Descriptive Analysis of LAP1 Distribution and That of Associated Proteins throughout Spermatogenesis
Abstract
:1. Introduction
2. Results
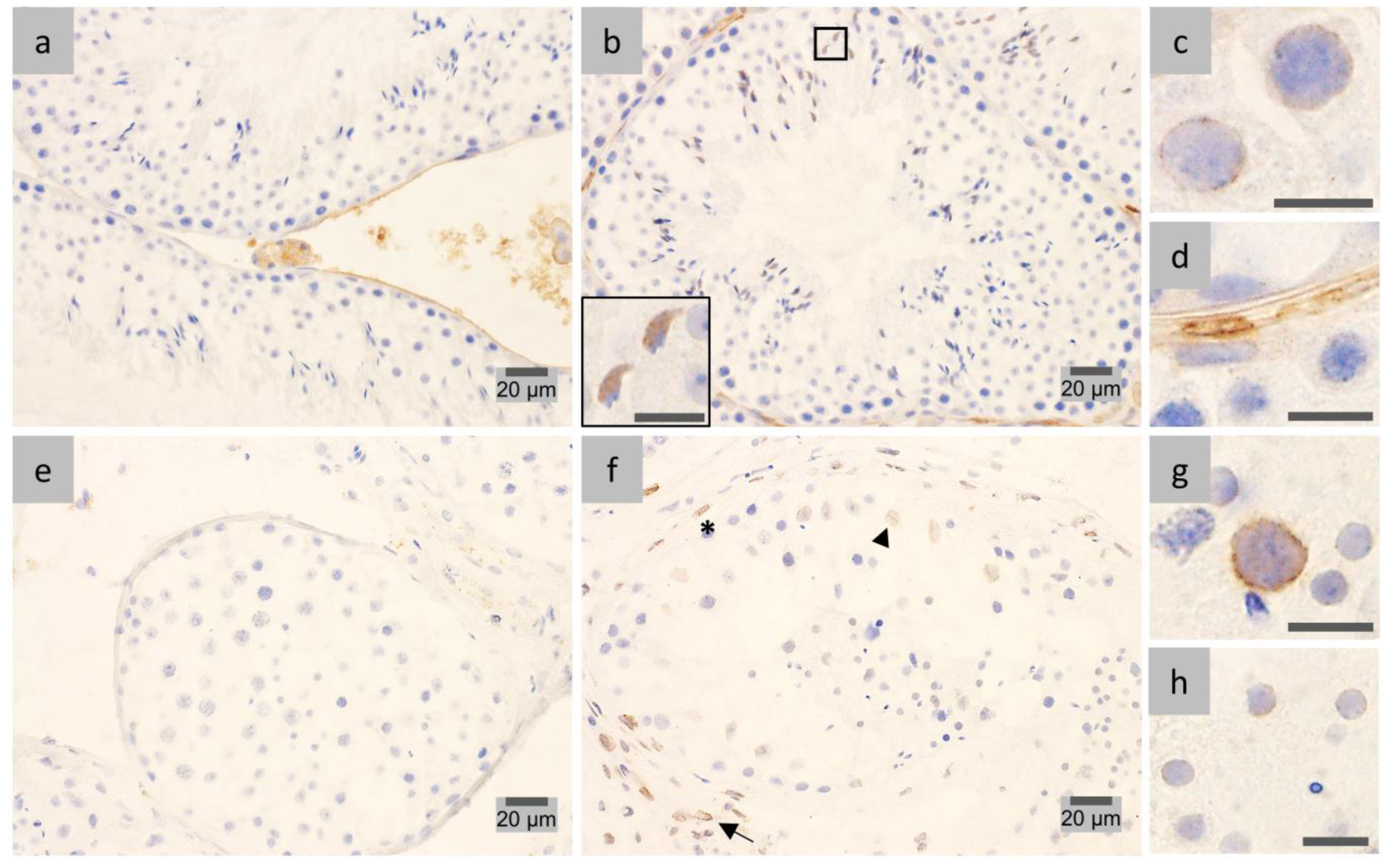
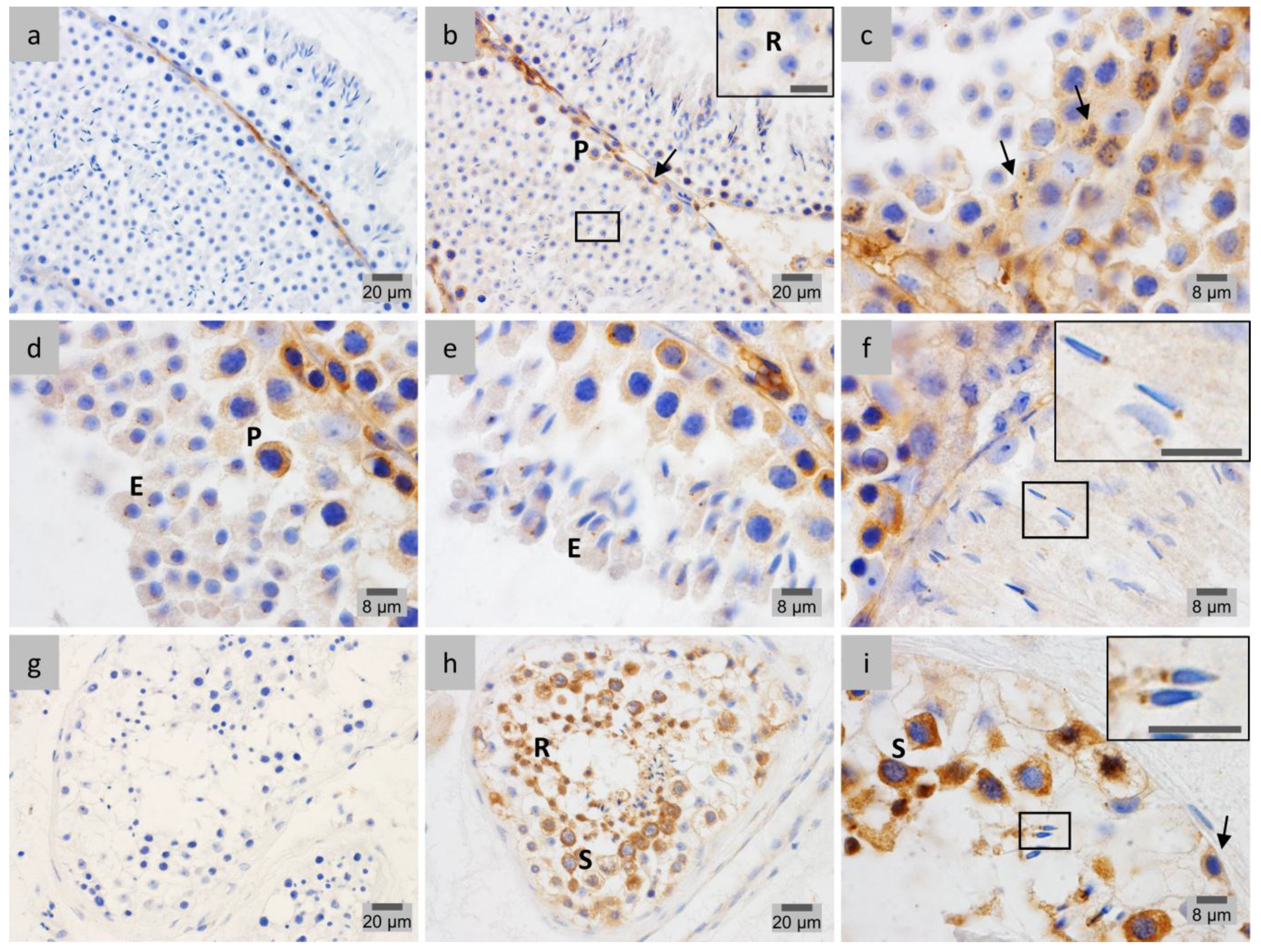
2.1. LAP1 Expression and Localization in the Testis
2.2. LAP1 as a New Player in Manchette Development
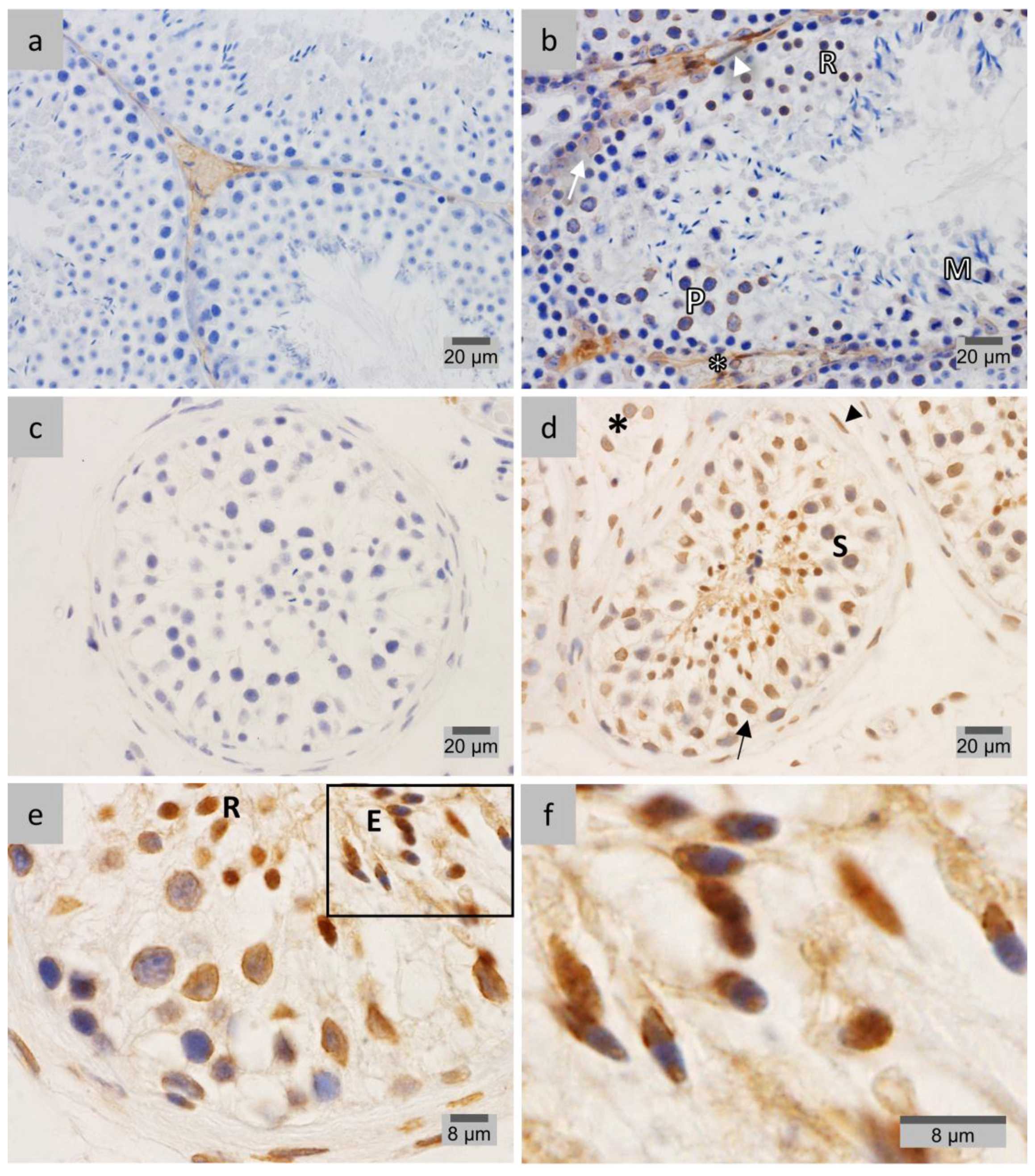
2.3. PP1γ2 Shares Similar Subcellular Distribution with LAP1 in the Testis
2.4. LAP1 Chromatin Regulation through Lamin Interaction
2.5. γ-Tubulin Localizes in the Centriolar Pole during Spermiogenesis
3. Discussion
4. Materials and Methods
4.1. Antibodies
4.2. Animals
4.3. Patient Material
4.4. Tissue Preparation for IHC
4.5. Histological Staining
4.6. Tissue Homogenization for WB Analysis
4.7. SDS-PAGE and Immunoblotting
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guraya, S.S. Biology of Spermatogenesis and Spermatozoa in Mammals; Springer: Berlin/Heidelberg, Germany, 1987; Volume 20. [Google Scholar]
- Hess, R.A.; de Franca, L.R. Spermatogenesis and Cycle of the Seminiferous Epithelium. In Molecular Mechanisms in Spermatogenesis; Cheng, C.Y., Ed.; Landes Bioscience and Springer Science+Business Media: Philadelphia, NY, USA, 2008; pp. 1–15. [Google Scholar]
- Bellvé, A.R.; Cavicchia, J.C.; Millette, C.F.; O’Brien, D.A.; Bhatnagar, Y.M.; Dym, M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J. Cell Biol. 1977, 74, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Pelletier, R.M.; Cyr, D.G.; Smith, C.E. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: Background to spermatogenesis, spermatogonia, and spermatocytes. Microsc. Res. Tech. 2010, 73, 241–278. [Google Scholar] [CrossRef] [PubMed]
- Muciaccia, B.; Boitani, C.; Berloco, B.P.; Nudo, F.; Spadetta, G.; Stefanini, M.; de Rooij, D.G.; Vicini, E. Novel Stage Classification of Human Spermatogenesis Based on Acrosome Development. Biol. Reprod. 2013, 89, 60. [Google Scholar] [CrossRef] [PubMed]
- Alsheimer, M.; Fecher, E.; Benavente, R. Nuclear envelope remodelling during rat spermiogenesis: Distribution and expression pattern of LAP2/thymopoietins. J. Cell Sci. 1998, 2234, 2227–2234. [Google Scholar]
- Alsheimer, M.; Von Glasenapp, E.; Hock, R.; Benavente, R. Architecture of the Nuclear Periphery of Rat Pachytene Spermatocytes: Distribution of Nuclear Envelope Proteins in Relation to Synaptonemal Complex Attachment Sites. Am. Soc. Cell Biol. 1999, 10, 1235–1245. [Google Scholar] [CrossRef]
- Alsheimer, M.; Jahn, D.; Schramm, S.; Benavente, R. Nuclear Lamins in Mammalian Spermatogenesis. In Epigenetics and Human Reproduction; Rousseaux, S., Khochbin, S., Eds.; Springer: Berlin/Heidelberg, Germary, 2011; Volume 2148, pp. 279–288. [Google Scholar]
- Dernburg, A.F. Pushing the (nuclear) envelope into meiosis. Genome Biol. 2013, 14. [Google Scholar] [CrossRef]
- Link, J.; Leubner, M.; Schmitt, J.; Go, E.; Benavente, R.; Jeang, K.; Xu, R.; Alsheimer, M. Analysis of Meiosis in SUN1 Deficient Mice Reveals a Distinct Role of SUN2 in Mammalian Meiotic LINC Complex Formation and Function. PLoS Genet. 2014, 10, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Mylonis, I. Temporal Association of Protamine 1 with the Inner Nuclear Membrane Protein Lamin B Receptor during Spermiogenesis. J. Biol. Chem. 2004, 279, 11626–11631. [Google Scholar] [CrossRef] [PubMed]
- Göb, E.; Schmitt, J.; Benavente, R.; Alsheimer, M. Mammalian Sperm Head Formation Involves Different Polarization of Two Novel LINC Complexes. PLoS ONE 2010, 5, e12072. [Google Scholar] [CrossRef] [PubMed]
- Senior, A.; Gerace, L. Integral membrane proteins specific to the inner nuclear membrane and associated with the nuclear lamina. J. Cell Biol. 1988, 107, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Foisner, R.; Gerace, L. Integral membrane proteins of the nuclear envelope interact with lamins and chromosomes, and binding is modulated by mitotic phosphorylation. Cell 1993, 73, 1267–1279. [Google Scholar] [CrossRef]
- Santos, M.; Costa, P.; Martins, F.; da Cruz e Silva, E.F.; da Cruz e Silva, O.A.B.; Rebelo, S. LAP1 is a crucial protein for the maintenance of the nuclear envelope structure and cell cycle progression. Mol. Cell. Biochem. 2015, 399, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, S.; da Cruz e Silva, E.F.; da Cruz e Silva, O.A.B. Genetic mutations strengthen functional association of LAP1 with DYT1 dystonia and muscular dystrophy. Mutat. Res. Mutat. Res. 2015, 766, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; da Cruz e Silva, O.; Rebelo, S. Lamina Associated Polypeptide 1 (LAP1) Interactome and Its Functional Features. Membranes 2016, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Rebelo, S.; van Kleeff, P.J.M.; Kim, C.E.; Dauer, W.T.; Fardilha, M.; da Cruz e Silva, O.A.; da Cruz e Silva, E.F. The nuclear envelope protein, LAP1B, is a novel protein phosphatase 1 substrate. PLoS ONE 2013, 8, e76788. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Domingues, S.C.; Costa, P.; Muller, T.; Galozzi, S.; Marcus, K.; da Cruz e Silva, E.F.; da Cruz e Silva, O.A.; Rebelo, S. Identification of a Novel Human LAP1 Isoform That Is Regulated by Protein Phosphorylation. PLoS ONE 2014, 9, e113732. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, S.; Santos, M.; Martins, F.; da Cruz e Silva, E.F.; da Cruz e Silva, O.A.B. Protein phosphatase 1 is a key player in nuclear events. Cell. Signal. 2015, 27, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Sasaki, K.; Shima, H.; Shibuya, M.; Sugimura, T.; Nagao, M. Protein phosphatases possibly involved in rat spermatogenesis. Biochem. Biophys. Res. Commun. 1990, 171, 230–235. [Google Scholar] [CrossRef]
- Smith, G.D.; Wolf, D.P.; Trautman, K.C.; da Cruz e Silva, E.F.; Greengard, P.; Vijayaraghavan, S. Primate sperm contain protein phosphatase 1, a biochemical mediator of motility. Biol. Reprod. 1996, 54, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, R.; Kline, D.; Lu, J.; Orth, J.; Pilder, S.; Vijayaraghavan, S. Analysis of Ppp1cc-null mice suggests a role for PP1gamma2 in sperm morphogenesis. Biol. Reprod. 2007, 76, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Puri, P.; Nairn, A.C.; Vijayaraghavan, S. Selective ablation of Ppp1cc gene in testicular germ cells causes oligo-teratozoospermia and infertility in mice. Biol. Reprod. 2013, 89, 128. [Google Scholar] [CrossRef] [PubMed]
- Vester, B.; Smith, A.; Krohne, G.; Benavente, R. Presence of a nuclear lamina in pachytene spermatocytes of the rat. J. Cell Sci. 1993, 104, 557–563. [Google Scholar] [PubMed]
- Elkhatib, R.; Longepied, G.; Paci, M.; Achard, V.; Grillo, J.-M.; Levy, N.; Mitchell, M.J.; Metzler-Guillemain, C. Nuclear envelope remodelling during human spermiogenesis involves somatic B-type lamins and a spermatid-specific B3 lamin isoform. Mol. Hum. Reprod. 2015, 21, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.B.; Burnham, B.L.; Bellvé, A.R. The differential expression of lamin epitopes during mouse spermatogenesis. Mol. Reprod. Dev. 1993, 34, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Maul, G.G.; French, B.T.; Bechtol, K.B. Identification and redistribution of lamins during nuclear differentiation in mouse spermatogenesis. Dev. Biol. 1986, 115, 68–77. [Google Scholar] [CrossRef]
- Shen, J.; Chen, W.; Shao, B.; Qi, Y.; Xia, Z.; Wang, F.; Wang, L.; Guo, X.; Huang, X.; Sha, J. Lamin A/C proteins in the spermatid acroplaxome are essential in mouse spermiogenesis. Reproduction 2014, 148, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Alsheimer, M.; Benavente, R. Change of karyoskeleton during mammalian spermatogenesis: Expression pattern of nuclear lamin C2 and its regulation. Exp. Cell Res. 1996, 228, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Link, J.; Jahn, D.; Schmitt, J.; Göb, E.; Baar, J.; Ortega, S.; Benavente, R.; Alsheimer, M. The meiotic nuclear lamina regulates chromosome dynamics and promotes efficient homologous recombination in the mouse. PLoS Genet. 2013, 9, e1003261. [Google Scholar] [CrossRef] [PubMed]
- Schütz, W.; Alsheimer, M.; Ollinger, R.; Benavente, R. Nuclear envelope remodeling during mouse spermiogenesis: Postmeiotic expression and redistribution of germline lamin B3. Exp. Cell Res. 2005, 307, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Machiels, B.M.; Ramaekers, F.C.; Kuijpers, H.J.; Groenewoud, J.S.; Oosterhuis, J.W.; Looijenga, L.H. Nuclear lamin expression in normal testis and testicular germ cell tumours of adolescents and adults. J. Pathol. 1997, 182, 197–204. [Google Scholar] [CrossRef]
- Neumann, B.; Walter, T.; Hériché, J.-K.; Bulkescher, J.; Erfle, H.; Conrad, C.; Rogers, P.; Poser, I.; Held, M.; Liebel, U.; et al. Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature 2010, 464, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, J.P.; Kann, M.L.; Combeau, C.; Melki, R. Gamma-tubulin during the differentiation of spermatozoa in various mammals and man. Mol. Hum. Reprod. 1998, 4, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-Y.; Méndez-López, I.; Hong, M.; Wang, Y.; Tanji, K.; Wu, W.; Shugol, L.; Krauss, R.S.; Dauer, W.T.; Worman, H.J. Lamina-associated polypeptide 1 is dispensable for embryonic myogenesis but required for postnatal skeletal muscle growth. Hum. Mol. Genet. 2016, 26, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Crimaudo, C.; Gerace, L. cDNA cloning and characterization of lamina-associated polypeptide 1C (LAP1C), an integral protein of the inner nuclear membrane. J. Biol. Chem. 1995, 270, 8822–8828. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.E.; Perez, A.; Perkins, G.; Ellisman, M.H.; Dauer, W.T. A molecular mechanism underlying the neural-specific defect in torsinA mutant mice. Proc. Natl. Acad. Sci. USA 2010, 107, 9861–9866. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Yu, L.; Zhang, H. Morphology of rat testis preserved in three different fixatives. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Mochida, K.; Tres, L.L.; Kierszenbaum, A.L. Structural and biochemical features of fractionated spermatid manchettes and sperm axonemes of the Azh/Azh mutant mouse. Mol. Reprod. Dev. 1999, 52, 434–444. [Google Scholar] [CrossRef]
- Mochida, K.; Tres, L.L.; Kierszenbaum, A.L. Isolation of the rat spermatid manchette and its perinuclear ring. Dev. Biol. 1998, 200, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Kallio, M.; Mustalahti, T.; Yen, T.J.; Lähdetie, J. Immunolocalization of alpha-tubulin, gamma-tubulin, and CENP-E in male rat and male mouse meiotic divisions: Pathway of meiosis I spindle formation in mammalian spermatocytes. Dev. Biol. 1998, 195, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, G.; Sutovsky, P.; Joshi, H.C.; Stearns, T.; Schatten, G. Centrosome reduction during mouse spermiogenesis. Dev. Biol. 1998, 203, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, D.W.; Phillips, D.M. The fine structure and development of the neck region of the mammalian spermatozoon. Anat. Rec. 1969, 165, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Goodchild, R.E.; Dauer, W.T. The AAA+ protein torsinA interacts with a conserved domain present in LAP1 and a novel ER protein. J. Cell Biol. 2005, 168, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.L.; Berk, J.M. The nuclear envelope at a glance. J. Cell Sci. 2010, 123, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.; O’Bryan, M.K. Microtubules and spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.-H.; Voss, M.; Haug-Kröper, M.; Schröder, B.; Schepers, U.; Bräse, S.; Haass, C.; Lichtenthaler, S.F.; Fluhrer, R. Secretome Analysis Identifies Novel Signal Peptide Peptidase-Like 3 (SPPL3) Substrates and Reveals a Role of SPPL3 in Multiple Golgi Glycosylation Pathways. Mol. Cell. Proteomics 2015, 14, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
- Korrodi-Gregório, L.; Esteves, S.L.C.; Fardilha, M. Protein phosphatase 1 catalytic isoforms: Specificity toward interacting proteins. Transl. Res. 2014, 164, 366–391. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.D.; Coyaud, É.; Gonçalves, J.; Mojarad, B.A.; Liu, Y.; Wu, Q.; Gheiratmand, L.; Comartin, D.; Tkach, J.M.; Cheung, S.W.T.; et al. A Dynamic Protein Interaction Landscape of the Human Centrosome-Cilium Interface. Cell 2015, 163, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU—On the Protection of Animals Used for Scientific Purposes; European Parliament: Brussels, Belgium, 2010; L276/33.
- Rebelo, S.; Domingues, S.C.; Santos, M.; Fardilha, M.; Esteves, S.L.C.; Vieira, S.I.; Vintém, A.P.B.; Wu, W.; da Cruz e Silva, E.F.; da Cruz e Silva, O.A.B. Identification of a novel complex AβPP:Fe65:PP1 that regulates AβPP Thr668 phosphorylation levels. J. Alzheimer’s Dis. 2013, 35, 761–775. [Google Scholar]
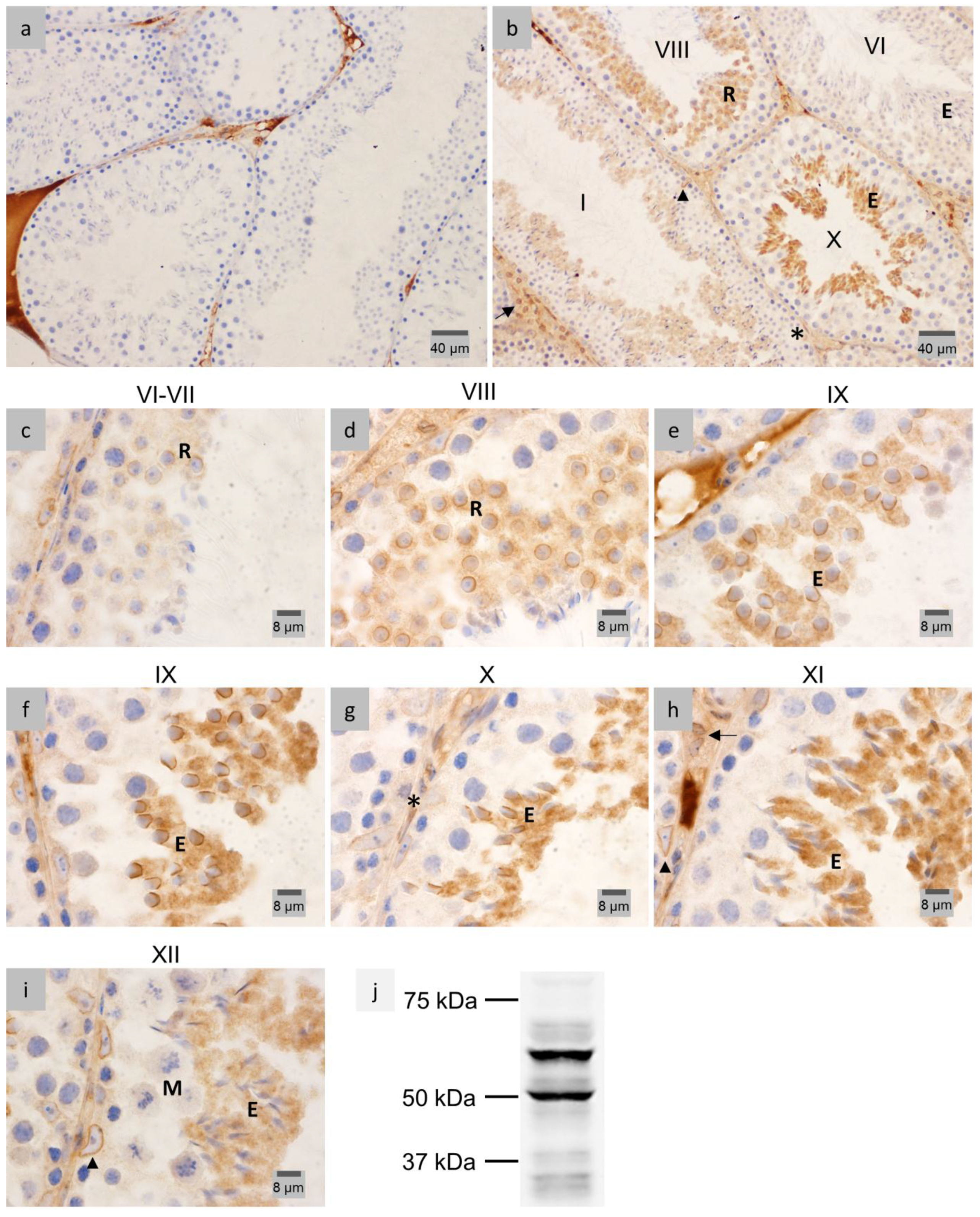
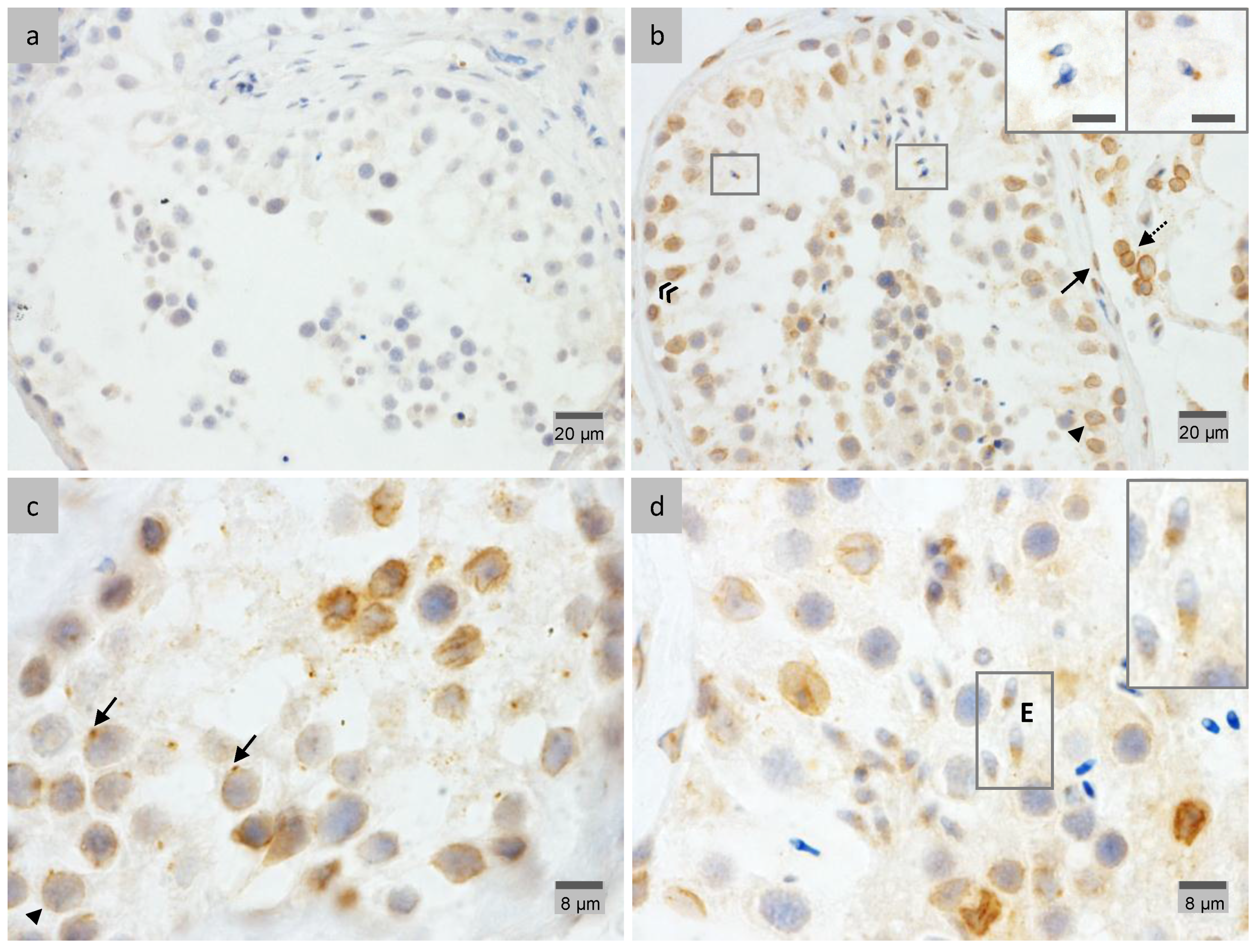
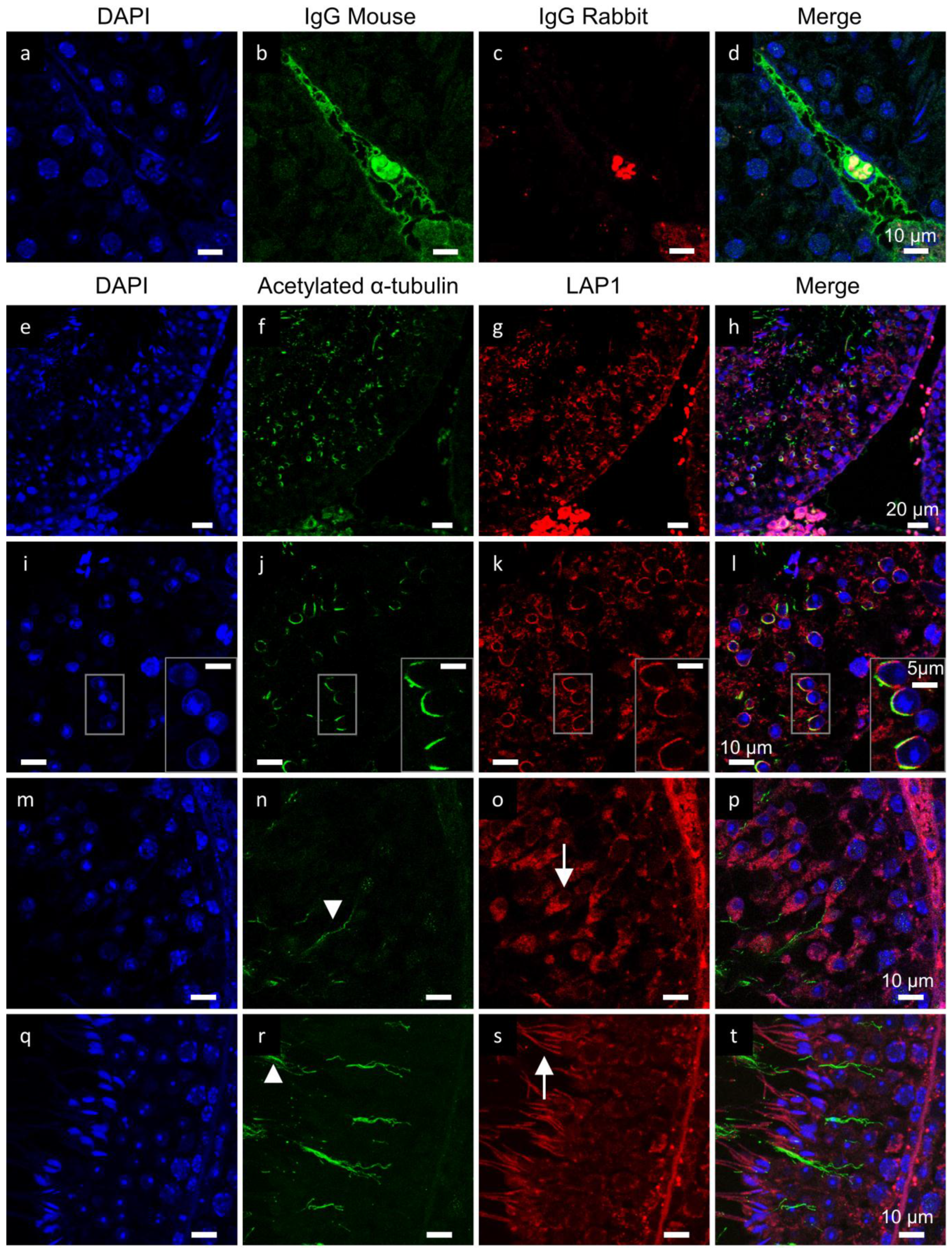
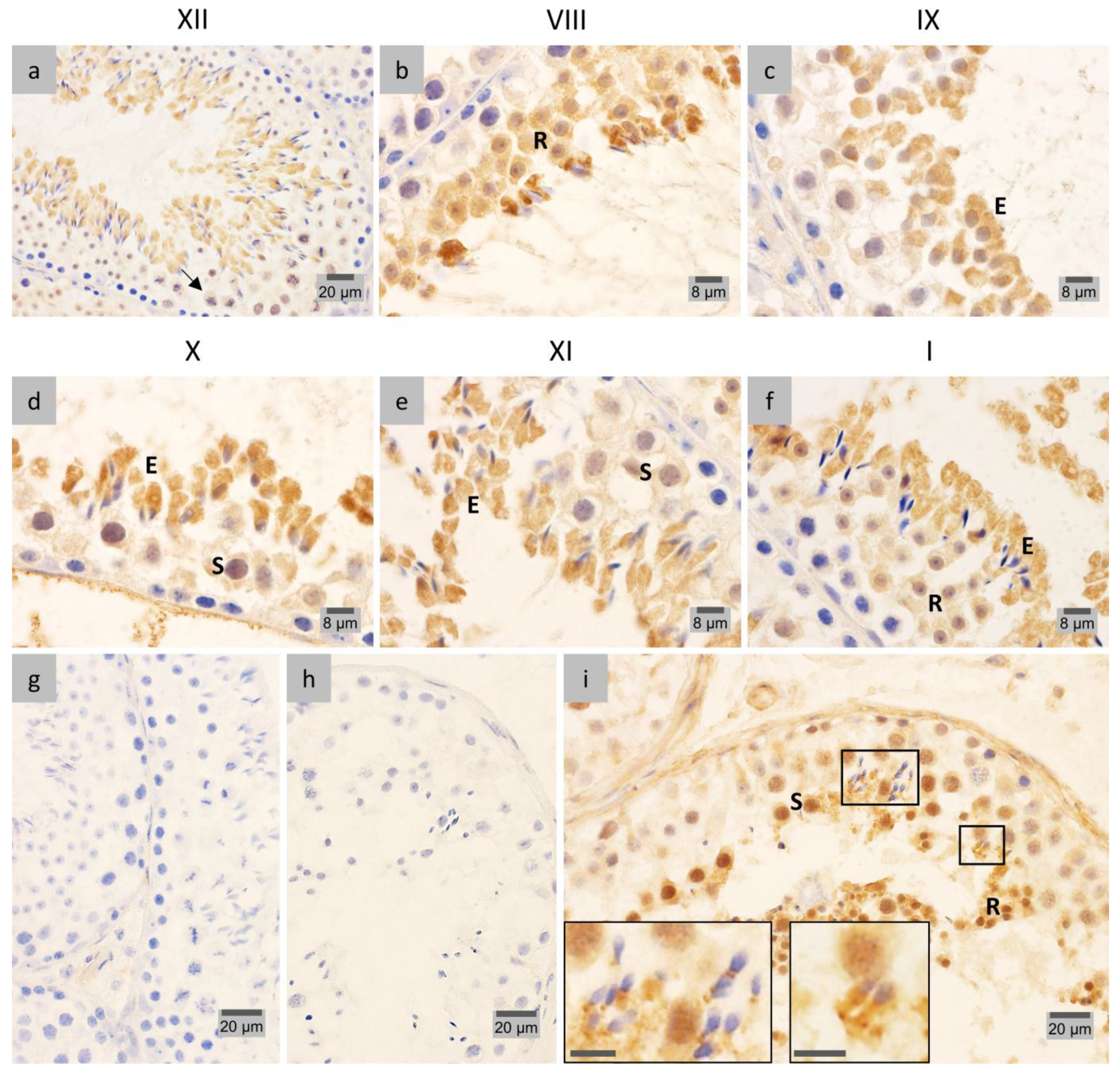
| Cell Type | Stage/Step | LAP1 | PP1γ2 | Lamin A/C | Lamin B1 | γ-Tubulin |
|---|---|---|---|---|---|---|
| Spermatogonia | – | NE | – | – | NE | Cy |
| Spermatocytes | All | NE | – | – | – | – |
| Pachytene | – | Nu, Cy | NE | NE | Cy | |
| Round spermatids | Steps 1–5 | Cy | Nu, Cy | – | NE | CP |
| Steps 6, 7 | NE, Cy | Nu, Cy | – | NE | CP | |
| Step 8 | NE, CP, Cy | Nu, Cy | – | NE | CP | |
| Elongating spermatids | Step 9 | CP, Cy | CP, Nu, Cy | – | – | CP |
| Step 10 | CP, Cy | Nu, Cy | – | – | CP | |
| Step 11 | CP, Cy | Cy | – | – | CP | |
| Steps 12, 13 | CP, Cy | Cy | – | – | CP | |
| Elongated mature spermatids | Steps 14, 15 | CP, Cy | Cy | Ac | – | CP |
| Step 16 | – | RB | Ac | – | CP |
| Cell Type | Stage | LAP1 | PP1γ2 | Lamin A/C | Lamin B1 | γ-Tubulin |
|---|---|---|---|---|---|---|
| Spermatogonia | – | NE | – | – | – | Cy |
| Spermatocytes | All | NE, CyN | – | – | NE | Cy |
| Pachytene | – | Nu, Cy | NE | – | – | |
| Round spermatids | – | CP, Cy | Nu, Cy | NE | Nu | CP, Cy |
| Elongating spermatids | – | CP | CP | – | CP | CP |
| Elongated mature spermatids | – | CP | CP | – | CP | CP |
| Antibody | Origin/Supplier | Antibody Type | Target | Dilution IHC | Blocking Solution |
|---|---|---|---|---|---|
| Anti-LAP1 | Goodchild and Dauer [45] | Rabbit polyclonal | LAP1 | 1:2000 | 5% BSA + 0.01% BSA-C |
| Anti-lamin A/C | Cell Signaling Technology #2023 (Beverly, MA, USA) | Rabbit polyclonal | Lamin A/C | 1:50 | 3% BSA |
| Anti-lamin B1 (H-90) | Santa Cruz Biotechnology sc-20682 (Dallas, TX, USA) | Rabbit polyclonal | Lamin B1 | 1:100 | 5% BSA |
| Anti-PP1γ2 (CBC502) | Smith et al. [22] | Rabbit polyclonal | PP1γ2 | 1:500 | 3% BSA |
| Anti-acetylated α-tubulin | Sigma T7451 (St. Louis, MO, USA) | Mouse monoclonal | Acetylated α-tubulin | 1:250 | 5% BSA + 0.01% BSA-C |
| Anti-γ-tubulin (GTU-88) | Sigma T5326 (St. Louis, MO, USA) | Mouse monoclonal | γ-Tubulin | 1:2000 | 5% BSA + 0.01% BSA-C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, J.B.; Martins, F.; Sousa, J.C.; Pereira, C.D.; Van Pelt, A.M.M.; Rebelo, S.; Da Cruz e Silva, O.A.B. Descriptive Analysis of LAP1 Distribution and That of Associated Proteins throughout Spermatogenesis. Membranes 2017, 7, 22. https://doi.org/10.3390/membranes7020022
Serrano JB, Martins F, Sousa JC, Pereira CD, Van Pelt AMM, Rebelo S, Da Cruz e Silva OAB. Descriptive Analysis of LAP1 Distribution and That of Associated Proteins throughout Spermatogenesis. Membranes. 2017; 7(2):22. https://doi.org/10.3390/membranes7020022
Chicago/Turabian StyleSerrano, Joana B., Filipa Martins, João C. Sousa, Cátia D. Pereira, Ans M. M. Van Pelt, Sandra Rebelo, and Odete A. B. Da Cruz e Silva. 2017. "Descriptive Analysis of LAP1 Distribution and That of Associated Proteins throughout Spermatogenesis" Membranes 7, no. 2: 22. https://doi.org/10.3390/membranes7020022
APA StyleSerrano, J. B., Martins, F., Sousa, J. C., Pereira, C. D., Van Pelt, A. M. M., Rebelo, S., & Da Cruz e Silva, O. A. B. (2017). Descriptive Analysis of LAP1 Distribution and That of Associated Proteins throughout Spermatogenesis. Membranes, 7(2), 22. https://doi.org/10.3390/membranes7020022








