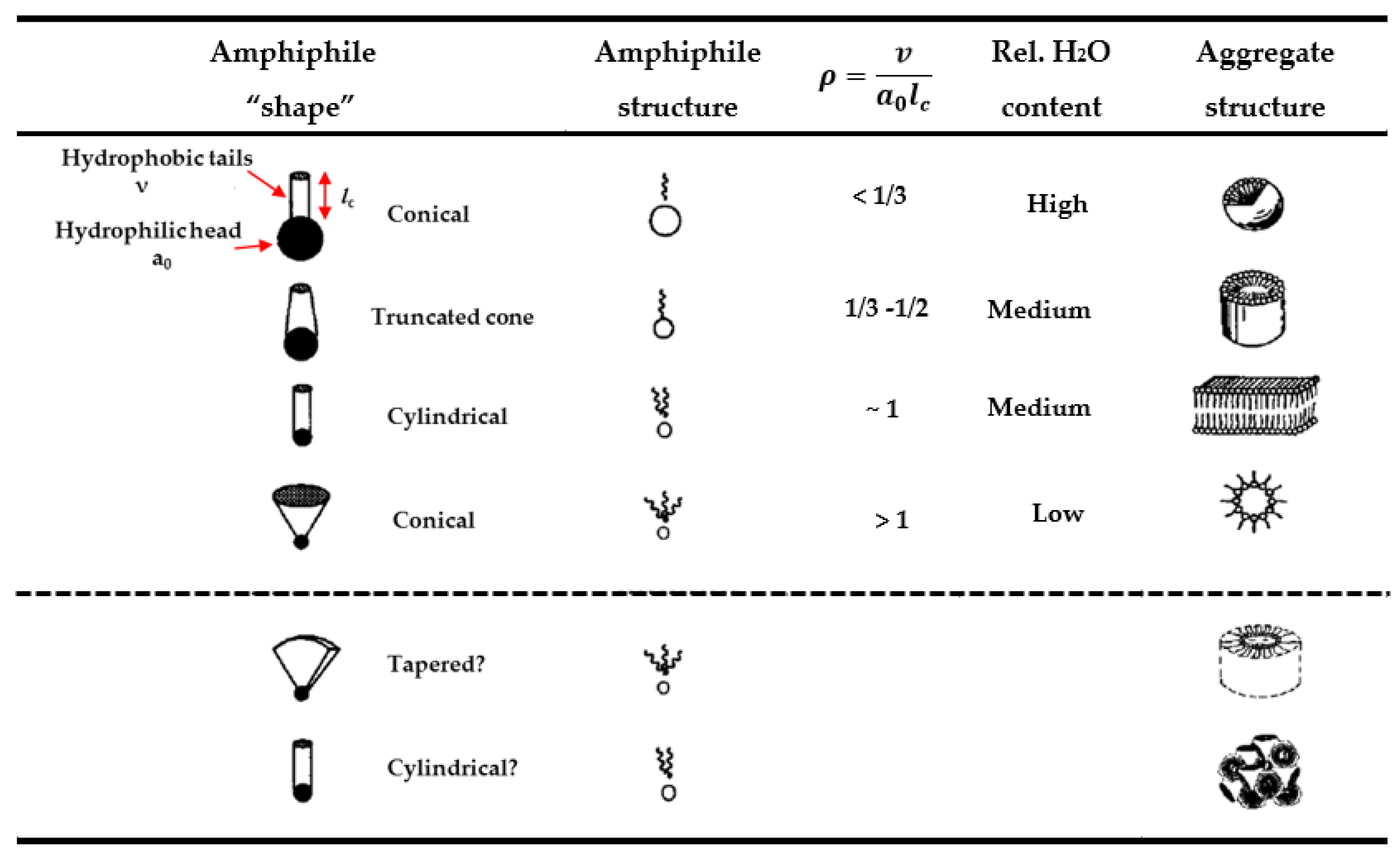
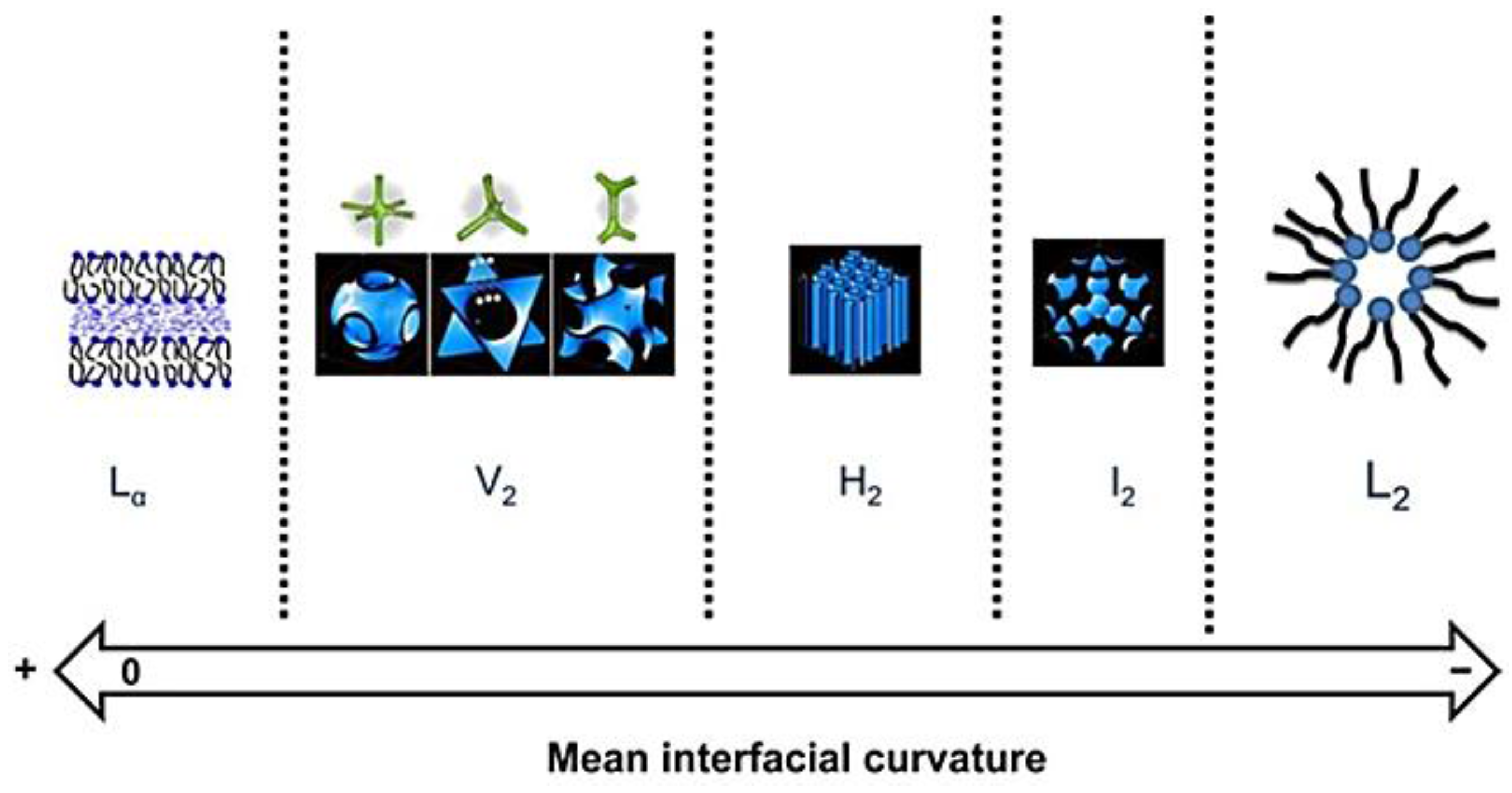
2.1. Phase Formation
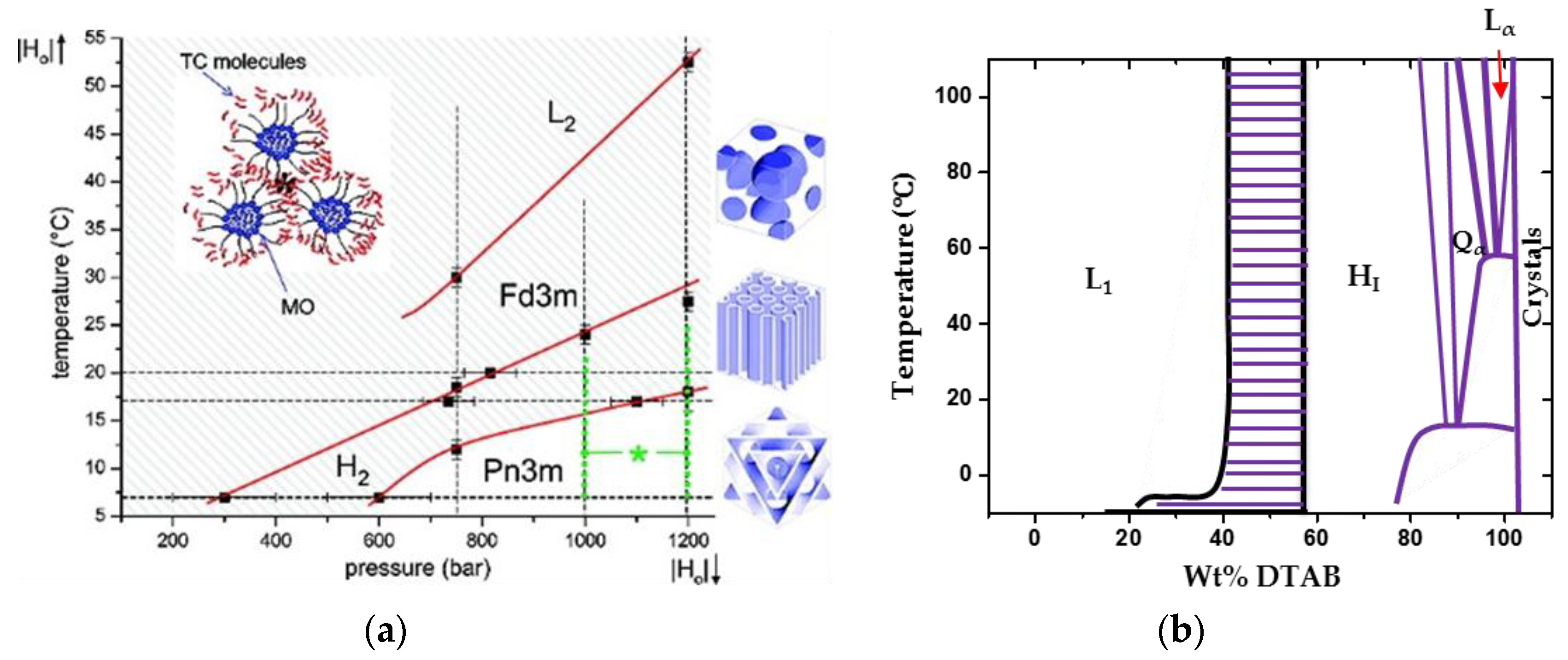
2.2. Phase Transition
2.3. Thermodynamic Behaviours during Polymerization
- (1)
- (2)
- LLCs phase behaviours are inherently sensitive to concentration, temperature, and pressure, as mentioned above.
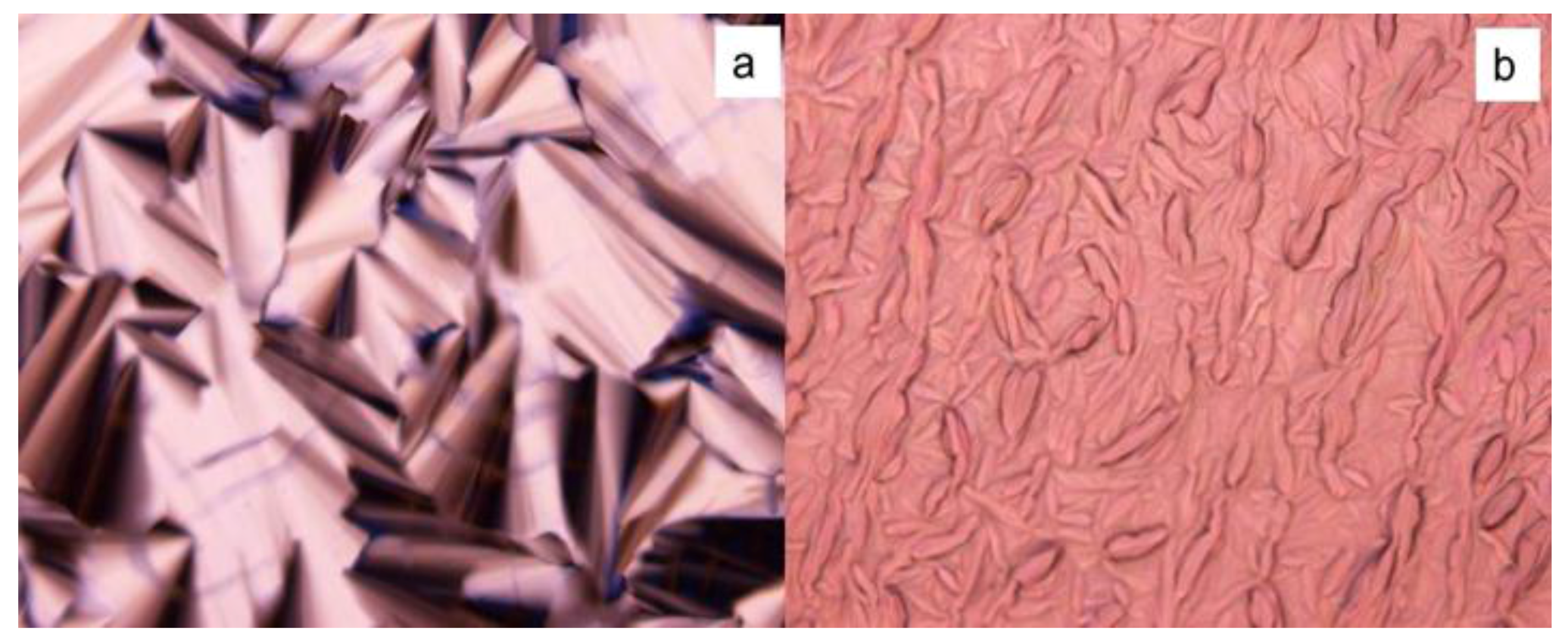
2.4. Phase Retention
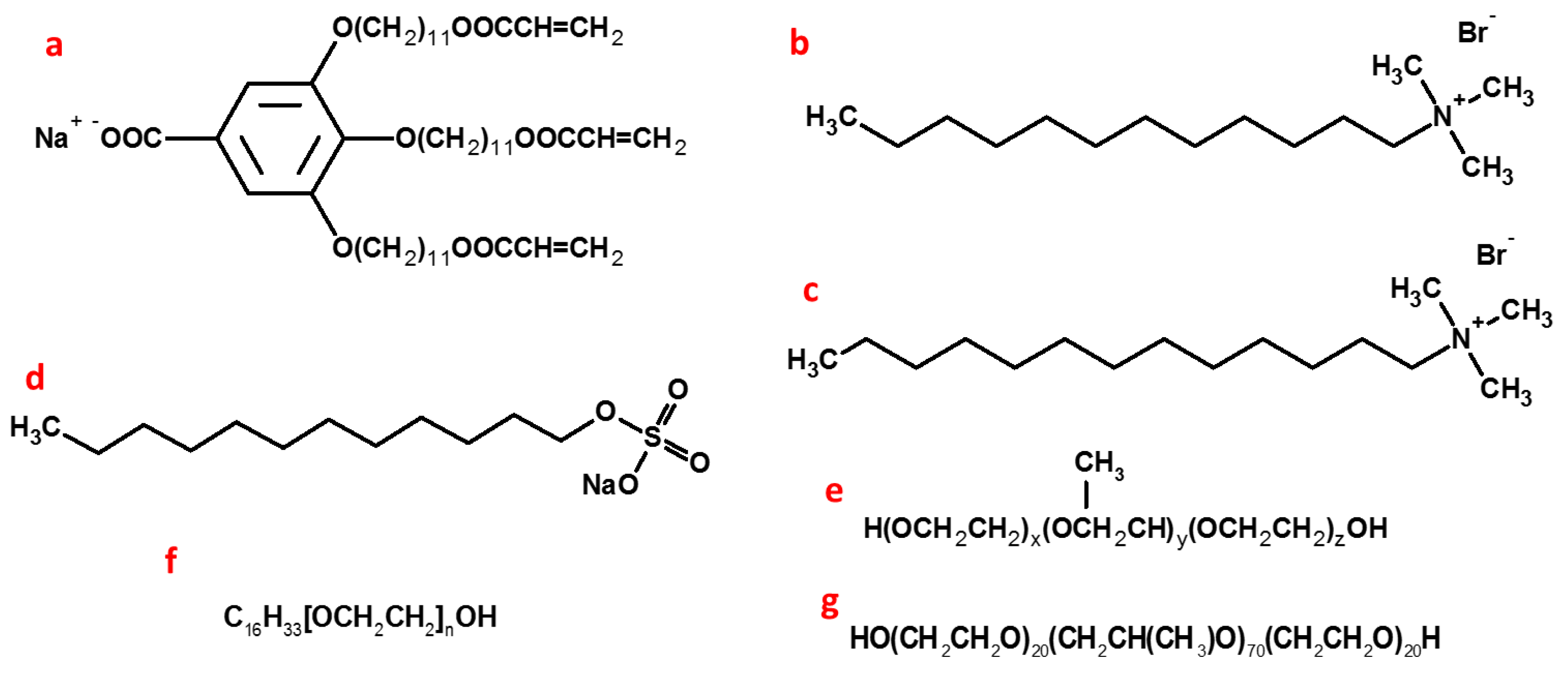
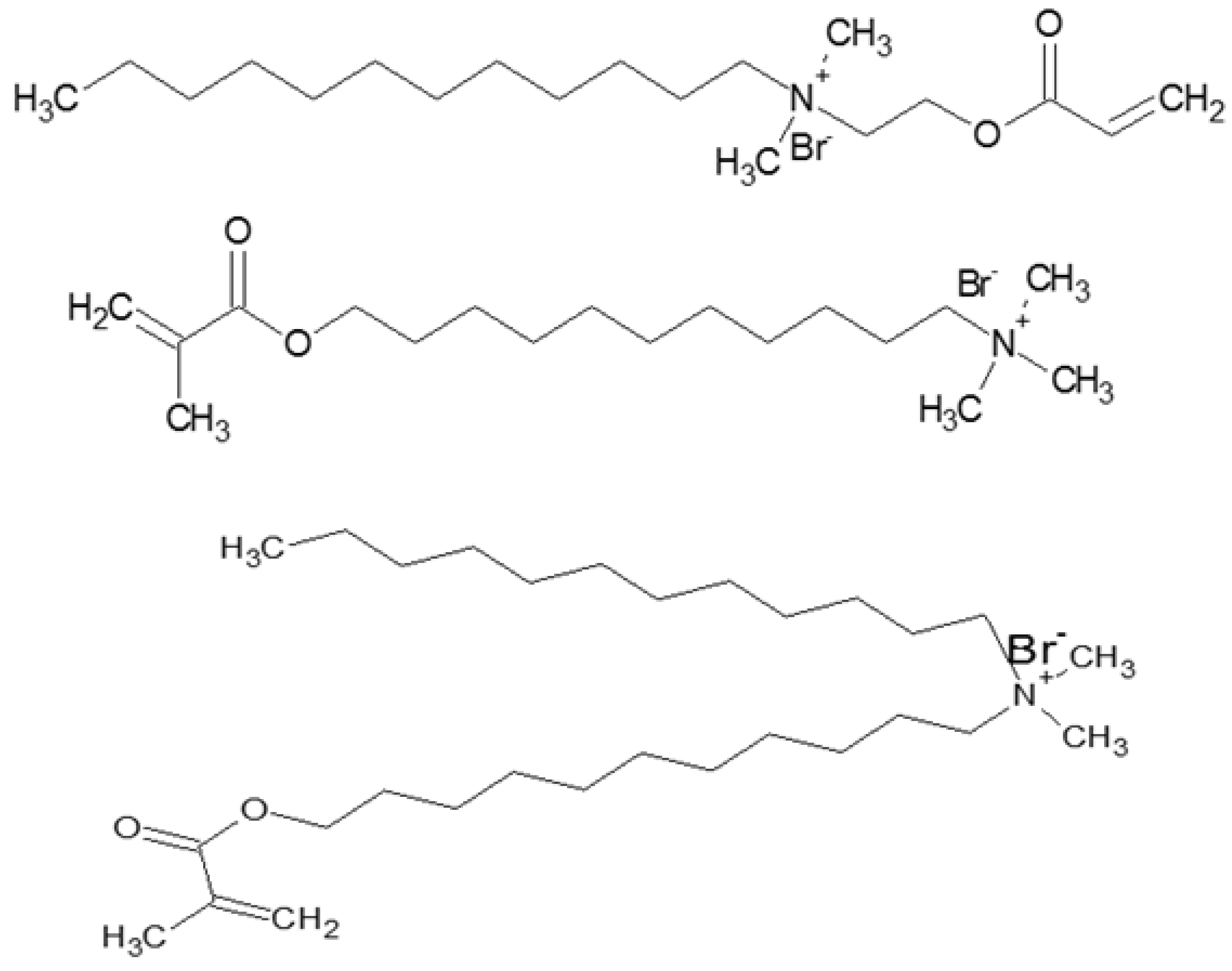
- The location of diene groups conjugated with the acyl chain carbonyl should not interfere with the biocompatibility of the interface and have less effect on the curvature;
- The entropy loss and enthalpy reduction should be compensated by using the monomers with a high number of reactive entities so that the monomers can be polymerized with the lowest conversion rate;
- A more thermodynamically stable template with long rearrangement times should be developed; and
- Some small multifunctional monomers with high mobility that form cross-linked networks easily could be used to enforce the instant interface curvature during polymerization.
2.4.1. Application of Cosurfactant
2.4.2. Surfactant Analogues
2.4.3. Special Drying Process
3. Reorientation
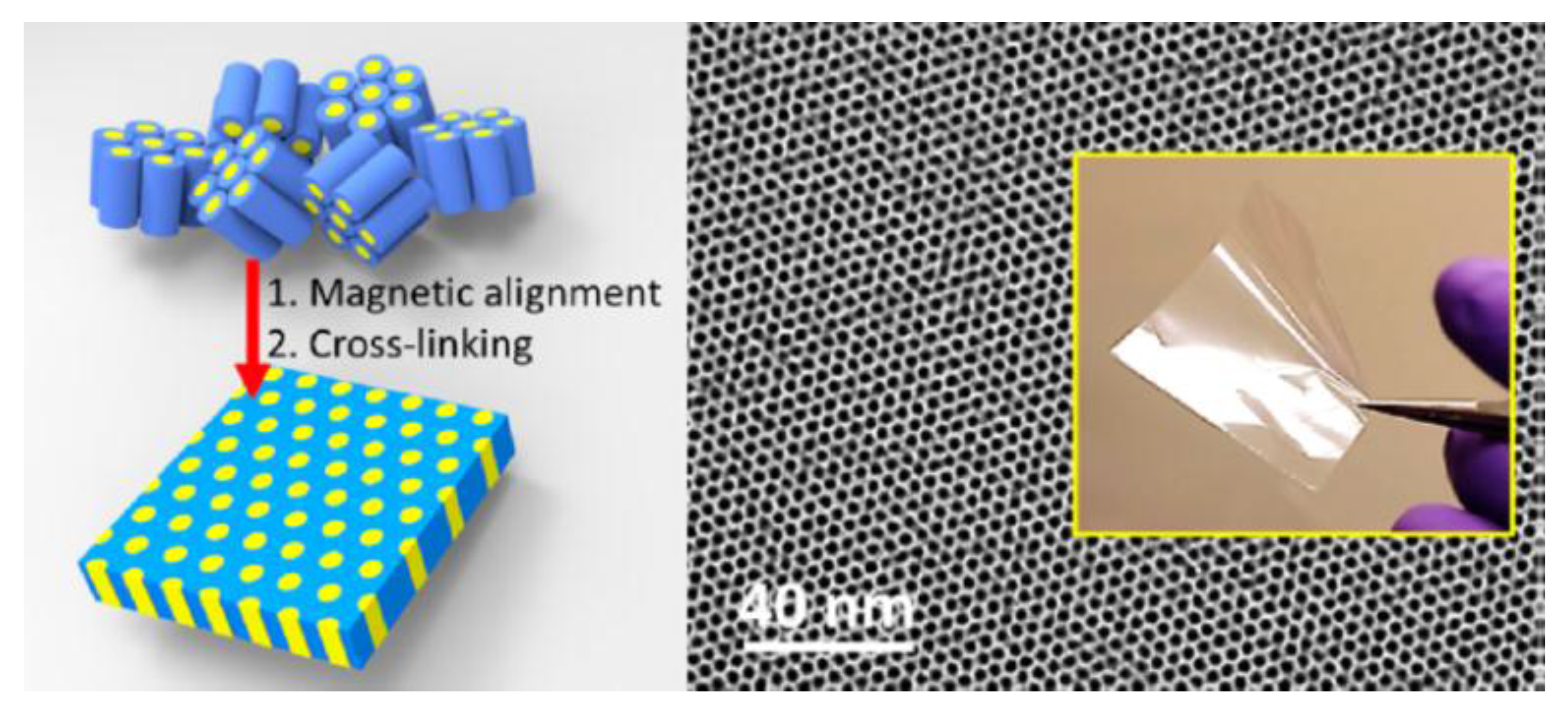
3.1. External Field
3.2. Confinement in a Small Space
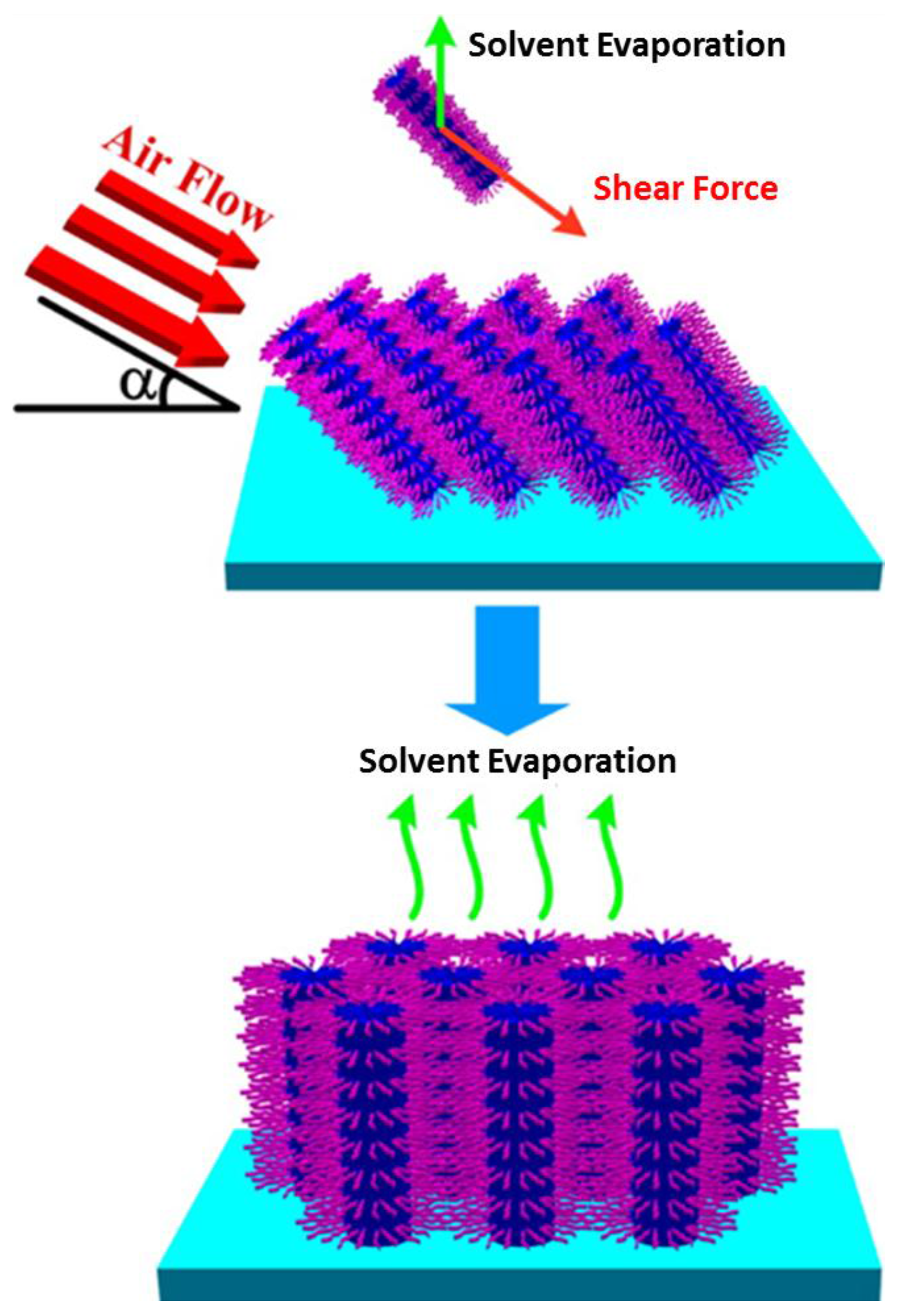
3.3. Other Methods
4. Future Directions and Conclusions
4.1. Future Directions
4.2. Conclusions
Acknowledgments
Author Contribution
Conflicts of Interest
References
- Gleick, P.H. Water in Crisis; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- Falconer, I.R.; Humpage, A.R. Health risk assessment of cyanobacterial (blue-green algal) toxins in drinking water. Int. J. Environ. Res. Public Health 2005, 2, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Rozell, D.J.; Reaven, S.J. Water pollution risk associated with natural gas extraction from the marcellus shale. Risk Anal. 2012, 32, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Al-Alshaikh, A. Seawater desalination in saudi arabia: An overview. In Proceedings of the International Conference on Desalination & Sustainability, Casablanca, Morocco, 1–2 March 2012. [Google Scholar]
- Dashtpour, R.; Al-Zubaidy, S.N. Energy efficient reverse osmosis desalination process. Int. J. Environ. Sci. Dev. 2012, 3, 339. [Google Scholar]
- Goldstein, R.; Smith, W. Water & Sustainability (volume 4): Us Electricity Consumption for Water Supply & Treatment-the Next Half Century; Electric Power Research Institute: Palo Alto, CA, USA, 2002. [Google Scholar]
- Zhou, Y.; Tol, R.S. Evaluating the costs of desalination and water transport. Water Resour. Res. 2005, 41, W03003. [Google Scholar] [CrossRef]
- Al Gobaisi, D. Encyclopedia of Desalination and Water Resources; EOLSS Publishers: Oxford, UK, 2000. [Google Scholar]
- Younos, T.; Tulou, K.E. Energy needs, consumption and sources. J. Contemp. Water Res. Educ. 2005, 132, 27–38. [Google Scholar] [CrossRef]
- Ghalavand, Y.; Hatamipour, M.S.; Rahimi, A. A review on energy consumption of desalination processes. Desalin. Water Treat. 2015, 54, 1526–1541. [Google Scholar] [CrossRef]
- Chung, H.J.; Park, T.G. Self-assembled and nanostructured hydrogels for drug delivery and tissue engineering. Nano Today 2009, 4, 429–437. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Nanostructured biomaterials for regeneration. Adv. Funct. Mater. 2008, 18, 3568–3582. [Google Scholar] [CrossRef] [PubMed]
- Vallooran, J.J.; Negrini, R.; Mezzenga, R. Controlling anisotropic drug diffusion in lipid-Fe3O4 nanoparticle hybrid mesophases by magnetic alignment. Langmuir 2013, 29, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Nguyen, V.; Zeng, X.; Elliott, B.J.; Gin, D.L. Selective rejection of a water-soluble nerve agent stimulant using a nanoporous lyotropic liquid crystal–butyl rubber vapor barrier material: Evidence for a molecular size-discrimination mechanism. J. Membr. Sci. 2008, 318, 397–404. [Google Scholar] [CrossRef]
- Mi, B. Graphene oxide membranes for ionic and molecular sieving. Science 2014, 343, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Fornasiero, F.; Park, H.G.; Holt, J.K.; Stadermann, M.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Ion exclusion by sub-2-nm carbon nanotube pores. Proc. Natl. Acad. Sci. USA 2008, 105, 17250–17255. [Google Scholar] [CrossRef] [PubMed]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Hinds, B.J.; Chopra, N.; Rantell, T.; Andrews, R.; Gavalas, V.; Bachas, L.G. Aligned multiwalled carbon nanotube membranes. Science 2004, 303, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Srivastava, O.; Talapatra, S.; Vajtai, R.; Ajayan, P. Carbon nanotube filters. Nat. Mater. 2004, 3, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Dotzauer, D.M.; Dai, J.; Sun, L.; Bruening, M.L. Catalytic membranes prepared using layer-by-layer adsorption of polyelectrolyte/metal nanoparticle films in porous supports. Nano Lett. 2006, 6, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Allabashi, R.; Arkas, M.; Hörmann, G.; Tsiourvas, D. Removal of some organic pollutants in water employing ceramic membranes impregnated with cross-linked silylated dendritic and cyclodextrin polymers. Water Res. 2007, 41, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Z.; Bai, H.; Sun, D.D. Concurrent filtration and solar photocatalytic disinfection/degradation using high-performance Ag/TiO2 nanofiber membrane. Water Res. 2012, 46, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Athanasekou, C.; Romanos, G.; Katsaros, F.; Kordatos, K.; Likodimos, V.; Falaras, P. Very efficient composite titania membranes in hybrid ultrafiltration/photocatalysis water treatment processes. J. Membr. Sci. 2012, 392, 192–203. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [PubMed]
- Gin, D.L.; Gu, W.; Pindzola, B.A.; Zhou, W.-J. Polymerized lyotropic liquid crystal assemblies for materials applications. Acc. Chem. Res. 2001, 34, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, Z.; Hill, A.J.; She, F.H.; Thornton, A.W.; Hoang, M.; Kong, L.X. Structure retention in cross-linked poly(ethylene glycol) diacrylate hydrogel templated from a hexagonal lyotropic liquid crystal by controlling the surface tension. Soft Matter 2012, 8, 2087–2094. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: San Diego, CA, USA, 2011. [Google Scholar]
- Hyde, S.; Blum, Z.; Landh, T.; Lidin, S.; Ninham, B.; Andersson, S.; Larsson, K. The Language of Shape: The Role of Curvature in Condensed Matter: Physics, Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Yaghmur, A.; Sartori, B.; Rappolt, M. Self-assembled nanostructures of fully hydrated monoelaidin-elaidic acid and monoelaidin-oleic acid systems. Langmuir 2012, 28, 10105–10119. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Kriechbaum, M.; Amenitsch, H.; Steinhart, M.; Laggner, P.; Rappolt, M. Effects of pressure and temperature on the self-assembled fully hydrated nanostructures of monoolein—Oil systems. Langmuir 2009, 26, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- McGrath, K. Phase behavior of dodecyltrimethylammonium bromide/water mixtures. Langmuir 1995, 11, 1835–1839. [Google Scholar] [CrossRef]
- Israelachvili, J. Intermolecular and Surface Forces with Applications to Colloidal and Biological Systems; Academic Press: London, England, 1985; pp. 249–257. [Google Scholar]
- Shearman, G.; Tyler, A.; Brooks, N.; Templer, R.; Ces, O.; Law, R.; Seddon, J. Ordered micellar and inverse micellar lyotropic phases. Liq. Cryst. 2010, 37, 679–694. [Google Scholar] [CrossRef]
- Gruner, S.M. Intrinsic curvature hypothesis for biomembrane lipid composition: A role for nonbilayer lipids. Proc. Natl. Acad. Sci. USA 1985, 82, 3665–3669. [Google Scholar] [CrossRef] [PubMed]
- Gruner, S.M. Stability of lyotropic phases with curved interfaces. J. Phys. Chem. 1989, 93, 7562–7570. [Google Scholar] [CrossRef]
- Hyde, S.T. Identification of lyotropic liquid crystalline mesophases. Handb. Appl. Surf. Colloid Chem. 2001, 2, 299–332. [Google Scholar]
- Rappolt, M. The biologically relevant lipid mesophases as “seen” by X-rays. Adv. Planar Lipid Bilayers Liposomes 2006, 5, 253–283. [Google Scholar]
- Yaghmur, A.; Rappolt, M.; Østergaard, J.; Larsen, C.; Larsen, S.W. Characterization of bupivacaine-loaded formulations based on liquid crystalline phases and microemulsions: The effect of lipid composition. Langmuir 2012, 28, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Iglic, A.; Kulkarni, C. Advances in Planar Lipid Bilayers and Liposomes Volume 18; Academic Press: Oxford, UK, 2013; p. 115. [Google Scholar]
- Mauter, M.S.; Elimelech, M.; Osuji, C.O. Nanocomposites of vertically aligned single-walled carbon nanotubes by magnetic alignment and polymerization of a lyotropic precursor. ACS Nano 2010, 4, 6651–6658. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zheng, G.; Dou, Y.; Li, W.; Mou, C.Y.; Zhang, X.; Asiri, A.M.; Zhao, D. Highly ordered mesoporous silica films with perpendicular mesochannels by a simple stöber-solution growth approach. Angew. Chem. Int. Ed. 2012, 51, 2173–2177. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Han, L.; Jiang, Z.; Huang, Z.; Feng, J.; Yao, Y.; Che, S. Growth of mesoporous silica film with vertical channels on substrate using gemini surfactants. Chem. Mater. 2011, 23, 3583–3586. [Google Scholar] [CrossRef]
- Shan, F.; Lu, X.; Zhang, Q.; Wu, J.; Wang, Y.; Bian, F.; Lu, Q.; Fei, Z.; Dyson, P.J. A facile approach for controlling the orientation of one-dimensional mesochannels in mesoporous titania films. J. Am. Chem. Soc. 2012, 134, 20238–20241. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Tousley, M.E.; Cowan, M.G.; Wiesenauer, B.R.; Nejati, S.; Choo, Y.; Noble, R.D.; Elimelech, M.; Gin, D.L.; Osuji, C.O. Scalable fabrication of polymer membranes with vertically aligned 1 nm pores by magnetic field directed self-assembly. ACS Nano 2014, 8, 11977–11986. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Nejati, S.; Cowan, M.G.; Tousley, M.E.; Wiesenauer, B.R.; Noble, R.D.; Elimelech, M.; Gin, D.L.; Osuji, C.O. Thin polymer films with continuous vertically aligned 1 nm pores fabricated by soft confinement. ACS Nano 2015, 10, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Asghar, K.A.; Elliott, J.M.; Squires, A.M. 2D hexagonal mesoporous platinum films exhibiting biaxial, in-plane pore alignment. J. Mater. Chem. 2012, 22, 13311–13317. [Google Scholar] [CrossRef]
- Vallooran, J.J.; Bolisetty, S.; Mezzenga, R. Macroscopic alignment of lyotropic liquid crystals using magnetic nanoparticles. Adv. Mater. 2011, 23, 3932–3937. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zvelindovsky, A.; Sevink, G.; Gang, O.; Ocko, B.; Zhu, Y.; Gido, S.P.; Russell, T.P. Electric field induced sphere-to-cylinder transition in diblock copolymer thin films. Macromolecules 2004, 37, 6980–6984. [Google Scholar] [CrossRef]
- Peng, S.; Guo, Q.; Hughes, T.C.; Hartley, P.G. Reversible photorheological lyotropic liquid crystals. Langmuir 2013, 30, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, F.C.; da Silveira, N.D.P.; Nallet, F.D.R.; Černoch, P.; Steinhart, M.; Štěpánek, P. Cubic to hexagonal phase transition induced by electric field. Macromolecules 2010, 43, 4261–4267. [Google Scholar] [CrossRef]
- Kijima, T.; Nishida, Y.; Fujikawa, D.; Uota, M.; Yoshimura, T.; Sakai, G. Long-chain alcohol induced phase transition in lyotropic mixed polyoxyethylene-type surfactant liquid-crystals. J. Mol. Liq. 2007, 133, 54–60. [Google Scholar] [CrossRef]
- Decker, C. Light-induced crosslinking polymerization. Polym. Int. 2002, 51, 1141–1150. [Google Scholar] [CrossRef]
- Odian, G. Radical chain polymerization. In Principles of Polymerization, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 198–349. [Google Scholar]
- Thundathil, R.; Stoffer, J.O.; Friberg, S.E. Polymerization in lyotropic liquid crystals. I. Change of structure during polymerization. J. Polym. Sci. A Polym. Chem. Ed. 1980, 18, 2629–2640. [Google Scholar] [CrossRef]
- Forney, B.S.; Guymon, C.A. Nanostructure evolution during photopolymerization in lyotropic liquid crystal templates. Macromolecules 2010, 43, 8502–8510. [Google Scholar] [CrossRef]
- Forney, B.S.; Baguenard, C.L.; Guymon, C.A. Effects of controlling polymer nanostructure using photopolymerization within lyotropic liquid crystalline templates. Chem. Mater. 2013, 25, 2950–2960. [Google Scholar] [CrossRef]
- Lester, C.L.; Guymon, C.A. Ordering effects on the photopolymerization of a lyotropic liquid crystal. Polymer 2002, 43, 3707–3715. [Google Scholar] [CrossRef]
- DePierro, M.A.; Guymon, C.A. Photoinitiation and monomer segregation behavior in polymerization of lyotropic liquid crystalline systems. Macromolecules 2006, 39, 617–626. [Google Scholar] [CrossRef]
- DePierro, M.A.; Olson, A.J.; Guymon, C.A. Effect of photoinitiator segregation on polymerization kinetics in lyotropic liquid crystals. Polymer 2005, 46, 335–345. [Google Scholar] [CrossRef]
- Guymon, C.A.; Bowman, C.N. Kinetic analysis of polymerization rate acceleration during the formation of polymer/smectic liquid crystal composites. Macromolecules 1997, 30, 5271–5278. [Google Scholar] [CrossRef]
- Lester, C.L.; Colson, C.D.; Guymon, C.A. Photopolymerization kinetics and structure development of templated lyotropic liquid crystalline systems. Macromolecules 2001, 34, 4430–4438. [Google Scholar] [CrossRef]
- Lester, C.L.; Smith, S.M.; Jarrett, W.L.; Guymon, C.A. Effects of monomer organization on the photopolymerization kinetics of acrylamide in lyotropic liquid crystalline phases. Langmuir 2003, 19, 9466–9472. [Google Scholar] [CrossRef]
- Zhou, M.J.; Kidd, T.J.; Noble, R.D.; Gin, D.L. Supported lyotropic liquid-crystal polymer membranes: Promising materials for molecular-size-selective aqueous nanofiltration. Adv. Mater. 2005, 17, 1850–1853. [Google Scholar] [CrossRef]
- Rappolt, M.; Hickel, A.; Bringezu, F.; Lohner, K. Mechanism of the lamellar/inverse hexagonal phase transition examined by high resolution X-ray diffraction. Biophys. J. 2003, 84, 3111–3122. [Google Scholar] [CrossRef]
- Hara, M.; Nagano, S.; Seki, T. π−π interaction-induced vertical alignment of silica mesochannels templated by a discotic lyotropic liquid crystal. J. Am. Chem. Soc. 2010, 132, 13654–13656. [Google Scholar] [CrossRef] [PubMed]
- Melosh, N.; Davidson, P.; Feng, P.; Pine, D.; Chmelka, B. Macroscopic shear alignment of bulk transparent mesostructured silica. J. Am. Chem. Soc. 2001, 123, 1240–1241. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Bu, X.; Pine, D.J. Control of pore sizes in mesoporous silica templated by liquid crystals in block copolymer-cosurfactant-water systems. Langmuir 2000, 16, 5304–5310. [Google Scholar] [CrossRef]
- Clapper, J.D.; Sievens-Figueroa, L.; Guymon, C.A. Photopolymerization in polymer templating. Chem. Mater. 2007, 20, 768–781. [Google Scholar] [CrossRef]
- Kumaraswamy, G.; Wadekar, M.N.; Agrawal, V.V.; Pasricha, R. Polycondensation in liquid crystalline phases of nonionic surfactants. Kinetics and morphology. Polymer 2005, 46, 7961–7968. [Google Scholar] [CrossRef]
- Gin, D.L.; Pecinovsky, C.S.; Bara, J.E.; Kerr, R.L. Functional lyotropic liquid crystal materials. In Liquid Crystalline Functional Assemblies and Their Supramolecular Structures; Springer: Berlin, Germany, 2008; Volume 128, pp. 181–222. [Google Scholar]
- Hoag, B.P.; Gin, D.L. Cross-linkable liquid crystal monomers containing hydrocarbon 1,3-diene tail systems. Macromolecules 2000, 33, 8549–8558. [Google Scholar] [CrossRef]
- Eastoe, J.; Summers, M.; Heenan, R.K. Control over phase curvature using mixtures of polymerizable surfactants. Chem. Mater. 2000, 12, 3533–3537. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Hoang, M.; Hill, A.; Cong, W.; She, F.H.; Gao, W.; Kong, L. Retention of original lyotropic liquid crystal structure in cross-linked poly (ethylene glycol) diacrylate hydrogels reinforced via silica networks. Soft Matter 2014, 10, 5192–5200. [Google Scholar] [CrossRef] [PubMed]
- Valentin, R.; Molvinger, K.; Viton, C.; Domard, A.; Quignard, F. From hydrocolloids to high specific surface area porous supports for catalysis. Biomacromolecules 2005, 6, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.E.; Kolasinski, K.W. Structure and photoluminescence studies of porous silicon formed in ferric ion containing stain etchants. Phys. Status Solidi A 2009, 206, 1240–1244. [Google Scholar] [CrossRef]
- Luzzati, V.; Husson, F. The structure of the liquid-crystalline phases of lipid-water systems. J. Cell Biol. 1962, 12, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.W.; Gruner, S.M. Temperature dependence of the structural dimensions of the inverted hexagonal (HII) phase of phosphatidylethanolamine-containing membranes. Biochemistry 1989, 28, 4245–4253. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-J.; Chu, K.-C.; Chao, C.-Y.; Chen, Y.-F.; Lai, C.-W.; Kang, C.-C.; Chen, C.-Y.; Chou, P.-T. CdS nanorods imbedded in liquid crystal cells for smart optoelectronic devices. Nano Lett. 2007, 7, 1908–1913. [Google Scholar] [CrossRef]
- Gibbons, W.; Kosa, T.; Palffy-Muhoray, P.; Shannon, P.; Sun, S. Continuous grey-scale image storage using optically aligned nematic liquid crystals. Nature 1995, 377, 43–46. [Google Scholar] [CrossRef]
- Miller, S.A.; Kim, E.; Gray, D.H.; Gin, D.L. Heterogeneous catalysis with cross-linked lyotropic liquid crystal assemblies: Organic analogues to zeolites and mesoporous sieves. Angew. Chem. Int. Ed. 1999, 38, 3021–3026. [Google Scholar] [CrossRef]
- Firouzi, A.; Schaefer, D.J.; Tolbert, S.H.; Stucky, G.D.; Chmelka, B.F. Magnetic-field-induced orientational ordering of alkaline lyotropic silicate—Surfactant liquid crystals. J. Am. Chem. Soc. 1997, 119, 9466–9477. [Google Scholar] [CrossRef]
- Clawson, J.S.; Holland, G.P.; Alam, T.M. Magnetic alignment of aqueous ctab in nematic and hexagonal liquid crystalline phases investigated by spin-1 NMR. Phys. Chem. Chem. Phys. 2006, 8, 2635–2641. [Google Scholar] [CrossRef] [PubMed]
- Majewski, P.W.; Osuji, C.O. Controlled alignment of lamellar lyotropic mesophases by rotation in a magnetic field. Langmuir 2010, 26, 8737–8742. [Google Scholar] [CrossRef] [PubMed]
- Tousley, M.E.; Feng, X.; Elimelech, M.; Osuji, C.O. Aligned nanostructured polymers by magnetic-field-directed self-assembly of a polymerizable lyotropic mesophase. ACS Appl. Mater. Interfaces 2014, 6, 19710–19717. [Google Scholar] [CrossRef] [PubMed]
- Amundson, K.; Helfand, E.; Quan, X.; Hudson, S.D.; Smith, S.D. Alignment of lamellar block copolymer microstructure in an electric field. 2. Mechanisms of alignment. Macromolecules 1994, 27, 6559–6570. [Google Scholar] [CrossRef]
- Ku, A.Y.; Saville, D.A.; Aksay, I.A. Electric-field-induced orientation of surfactant-templated nanoscopic silica. Langmuir 2007, 23, 8156–8162. [Google Scholar] [CrossRef] [PubMed]
- Korner, H.; Shiota, A.; Bunning, T.J.; Ober, C.K. Orientation-on-demand thin films: Curing of liquid crystalline networks in ac electric fields. Science 1996, 272, 252. [Google Scholar] [CrossRef]
- Tsori, Y.; Tournilhac, F.; Andelman, D.; Leibler, L. Structural changes in block copolymers: Coupling of electric field and mobile ions. Phys. Rev. Lett. 2003, 90, 145504. [Google Scholar] [CrossRef] [PubMed]
- Hillhouse, H.W.; Okubo, T.; van Egmond, J.W.; Tsapatsis, M. Preparation of supported mesoporous silica layers in a continuous flow cell. Chem. Mater. 1997, 9, 1505–1507. [Google Scholar] [CrossRef]
- Hillhouse, H.W.; van Egmond, J.W.; Tsapatsis, M. Highly oriented mesostructured thin films: Shear-induced deposition of optically anisotropic coatings of tungsten oxide/surfactant composites. Langmuir 1999, 15, 4544–4550. [Google Scholar] [CrossRef]
- Su, B.; Lu, X.M.; Lu, Q.H. A facile method to prepare macroscopically oriented mesostructured silica film: Controlling the orientation of mesochannels in multilayer films by air flow. J. Am. Chem. Soc. 2008, 130, 14356–14357. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Uejo, F.; Yoda, T.; Uchida, T.; Tanamura, Y.; Yamashita, T.; Teramae, N. Self-assembly of a silica–surfactant nanocomposite in a porous alumina membrane. Nat. Mater. 2004, 3, 337–341. [Google Scholar] [CrossRef] [PubMed]
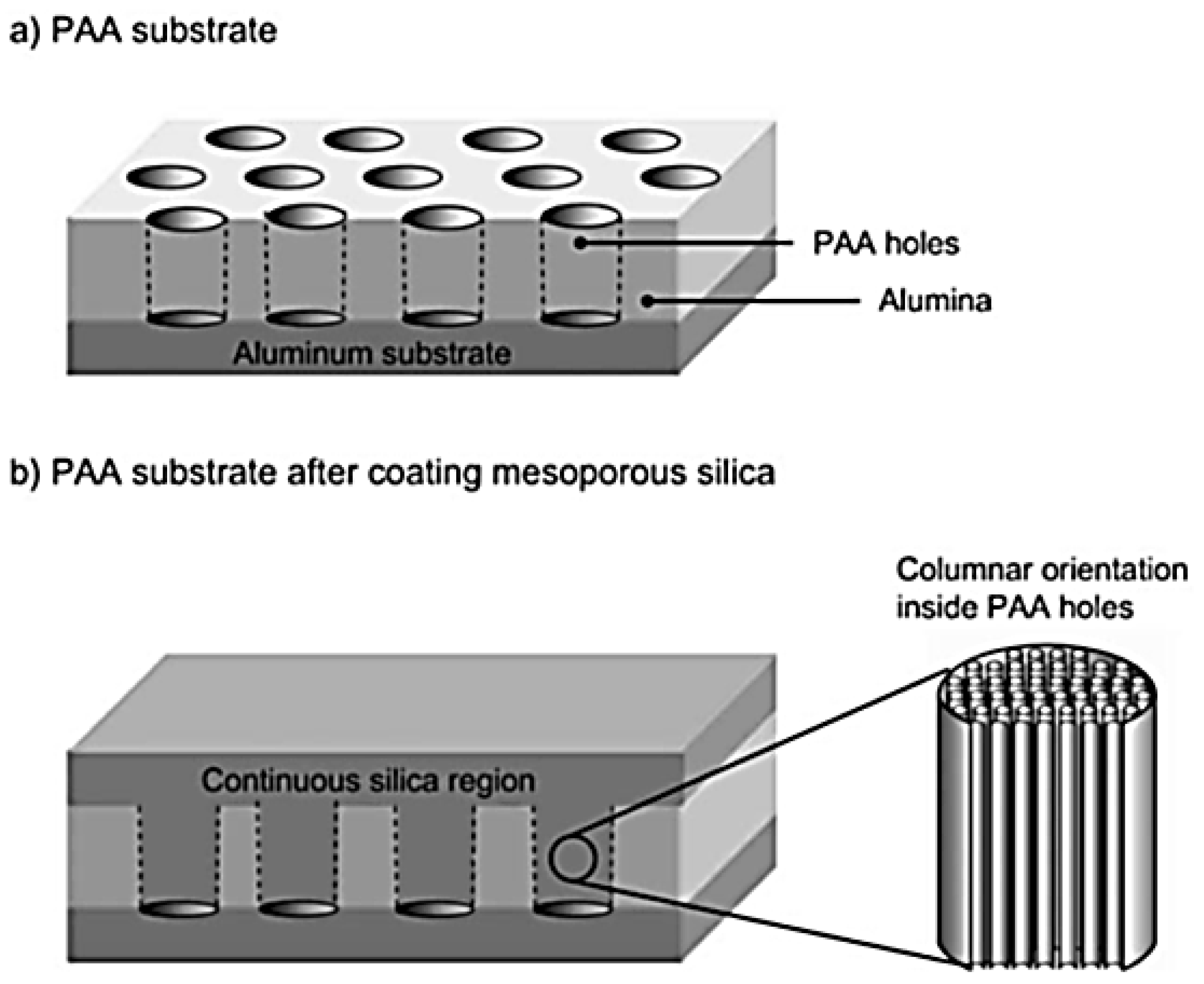
- Yamauchi, Y.; Nagaura, T.; Inoue, S. Oriented growth of small mesochannels utilizing a porous anodic alumina substrate: Preparation of continuous film with standing mesochannels. Chem. Asian J. 2009, 4, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Trau, M.; Yao, N.; Kim, E.; Xia, Y.; Whitesides, G.; Aksay, I. Microscopic patterning of orientated mesoscopic silica through guided growth. Nature 1997, 390, 674–676. [Google Scholar]
- Wu, C.W.; Ohsuna, T.; Edura, T.; Kuroda, K. Orientational control of hexagonally packed silica mesochannels in lithographically designed confined nanospaces. Angew. Chem. Int. Ed. 2007, 46, 5364–5368. [Google Scholar] [CrossRef] [PubMed]
- Aksay, I.; Trau, M.; Manne, S.; Honma, I.; Yao, N.; Zhou, L.; Fenter, P.; Eisenberger, P.; Gruner, S. Biomimetic pathways for assembling inorganic thin films. Science 1996, 273, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Miyata, H.; Kuroda, K. Preferred alignment of mesochannels in a mesoporous silica film grown on a silicon (110) surface. J. Am. Chem. Soc. 1999, 121, 7618–7624. [Google Scholar] [CrossRef]
- Yang, H.; Kuperman, A.; Coombs, N.; Mamiche-Afara, S.; Ozin, G.A. Synthesis of oriented films of mesoporous silica on mica. Nature 1996, 379, 703–705. [Google Scholar] [CrossRef]
- Walcarius, A.; Sibottier, E.; Etienne, M.; Ghanbaja, J. Electrochemically assisted self-assembly of mesoporous silica thin films. Nat. Mater. 2007, 6, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Higuchi, T.; Suzuki, M.; Fukuoka, T.; Shimomura, T.; Ono, M.; Radhakrishnan, L.; Wang, H.; Suzuki, N.; Oveisi, H. A high-speed passive-matrix electrochromic display using a mesoporous TiO2 electrode with vertical porosity. Angew. Chem. Int. Ed. 2010, 49, 3956–3959. [Google Scholar] [CrossRef] [PubMed]
- Hauß, T.; Dante, S.; Haines, T.H.; Dencher, N.A. Localization of coenzyme Q 10 in the center of a deuterated lipid membrane by neutron diffraction. Biochim. Biophys. Acta Bioenerg. 2005, 1710, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.; Hunt, T.; Darwish, T.A.; Hauß, T.; Garvey, C.J.; Bryant, G. Localization of trehalose in partially hydrated dopc bilayers: Insights into cryoprotective mechanisms. J. R. Soc. Interface 2014, 11, 20140069. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Kawabata, K.; Kaufman, G.; Elimelech, M.; Osuji, C.O. Highly selective vertically aligned nanopores in sustainably derived polymer membranes by molecular templating. ACS Nano 2017, 11, 3911–3921. [Google Scholar] [CrossRef] [PubMed]
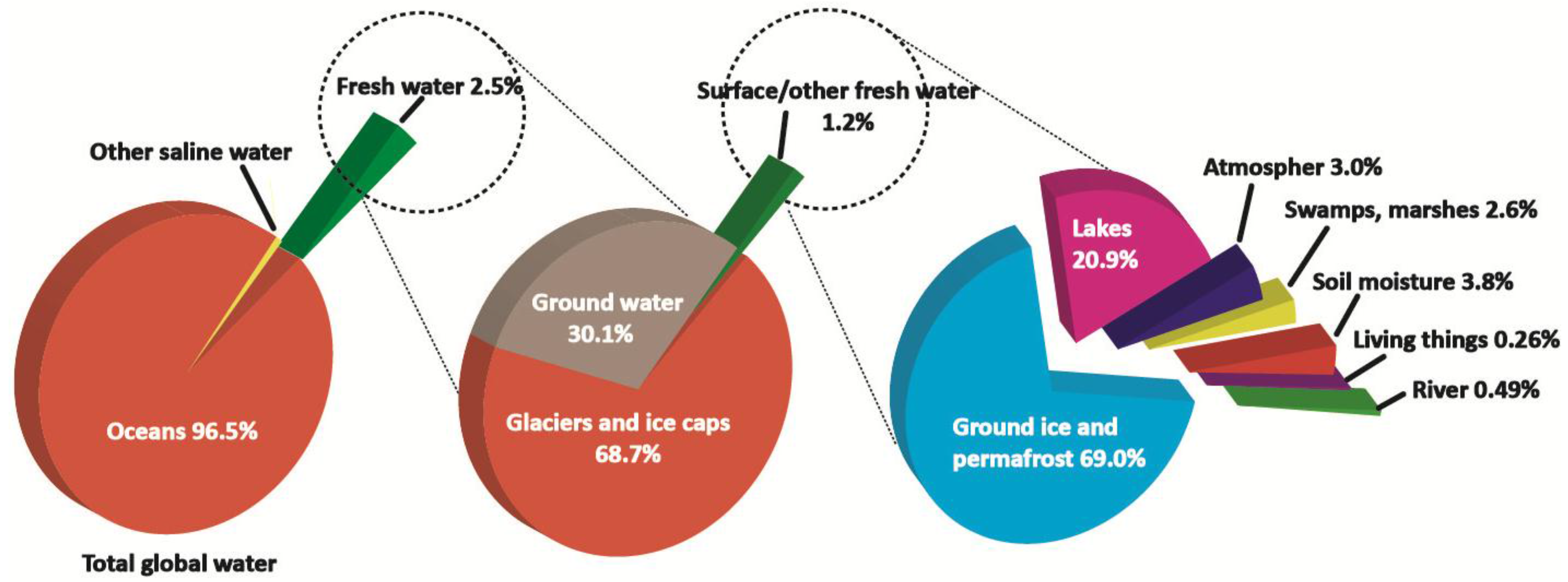
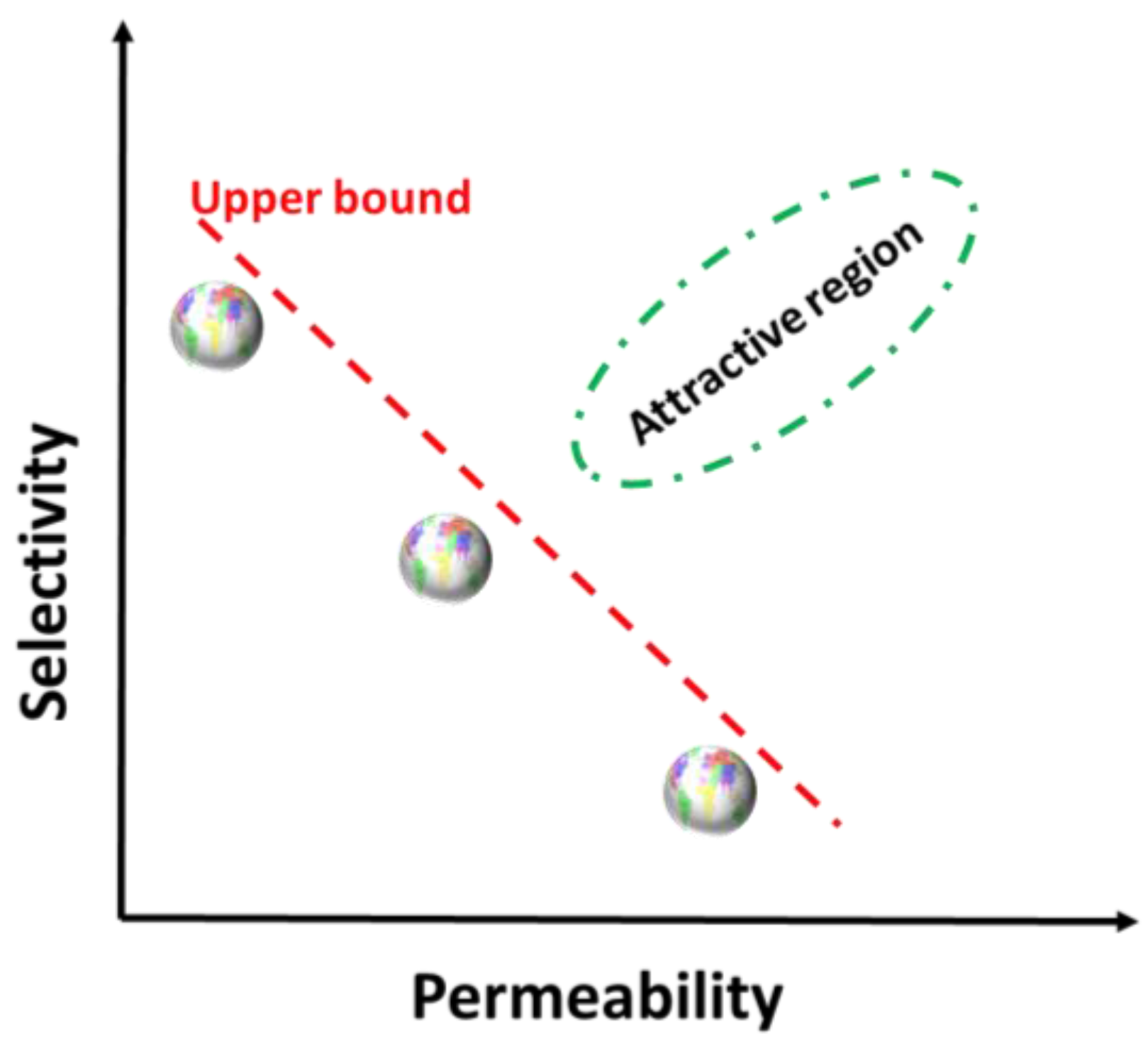
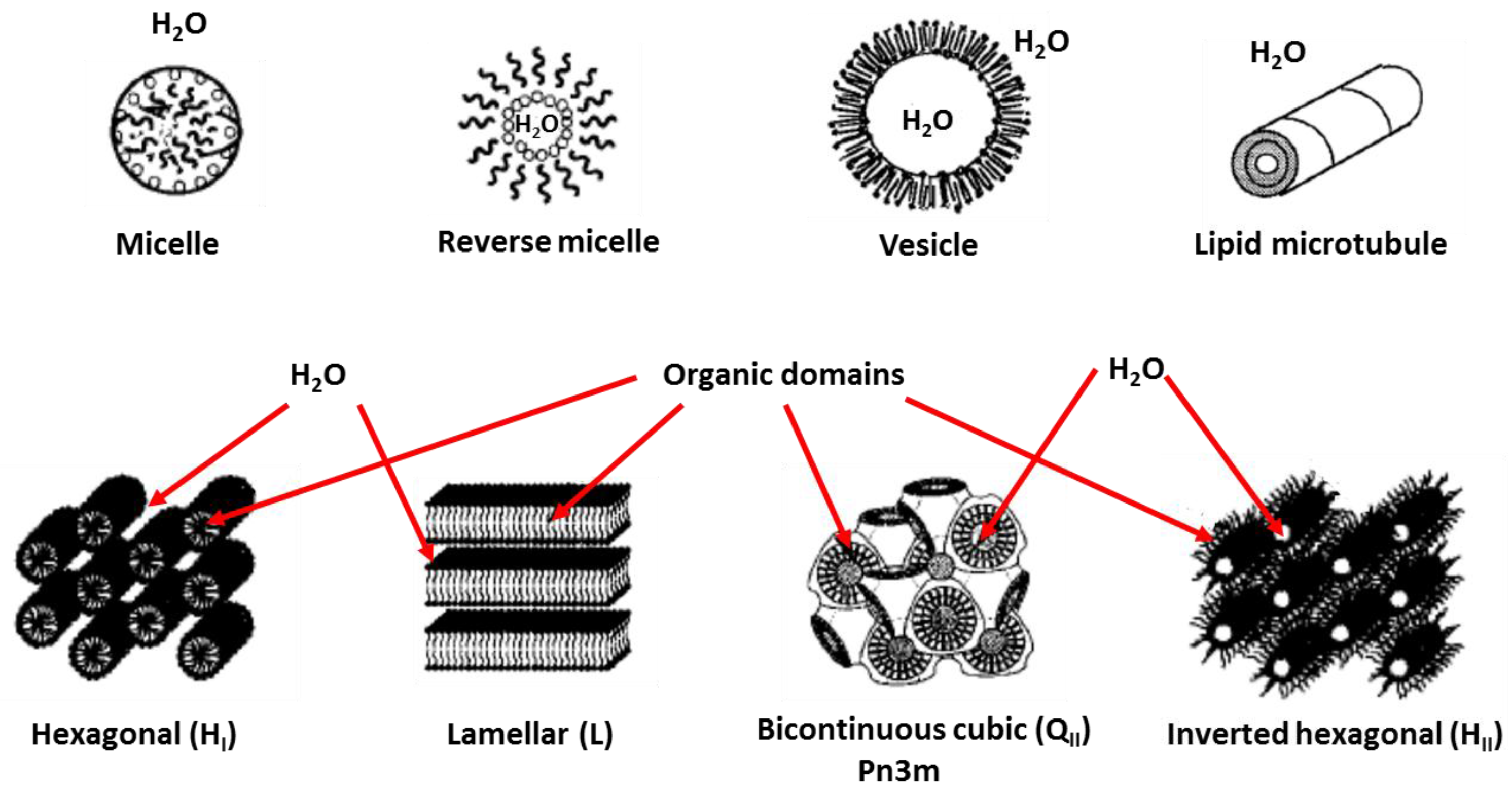
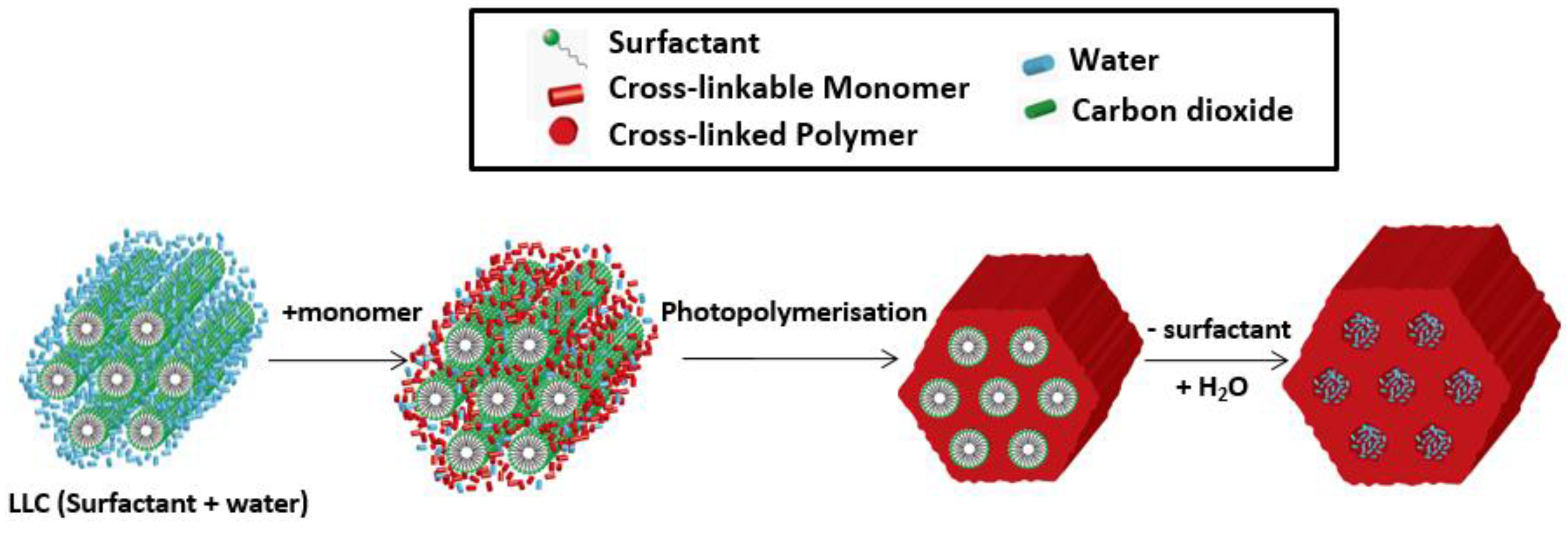
| Desalination Method | MSF | MED | MVC | RO |
|---|---|---|---|---|
| Electrical energy (kWh/m3) | 4–6 | 1.5–2.5 | 7–12 | 3–3.5 |
| Thermal energy (kWh/m3) | 50–100 | 60–100 | None | None |
| Total equivalent electrical energy (kWh/m3) | 13.5–25.5 | 6.5–11 | 7–12 | 3–5.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Garvey, C.J.; Zhao, H.; Huang, K.; Kong, L. Toward the Fabrication of Advanced Nanofiltration Membranes by Controlling Morphologies and Mesochannel Orientations of Hexagonal Lyotropic Liquid Crystals. Membranes 2017, 7, 37. https://doi.org/10.3390/membranes7030037
Wang G, Garvey CJ, Zhao H, Huang K, Kong L. Toward the Fabrication of Advanced Nanofiltration Membranes by Controlling Morphologies and Mesochannel Orientations of Hexagonal Lyotropic Liquid Crystals. Membranes. 2017; 7(3):37. https://doi.org/10.3390/membranes7030037
Chicago/Turabian StyleWang, Guang, Christopher J. Garvey, Han Zhao, Kang Huang, and Lingxue Kong. 2017. "Toward the Fabrication of Advanced Nanofiltration Membranes by Controlling Morphologies and Mesochannel Orientations of Hexagonal Lyotropic Liquid Crystals" Membranes 7, no. 3: 37. https://doi.org/10.3390/membranes7030037
APA StyleWang, G., Garvey, C. J., Zhao, H., Huang, K., & Kong, L. (2017). Toward the Fabrication of Advanced Nanofiltration Membranes by Controlling Morphologies and Mesochannel Orientations of Hexagonal Lyotropic Liquid Crystals. Membranes, 7(3), 37. https://doi.org/10.3390/membranes7030037






