Performance of Carbon Nanotube/Polysulfone (CNT/Psf) Composite Membranes during Oil–Water Mixture Separation: Effect of CNT Dispersion Method
Abstract
:1. Introduction
2. Experimental
2.1. Materials
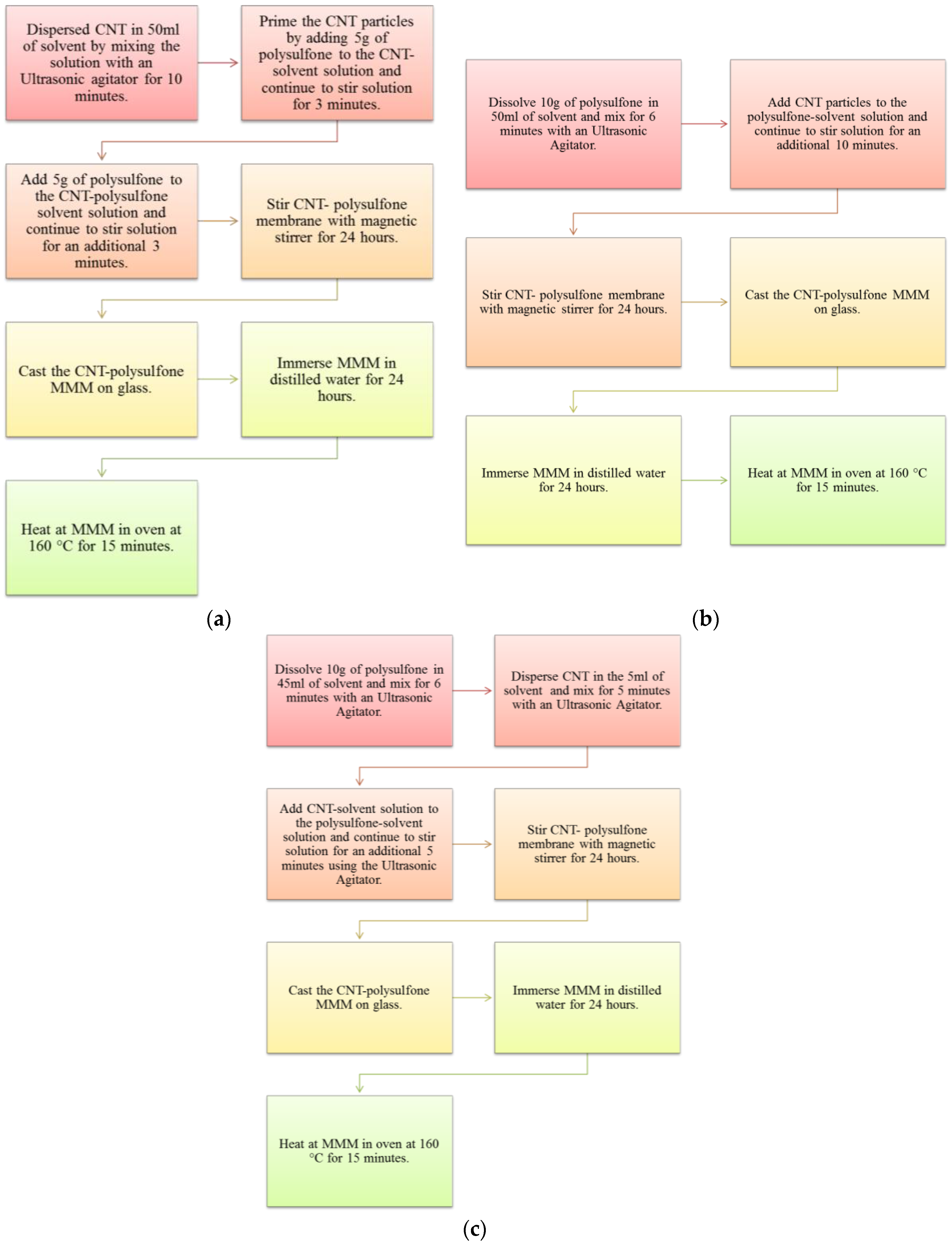
2.2. Membrane Synthesis
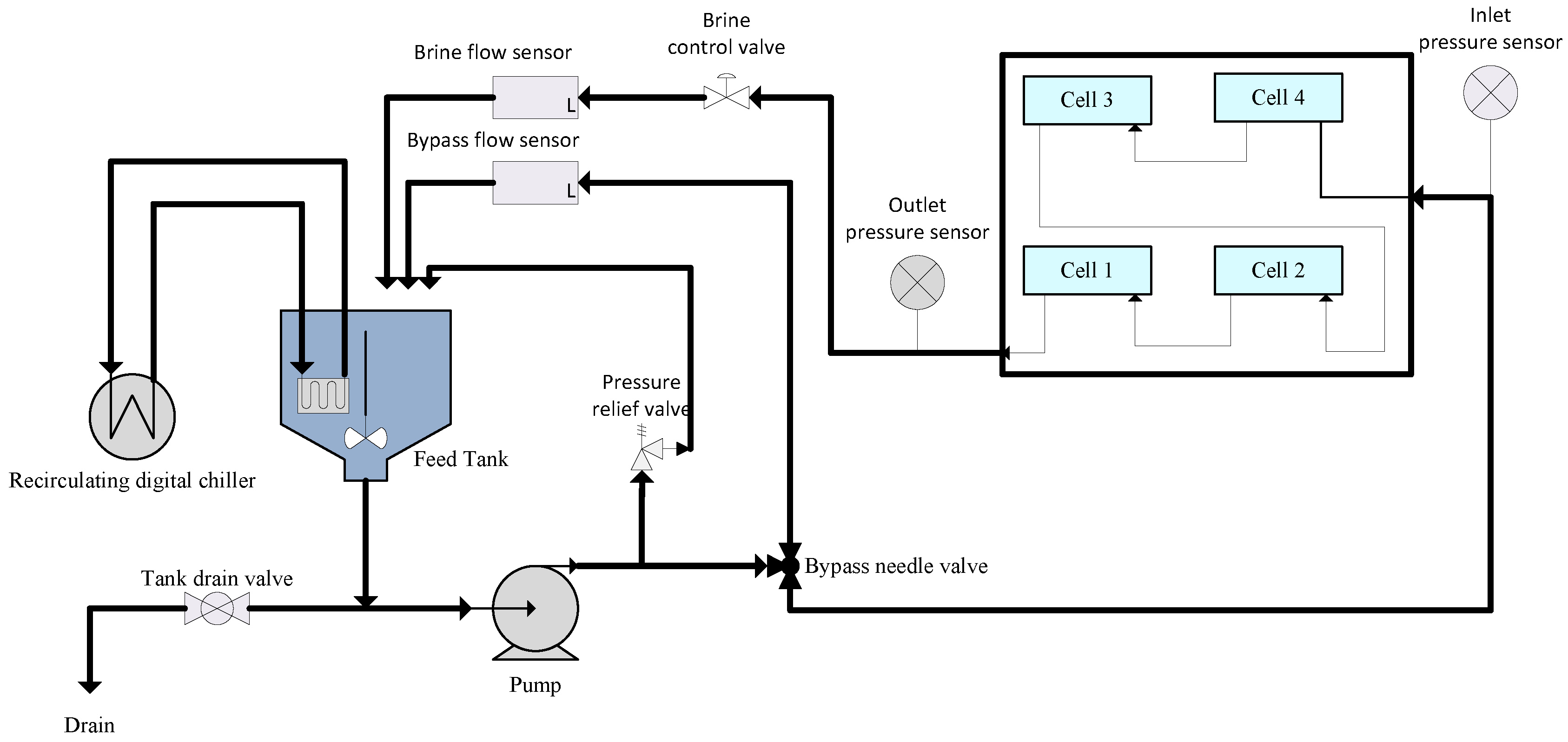
2.3. Performance Evaluation of the Synthesized Membranes
3. Results and Discussion
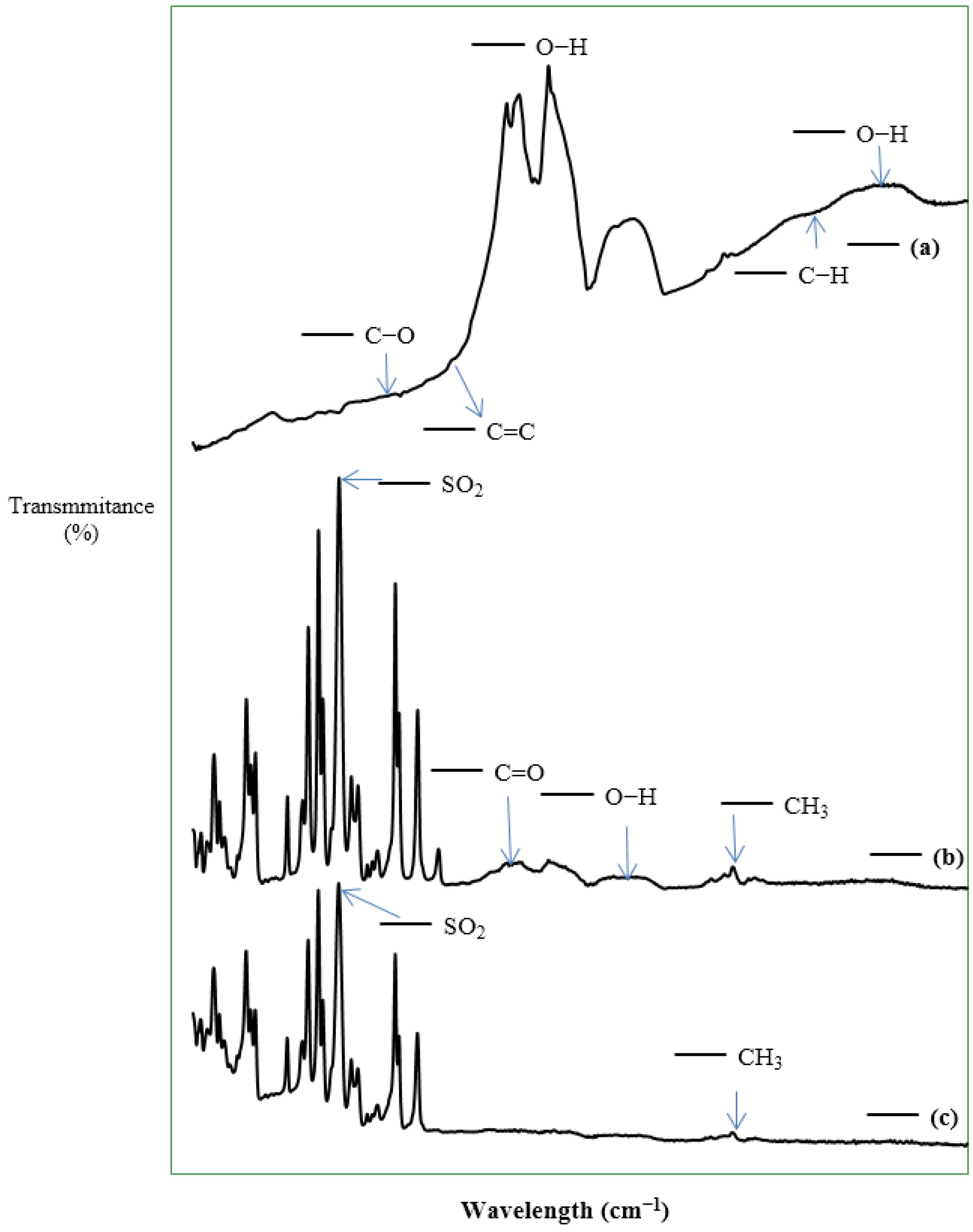
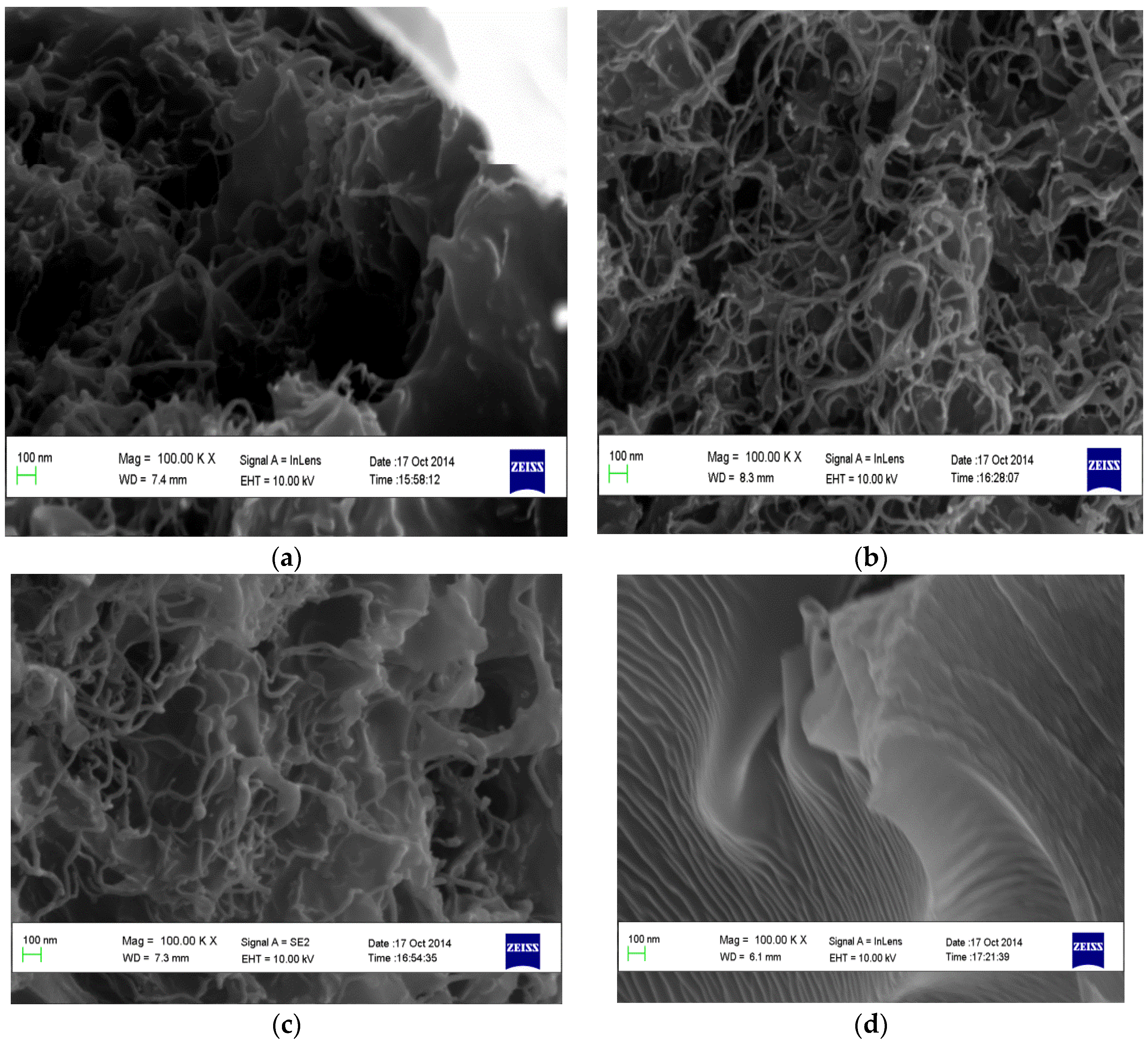
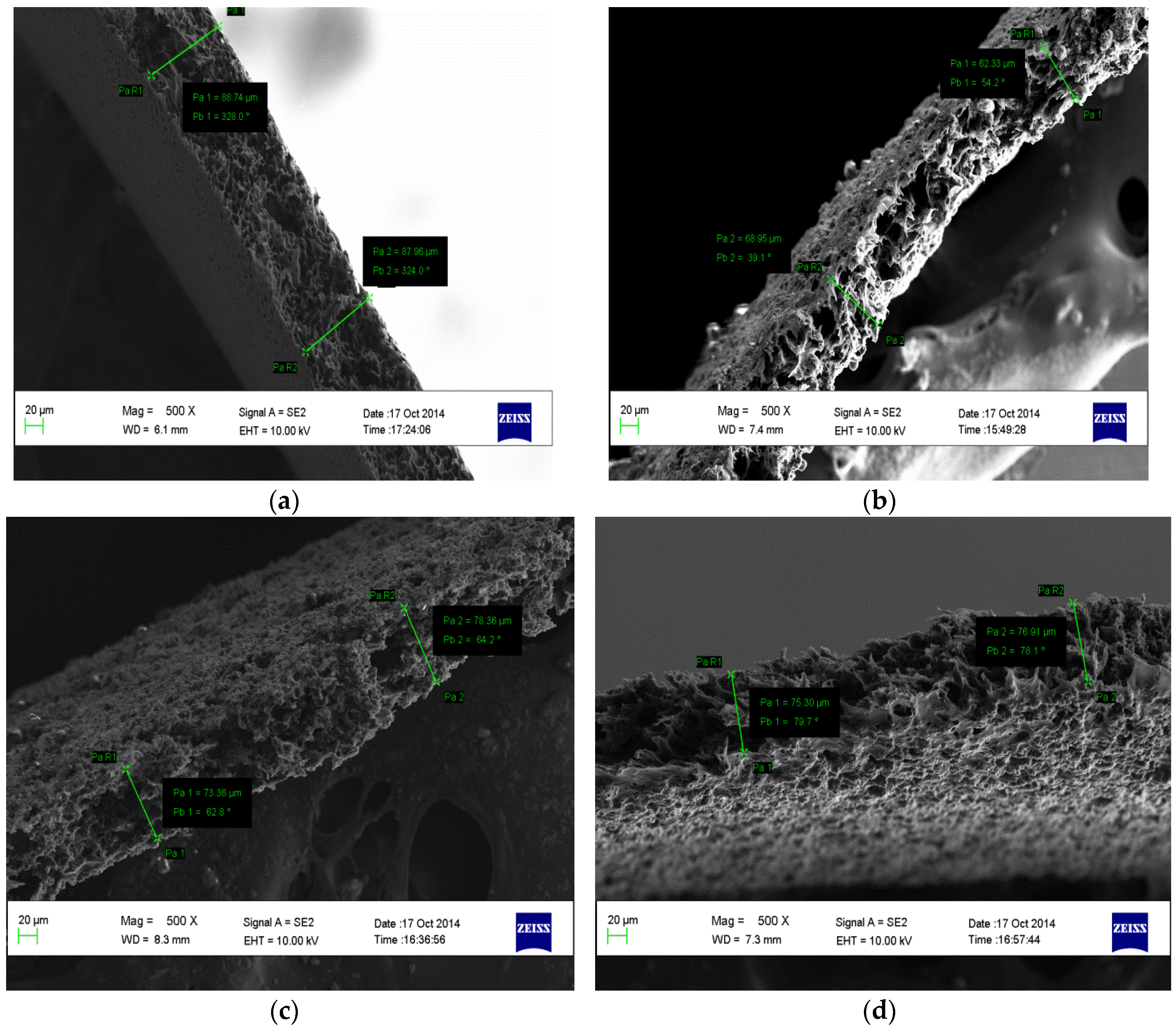
3.1. Characterization of the CNTs and the Fabricated Membranes
3.2. Hydrophilicity of the Fabricated Membranes
3.3. Performance Evaluation of the Membrane
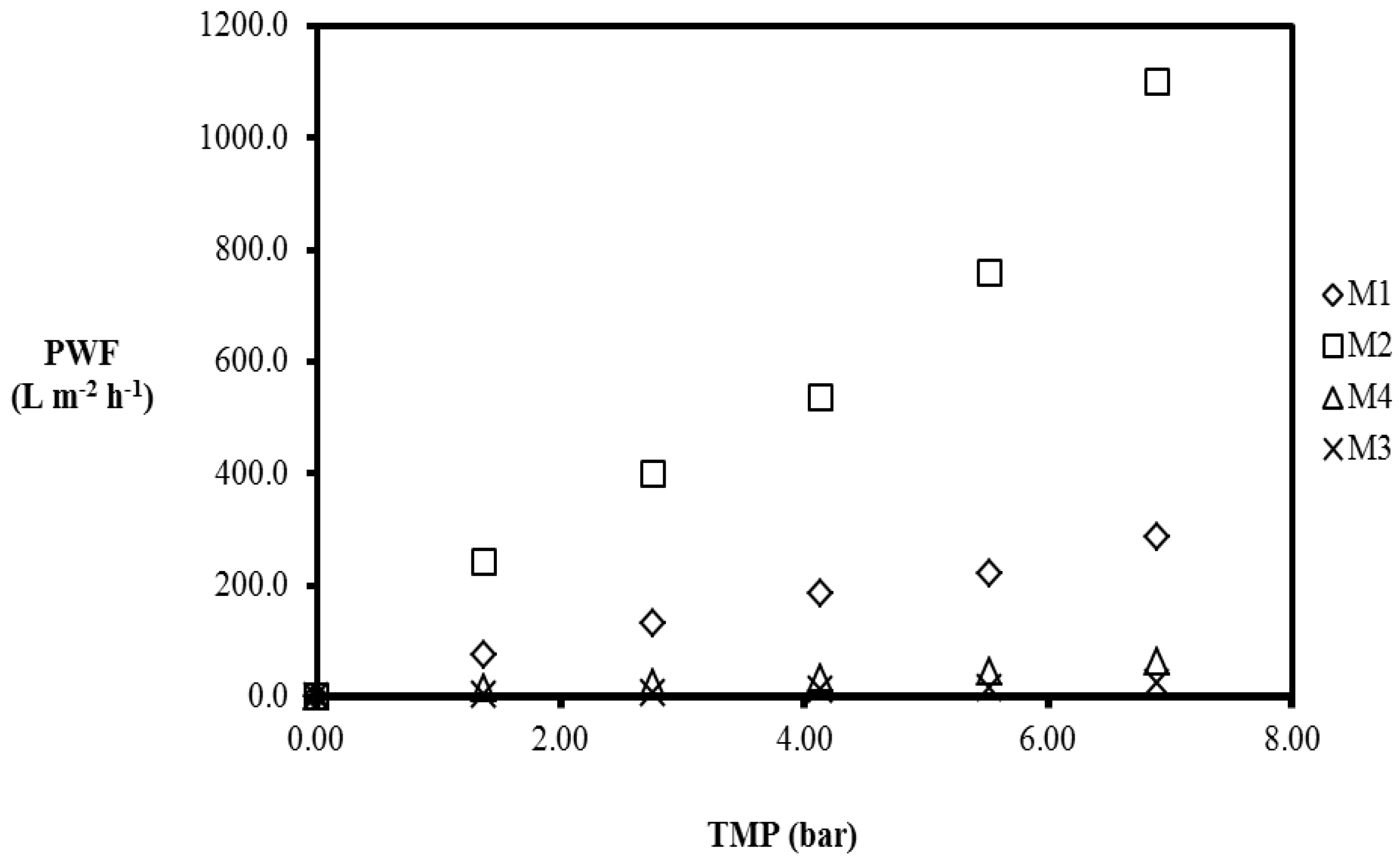
3.3.1. Pure Water Permeation
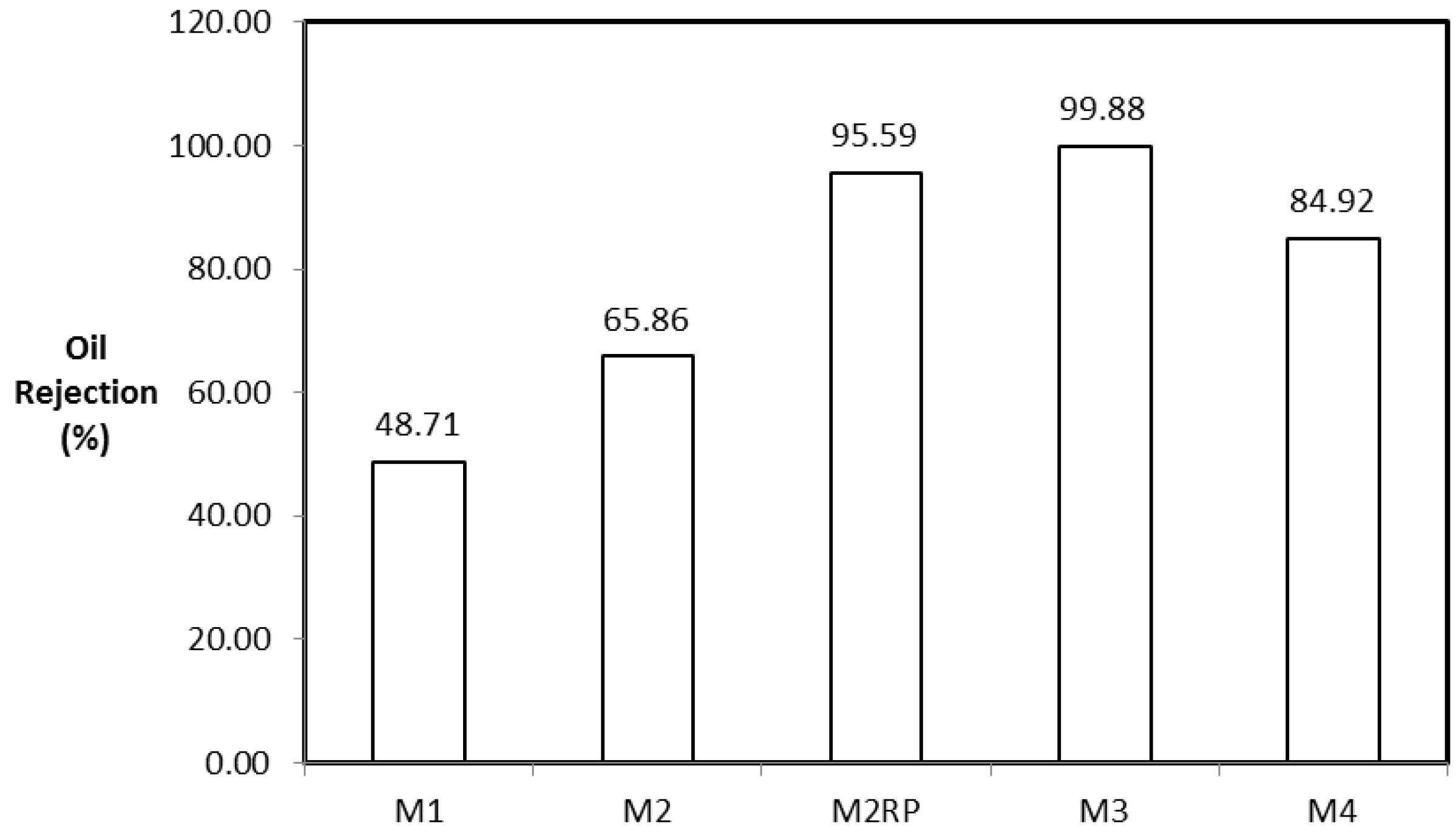
3.3.2. Oily Water Separation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aroon, M.A.; Ismaila, A.F.; Montazer-Rahmati, M.M.; Matsuura, T. Performance studies of mixed matrix membranes for gas separation. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Kołtuniewicz, A.B. The History and State of Art in Membrane Technologies. Available online: http://www.etseq.urv.es/assignatures/ops/presentacio_membranes.pdf (accessed on 4 May 2015).
- Damjanovica, L.; Rakic, V.; Rac, V.; Stosic, D.; Aurouxc, A. The investigation of phenol removal from aqueous solutions by zeolite as solid adsorbents. J. Hazard. Mater. 2010, 184, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.T.; Koros, W.J. Non-ideal effects inorganic-inorganic materials for gas separation membranes. J. Mol. Struct. 2005, 739, 87–98. [Google Scholar] [CrossRef]
- Abadi, S.R.H.; Sebzari, M.R.; Hemati, M.; Rekabdar, F.; Mohammadi, T. Ceramic performance in microfiltration of oily wastewater. Desalination 2010, 265, 222–228. [Google Scholar] [CrossRef]
- Tantekin-Ersolmaz, S.B.; Atalay-Oral, Ç.; Tatlıer, M.; Erdem-Senatalar, A.; Schoeman, B.; Sterte, J. Effect of Zeolite particle size on the performance of polymer-zeolite mixed matrix membrane. J. Membr. Sci. 2000, 175, 285–288. [Google Scholar] [CrossRef]
- Kumar, S.; Guria, C.; Mandal, A. Synthesis, characterization and performance studies of polysulfone/bentonite nanoparticles mixed-matrix ultra-filtration membranes using oil field produced water. Sep. Purif. Technol. 2015, 150, 145–158. [Google Scholar] [CrossRef]
- Shu, W.; Liangyin, C.; Wenmei, C. Fouling-resistant composite membranes for separation of oil-in-water microemulsions. Chin. J. Chem. Eng. 2006, 14, 37–45. [Google Scholar]
- Maphutha, S.; Moothi, K.; Meyyappan, M.; Iyuke, S.E. A carbon nanotube-infused polysulfone membrane with polyvinyl alcohol layer for treating oil-containing waste water. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Abdolmalekia, A.; Mallakpoura, S.; Borandeh, S. Amino acid-functionalized multiwalled carbon nanotubes for improving compatibility with chiral poly(amide-esterimide) containing 1-phenylalamine and 1-tyrosine linkages. Appl. Surf. Sci. 2013, 287, 117–123. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W.; Abdullah, P.; Jaafar, O. Potential tertiary treatment of produced water using highly hydrophilic nanofiltration and reverse osmosis membranes. J. Environ. Chem. Eng. 2013, 1, 1341–1349. [Google Scholar] [CrossRef]
- Slobodian, P.; Svoboda, P.; Riha, P.; Boruta, R.; Saha, P. Synthesis of PMMA-co-PMAA copolymer brush on multiwall carbon nanotubes. J. Surf. Eng. Mater. Adv. Technol. 2012, 2, 221–226. [Google Scholar]
- Yah, C.S.; Simate, G.S.; Moothi, K.; Sunny, K.S.; Iyuke, S.E. Synthesis of large carbon nanotubes from ferrocene: The chemical decomposition technique. Trends Appl. Sci. 2011, 6, 1270–1279. [Google Scholar] [CrossRef]
- Pourfayaz, F.; Khodadadi, A.A.; Mortazavi, Y.; Jafari, S.H. Plasma functionalization of MWCNTs in He followed by NH3 treatment and its application in PMMA based nanocomposites. Plasma Process. Polym. 2010, 7, 1001–1009. [Google Scholar] [CrossRef]
- Yudianti, R.; Onggo, H.; Sudirman, S.Y.; Iwata, T.; Azuma, J. Analysis of functional group sited on multi-wall carbon nanotubes surface. Open Mater. Sci. J. 2011, 5, 242–247. [Google Scholar] [CrossRef]
- McCurry, J. Organic Chemistry; Physical Science: Andover, MA, USA, 2008. [Google Scholar]
- Voicu, S.; Gales, I.; Nechifor, G.; Nechifor, A.C. 2011 Polymer Coat Improves the Detection Ability of Sensing Enzymes SPIE Newsroom. Available online: https://www.spie.org/x48500.xml?highlight=x2416&ArticleID=x48500 (accessed on 8 January 2015).
- Han, M.J.; Bhattacharyya, D. Changes in morphology and transport characteristics of polysulfone membranes prepared by different demixing conditions. J. Membr. Sci. 1995, 98, 191–200. [Google Scholar] [CrossRef]
- Richards, H.L.; Baker, P.G.L.; Iwuoha, E. Metal nanoparticles polysulfone membranes for use in wastewater treatment: A critical review. J. Surf. Eng. Mater. Adv. Technol. 2012, 2, 183–192. [Google Scholar] [CrossRef]
- Jeong, B.H.; Hoek, E.M.V.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Jawor, A. Interfacial polymerization of thin film nanoparticles. A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Yu, S.; Liu, X.; Liu, J.; Wu, D.; Liu, M.; Gao, C. Surface modification of thin-film composite polyamide reverse osmosis membranes with thermo-responsive polymer (TRP) for improved fouling resistance and cleaning efficiency. Sep. Purif. Technol. 2011, 76, 283–291. [Google Scholar] [CrossRef]
- Daramola, M.O.; Adeogun, A.G. Empirical modelling of chemically enhanced backwash during ultrafiltration process. Membr. Water Treat. 2011, 2, 225–237. [Google Scholar] [CrossRef]
- Barona, G.; Lim, J.; Choi, M.; Jong, B. Interfacial polymerization of polyamide-aluminosilicate SWNT nanocomposite membranes for reverse osmosis. Desalination 2013, 325, 138–147. [Google Scholar] [CrossRef]
- Yu, S.C.; Yao, G.H.; Dong, B.Y.; Zhu, H.W.; Peng, X.Y.; Liu, J.; Liu, M.H.; Gao, C.J. Improving fouling resistance of thin-film composite polyamide reverse osmosis membrane by coating natural hydrophilic polymer sericin. Sep. Purif. Technol. 2013, 118, 285–293. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Membr. Sci. 2008, 325, 325–427. [Google Scholar] [CrossRef]
- Daramola, M.O.; Dinat, A.; Hasrood, S. Synthesis and characterization of nanocomposite ceramic/hydroxyl sodalite membrane via pore-plugging technique. J. Membr. Sep. Technol. 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Shao, P.; Huang, R.Y.M. Polymeric membrane pervaporation. J. Membr. Sci. 2007, 287, 162–179. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Preparation and characterization of PVDF-PVP-TiO2 composite hollow fiber membranes for oily wastewater treatment using submerged membrane system. Desalin. Water Treat. 2013. [Google Scholar] [CrossRef]


| Membrane | Contact Angle (°) |
|---|---|
| M1 | 76.6 ± 5.0 |
| M2 | 77.9 ± 1.3 |
| M3 | 77.3 ± 4.5 |
| M4 | 88.1 ± 2.1 |
| Membrane | Contact Angle (°) | PWP (L·m−2·h−1·bar−1) | ||||
|---|---|---|---|---|---|---|
| 1.38 bar | 2.76 bar | 4.14 bar | 5.52 bar | 6.90 bar | ||
| M1 | 76.6 ± 5.0 | 53.9 | 47.6 | 44.7 | 40.2 | 41.7 |
| M2 | 77.9 ± 1.3 | 174.5 | 183.2 | 145.0 | 137.3 | 159.3 |
| M3 | 77.3 ± 4.5 | 3.2 | 3.0 | 3.0 | 2.9 | 3.7 |
| M4 | 88.1 ± 2.1 | 11.1 | 8.5 | 8.3 | 8.1 | 9.2 |
| Operating Pressure (bar) | OWP (L·m−2·h−1·bar−1) | ||||
|---|---|---|---|---|---|
| 1.38 | 2.76 | 4.14 | 5.52 | 6.90 | |
| M1 | 47.8 | 28.2 | 24.7 | 26.0 | 26.4 |
| M2 | 114.4 | 103.6 | 86.3 | 137.3 | 113.0 |
| M3 | 2.5 | 2.0 | 2.3 | 2.2 | 2.3 |
| M4 | 1.9 | 3.0 | 3.7 | 4.7 | 6.9 |
| Membrane Type | Material Treated | Filler | Oil Rejection (%) | Reference |
|---|---|---|---|---|
| Psf/bentonite membrane | Oil-Water mixture | Bentonite | >90 | [7] |
| PVDF/TiO2/polyvinylpyrrolidone membrane | Oily wastewater | TiO2 | 99.7 | [28] |
| Porous ceramic membrane/PVDF/PA/PVA | Oil-Water mixture | – | 98.5 | [8] |
| CNT/Psf/PVA membranes | Oil-Water mixture | CNTs | >95 | [9] |
| CNT/Psf membranes from M3 | Oil-Water mixture | CNTs | 99.88 | This study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daramola, M.O.; Hlanyane, P.; Sadare, O.O.; Oluwasina, O.O.; Iyuke, S.E. Performance of Carbon Nanotube/Polysulfone (CNT/Psf) Composite Membranes during Oil–Water Mixture Separation: Effect of CNT Dispersion Method. Membranes 2017, 7, 14. https://doi.org/10.3390/membranes7010014
Daramola MO, Hlanyane P, Sadare OO, Oluwasina OO, Iyuke SE. Performance of Carbon Nanotube/Polysulfone (CNT/Psf) Composite Membranes during Oil–Water Mixture Separation: Effect of CNT Dispersion Method. Membranes. 2017; 7(1):14. https://doi.org/10.3390/membranes7010014
Chicago/Turabian StyleDaramola, Michael Olawale, Palesa Hlanyane, Oluwafolakemi O. Sadare, Olugbenga O. Oluwasina, and Sunny E. Iyuke. 2017. "Performance of Carbon Nanotube/Polysulfone (CNT/Psf) Composite Membranes during Oil–Water Mixture Separation: Effect of CNT Dispersion Method" Membranes 7, no. 1: 14. https://doi.org/10.3390/membranes7010014
APA StyleDaramola, M. O., Hlanyane, P., Sadare, O. O., Oluwasina, O. O., & Iyuke, S. E. (2017). Performance of Carbon Nanotube/Polysulfone (CNT/Psf) Composite Membranes during Oil–Water Mixture Separation: Effect of CNT Dispersion Method. Membranes, 7(1), 14. https://doi.org/10.3390/membranes7010014






