2.3. Photo-Electrode Characterisation
According to the X-ray diffraction analysis (
Figure 3), the TiO
2 Degussa P90 powder (Frankfurt, Germany) used as photo-anode precursor mainly showed the presence of an anatase phase. The main characteristic peak at 25.4° 2θ was assigned to the (101) Miller index of anatase (JCPDS schedule: 21-1272) [
12,
15]. There is a small amount of evidence of the rutile structure (JCPDS schedule: 21-1276) with peaks at 27° (110) and 37° (101) 2θ; the occurrence of small percentages of brookite (JCPDS schedule: 16-617) is not excluded [
12]. According to the broadening of the X-ray diffraction peaks, the mean crystallite size for this sample, related to the anatase structure, was about 15 nm as determined from the Scherrer equation. Chemical reduction of the TiO
2 phase at 1050 °C gives rise to the formation of a mixture of sub-stoichiometric Ti-oxides [
13]. The occurrence of these processes was confirmed by XRD analysis (
Figure 3) showing typical peaks of Ti
nO
2n−1 (Magneli phase). In particular, the XRD patterns of the Ti-suboxide (
Figure 3) indicates the occurrence of the Ti
9O
17 (JCPDS: 18-1405) structure at 24°–24.5°. The Ti
6O
11 phase (JCPDS: 18-1401) was also present, whereas the Ti
4O
7 (JCPDS: 18-1402) was essentially present in traces. Thus, there was a high degree of sub-stoichiometry in the Ti-oxide reduced at high temperature. No evidence of peaks of Anatase or Rutile was observed in the XRD pattern of the reduced Ti-oxide. All of the assigned reflections belong to the Magneli phase.
The Brunauer–Emmett–Teller (BET) surface area of P90 was around 90 m
2/g according to the supplier, whereas the measured BET surface area of the in-house prepared Ti sub-oxide was 26 m
2/g. This relevant difference in surface area is essentially related to the sub-oxide reduction at 1050 °C, which promotes particle sintering [
13]. The morphology of the Ti-oxide materials was also investigated by TEM analysis (
Figure 4a,b). The P90 Ti-oxide showed spherical particles with relatively small particle size (around 15–20 nm), whereas the Ti-suboxide, due to the high temperature treatment, showed large particle agglomerates with primary particles of about 25–50 nm in size. However, the Ti-suboxide showed high surface roughness, which is possibly associated with a large fraction of surface defects.
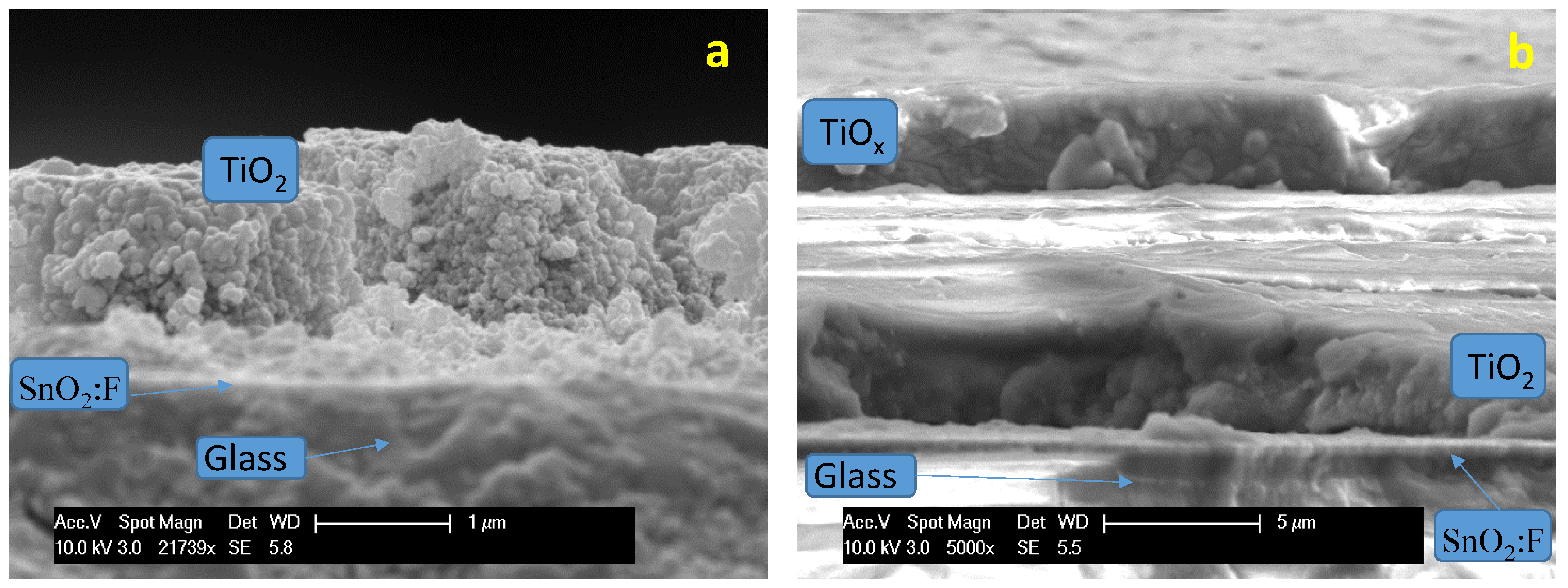
Two types of photoelectrodes were investigated. A bare TiO
2 layer was coated onto the TCO substrate and used in the first experiments (
Figure 5a). In a second set of experiments, the TiO
2 photo-anode layer was coated with a thin titanium sub-oxide layer (
Figure 5b). The cross-section of photoanodes prepared in a similar way was investigated by scanning electron microscopy. The thickness of the TiO
2 layer ranged between two and four microns, whereas the thickness of the Ti-suboxide layer was about three microns.
2.4. Photo-Electrolysis Behaviour
The influence of the membrane and ionomer characteristics on the water photo-splitting performance was investigated in a photo-electrolysis single cell. As discussed above, the photo-electrolysis device consisted of an assembly between the TiO2 photo-anode, the membrane and platinum as cathode. In this system, the hydrogen evolution occurs at the Pt cathode that was used as working/sense electrode, whereas, the photo-anode, where O2 evolution occurs, was used as counter/reference electrode. Since the aim of the work was to compare the effect of the ion exchange membrane on the water photo-splitting, a Titania-based photo-anode was initially used without any modification in terms of doping, surface treatment with reaction promoters, etc., as it would be required to achieve useful conversion efficiencies. Only a thermal sintering at 450 °C of the photo-anode was carried out to achieve good adhesion of the photo-electrode film to the substrate and proper continuity of the TiO2 network in the electrode layer. It is pointed out that an un-doped TiO2 according to its wide energy gap can absorb just a small fraction of the solar spectrum in the UV region. This system thus generates low photocurrents; however, at the same time, the high energy gap allows for achieving large spontaneous photo-voltage.
According to the sketch presented in
Figure 1, being the TiO
2 semiconductor (SC) brought in contact with the water film that is embedded into the ionomer and membrane electrolyte, a depletion of majority charge carriers (n-type in TiO
2) across the interface occurs due to the difference in the chemical potentials of the two phases [
15,
16]. This results in a band bending phenomena in the semiconductor near the interface. The illumination of the semiconductor–electrolyte interface causes a modification of the electrode potential as a consequence of the generation of electron–hole pairs, which are thus separated by the effect of the electric field across the depletion region [
15,
16]. At the open circuit (OCP), the illumination causes an accumulation of photo-generated charge carriers across the depletion region with a corresponding upward band bending producing a spontaneous photo-voltage [
16]. The band bending can be increased by polarising the electrode-electrolyte interface (
Figure 1 is showing the band bending in TiO
2 at the short circuit under illumination).
Accordingly, the photo-electrochemical behaviour of the electrode-polymer electrolyte membrane junction is characterised by a specific photocurrent-photo-voltage (I–V) profile that is very similar to a photodiode characteristic (
Figure 6,
Figure 7 and
Figure 8). The spontaneous photo-voltage drives the water photo-splitting process up to the short circuit (short circuit photocurrent, Isc). No hydrogen evolution occurs at the OCP, and the observed open circuit photo-voltage represents an electromotive force for the water photo-splitting process; whereas, the spontaneous hydrogen production is, in general, maximised at the short circuit (cell potential equal to zero in
Figure 6,
Figure 7 and
Figure 8). The potential region between the OCP and Isc is characterised by negative variation of the free energy, ∆
G, corresponding to a spontaneous hydrogen evolution and concomitant production of electricity (positive potential region when the cathode is used as working/sense electrode) [
15]. Under such conditions, the Fermi level in the semiconductor is higher in potential energy than the Fermi level in the electrolyte. This difference is the spontaneous photo-voltage [
15]. The production of electricity is null at the Isc where the Fermi levels in the semiconductor and the electrolyte are the same. The polarisation range between the OCP and Isc is also called photo-voltage driven region and the photo-electrolysis cell operating under such conditions is assisted only by the solar radiation. If an external bias is applied the band banding in the semiconductor increases and the photocurrent can persist or may increase further. In this case the cell voltage is negative because the cathode, as working electrode, and its associated Fermi level in the electrolyte is higher in terms of potential energy with respect to the Fermi level in the photo-anode. Above the reversible potential (1.23 V) for water splitting, the endothermic water electrolysis occurs also in the dark. The potential region between the Isc and the reversible potential is the bias-assisted photo-electrolysis region where most of the photo-electrolysis semiconductors characterised by small band gap or un-appropriate band levels usually operate.
The I–V characteristics of the photo-electrolysis cells under investigation are shown in
Figure 6,
Figure 7 and
Figure 8. The I–V response is reported under illumination only; the dark current was quite low and similar in all cases. As reported elsewhere [
14], for a large band gap n-type TiO
2 semiconductor, the equilibrium concentration of holes is very low. Accordingly, the anodic dark current that derives from the diffusion of holes towards the surface is also low.
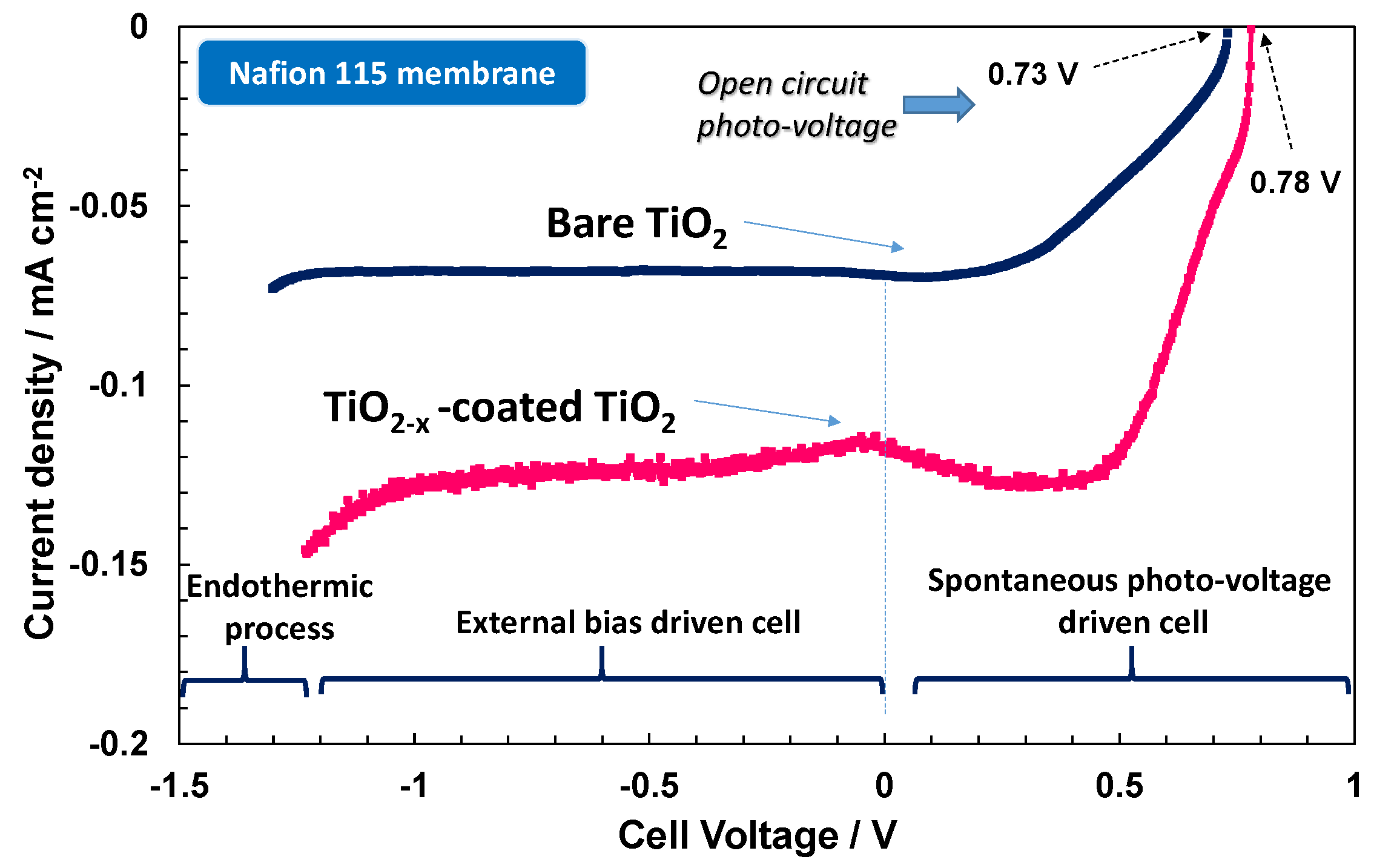
The photo-electrolytic characteristics for the bare TiO
2 photo-anode combined with proton exchange Nafion
® 115 and anion exchange Fumion
® FAA3-20 polymer electrolyte membranes are shown in
Figure 6. Upon illumination (100 mW cm
−2), the spontaneous photo-voltage registered for the Nafion
®-based cell is about 0.73 V, where it reaches 0.91 V with the alkaline membrane. More relevant is the increase of photocurrent with the alkaline system in the overall potential region. The short circuit photocurrent recorded with the anionic membrane is about twice that observed with Nafion
®. The higher slope of the photo-electrolytic characteristic recorded with the anionic membrane in the photo-voltage-driven region is indicative of a better fill factor corresponding to a lower occurrence of recombination phenomena [
15,
16,
17,
18,
19,
20,
21,
22]. Thus, the minority charge carriers (holes for the n-type TiO
2) are more easily transferred to the electrolyte under alkaline conditions. This aspect may be related to the larger concentration of OH species under alkaline conditions that are involved in the intermediate steps of the oxygen evolution process [
15,
16]. A large concentration of these species at the interface favours their adsorption on the photo-anode surface favouring the capture of holes by the adsorbed OH species with respect to the recombination with photo-generated electrons inside the semiconductor.
The large spontaneous photo-voltage recorded for the present electrolytic cells is associated with a relevant upper band bending occurring at the TiO
2 semiconductor upon illumination. This produces the onset of photocurrents at potentials quite negative with respect to the equilibrium potential of water splitting, which is the same in acid and alkaline electrolytes, i.e., 1.23 V at 25 °C. Thus, the energy required for water splitting is provided by the illumination and the generated high energy holes (
Figure 1 bottom) can oxidise water. Accordingly, water splitting can occur under conditions more favourably than at the equilibrium, and electricity is also generated together with hydrogen at positive cell potentials. The protonic or anionic membranes do not affect this mechanism but they influence the charge transfer at the photo-anode–electrolyte interface that must compete with the recombination processes inside the semiconductor. Besides the above-discussed mechanism, another phenomenon may occur in the photo-electrolysis device that can explain the large spontaneous photo-voltages observed here. However, specific efforts were adopted to avoid exposure to air of the water filled to the cell, since the cell itself is not gastight (the cell is designed to favour gas escape), and it is not excluded that some air infiltration may have occurred during this procedure and when the cell was operated. This would mean that part of the large spontaneous photo-voltage observed here could also derive from a shift of the Fermi level of the Pt electrode to positive values associated with the adsorption of oxygen species on Pt.
In principle, the photo-voltage is caused by a shift of the Fermi level of TiO
2 upon illumination from its rest potential in the dark, but the Fermi level positioning in the dark is determined by a specific interaction between the semiconductor surface states and the electrolyte. In our case, the electrolyte is composed of pure water in combination with a solid polymer electrolyte. The thermodynamic basis for hydrogen production relies on the fact that the conduction band edge of TiO
2 is higher in energy or negative in potential with respect to RHE under the present experimental conditions (
Figure 1 bottom). On the other hand, the measured cell voltage under illumination results from the difference between the cathode potential and the anode potential. The anode potential is affected by the interaction between the semiconductor surface states and the electrolyte whereas the cathode potential may also be affected by the adsorption of traces of oxygen gas molecules on the Pt surface.
The oxygen evolution process occurring at the TiO
2 surface is widely considered to be the rate determining step for the photo-electrolysis configuration under study in both acidic and alkaline systems [
17,
18,
19,
20,
21,
22]. Light harvesting produces the intrinsic ionization of the titania semiconductor, which leads to the formation of holes in the valence band and electrons in the conduction band provided that the energy of photons (
hν) is larger than the energy gap:
The electric field at the electrode–electrolyte interface avoids recombination of the photo-generated charge carriers allowing the light-induced electron-hole pairs to produce the splitting of water into oxygen and hydrogen at the photo-anode and photocathode–electrolyte interface. Besides influencing the charge transfer at the interface, a different type of electrolyte can favour the formation of electronic levels at the surface causing a pinning of the Fermi level [
23]. Accordingly, the difference in the observed photo-voltages for acidic and alkaline environment is associated with these two effects i.e., surface states and charge transfer kinetics.
To get more insights on the surface effects and the influence of the different electrolytes on the adsorption properties of OH species, the TiO
2 photo-anode was coated with a thin Titanium sub-oxide layer. The presence of the sub-stoichiometric TiO
2−x species on the photo-anode surface promotes significantly the adsorption of OH species. These suboxides have been identified as promoters for the oxygen evolution in acidic environment [
13].
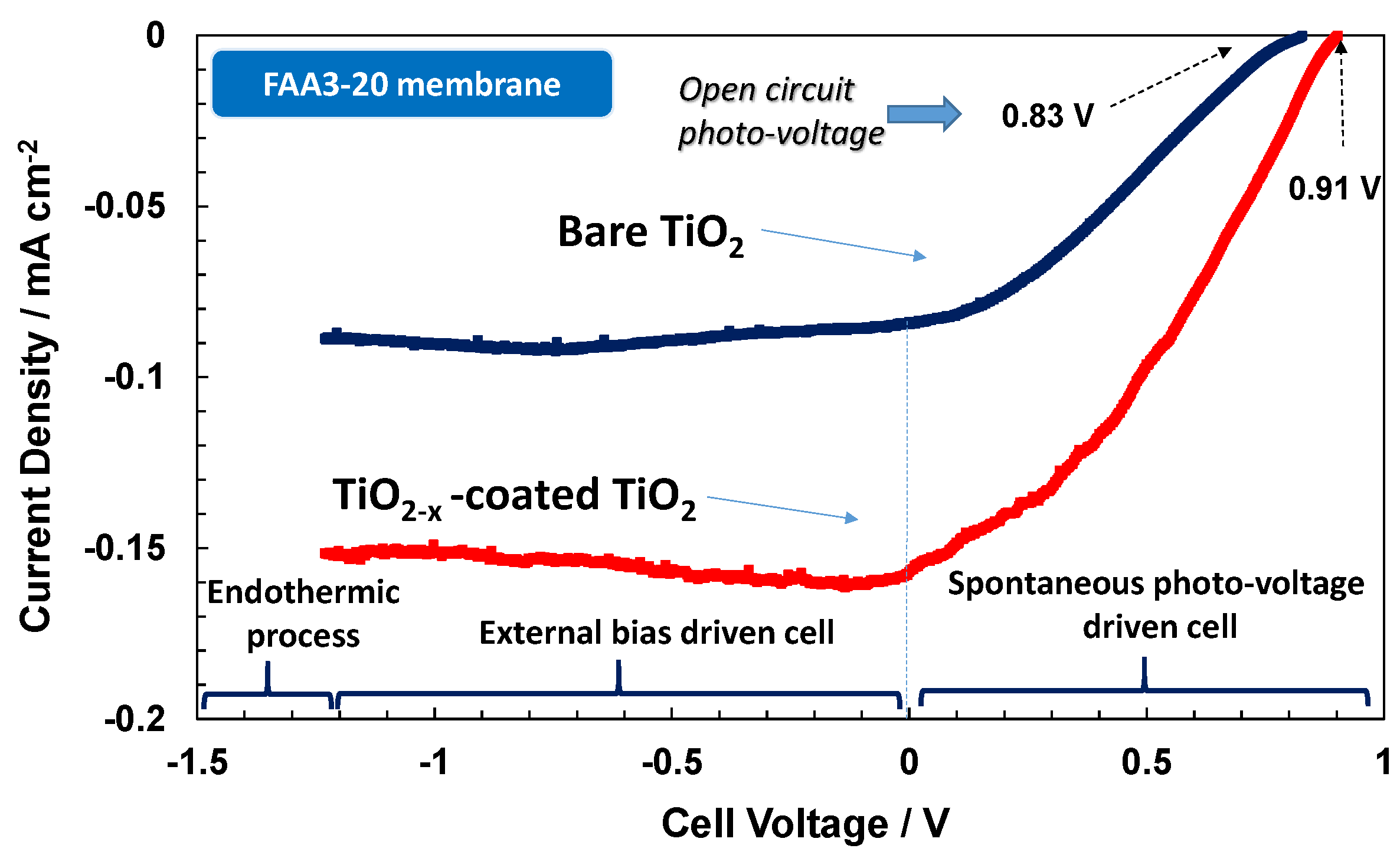
Figure 7 shows a small enhancement of photo-voltage for the Nafion
® based photo-electrolysis cell when TiO
2−x is coated on the TiO
2 photo-anode and a significant increase of photocurrent in the entire range, especially at potentials close to the reversible potential (−1.23 V). This provides a clear indication that OH adsorption is a rate determining step in the photo-electrolytic water splitting in acidic environment being this process promoted by the presence of sub-stoichiometric oxides. TiO
2−x surface states favour the adsorption of the oxygen species on the surface to saturate the defective sites. The presence of a wide coverage of OH species on the electrode surface favours the hole transfer to the electrolyte while avoiding the recombination phenomena [
22,
23,
24,
25]. The positive effect is observed in terms of both photo-voltage and photocurrent. The adsorption of OH species possibly shifts the flat-band potential producing an increase of the photo-voltage. In any case, the performance of the TiO
2−x-coated TiO
2 in acidic environment does not reach that of bare TiO
2 in alkaline environment both in terms of OCP and photocurrent.
Curiously, when the TiO
2−x-coated TiO
2 photoanode is used in the presence of the anionic system (
Figure 8), a strong decrease of performance both in terms of photo-voltage and photocurrent is observed. It appears that the large concentration of OH species in the alkaline environment causes a strong adsorption on the Ti sub-oxide surface, and this hinders the desorption of oxygen species to give rise to oxygen gas evolution. It is well known that in electro-catalytic reactions, the binding energy between the adsorbed species and the electrocatalyst surface should not be neither strong nor weak but intermediate to maximise the reaction rate [
7,
13]. Otherwise, the rate of adsorption or desorption becomes rate determining if the bond strength is weak or strong, respectively [
7,
13].
The presence of widely distributed electronic levels in the sub-stoichiometric oxide surface causes recombination and trapping effects for the photo-generated carriers if the oxygen species can not be easily desorbed after the capture of photo-generated holes, thus determining low photocurrent.
The present results clearly show how the type of electrolyte can drastically influence the choice of surface promoters in photo-electrocatalysis and the performance achievable with different photo-electrode systems.
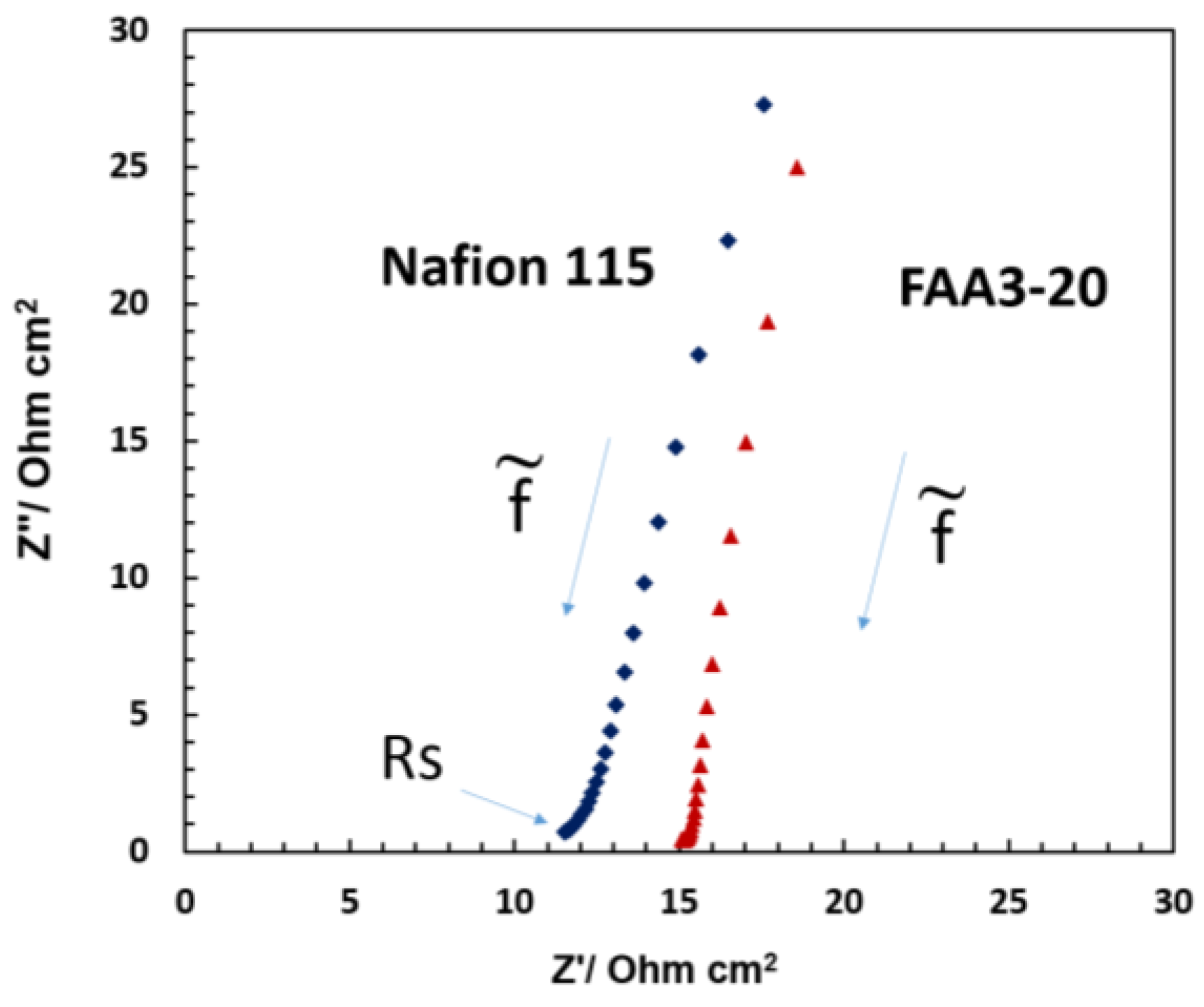
Ac-impedance analysis (
Figure 9) was carried out to understand if there was some relevant ohmic limitation associated with the different membranes used in the photo-electrolysis cells. The series resistance or the high frequency intercept in the Nyquist plot observed for the various systems under illumination was generally lower than 15 Ohm cm
2. Thus, this should not produce any relevant potential loss since the photocurrent measured in the photo-electrolysis device is relatively small. However, polarization resistance associated with both the charge transfer at the electrode–electrolyte interface and the charge transport in the space charge region appears dominant since no complete semicircle is observed. The impedance profile in the Nyquist plot is mainly capacitive and the high frequency intercept shifts to larger values for the alkaline membrane. The Nafion
® based cell shows slightly lower series resistance due to the better conductivity even if the Nafion
® 115 membrane thickness is much larger than the FAA3-20 membrane. Most of the contribution to the series resistance is generally due to the TCO substrate even if the membrane, interface and cell design can also have an impact on the ohmic properties of the cell.
The photo-electrolysis devices were also investigated in terms of time studies (
Figure 10). For these studies, only the cells providing the best performances during the initial experiments, i.e., the bare TiO
2 in combination with the anionic membrane and the Ti sub-oxide coated-TiO
2 combined with the protonic membrane, were selected. Chrono-amperomentric curves (
Figure 10) were carried out for both anionic and protonic devices for 4 h at the short circuit (0 V).
A gradual decrease of photocurrent with time was observed for both configurations even if the Nafion
®-based system appeared more stable (
Figure 10). After the time study, photoelectrochemical polarisations were repeated in the dark and under illumination (
Figure 11). The open circuit photovoltage decreased by about 170 and 140 mV in the case of the anionic and protonic cells, respectively, with respect to the initial measurements. However, the photocurrent decay was about 25% and 11% for the anionic and protonic cells, respectively, compared to the initial values. The curves registered in the dark showed a positive voltage of about 130 mV and 97 mV for the protonic and anionic cells, respectively (
Figure 11). However, the dark current was negligible in the photovoltage-driven region, increasing rapidly close to the reversible potential. This increase of dark current in the bias-assisted region close to the reversible potential was more evident for the anionic system compared to the protonic system.
Several hypotheses are formulated for the photocurrent and photo-voltage decay observed after the time studies. However, we have used deaerated water, since this is filled in the cell by a syringe system, water is necessarily exposed to the air. Thus, we do not exclude that some traces of dissolved oxygen may be presented in water. This may have possibly caused a depolarisation of the cathode by shifting the Fermi level to higher potentials. Such effect may explain the loss of photo-voltage after prolonged operation since the residual oxygen at the Pt electrode is consumed by the produced hydrogen. The decrease of photocurrent could be related to mass transport limitations for the evolution of the gas molecules inside the device or to some modification of the electrode–electrolyte interface. However, the performance decay was almost reversible in both cases since the photo-electrolysis cells re-gained the initial performance when the electrochemical operation under illumination was stopped for some hours. In this regard, we think that device design should be improved to address these recoverable losses.
The information gained from this study, using TiO2 as model system, and the selected membranes can be useful in developing photo-electrolysis cells based on smaller energy gap semiconductors than TiO2, thus providing a relevant increase of water splitting efficiency.