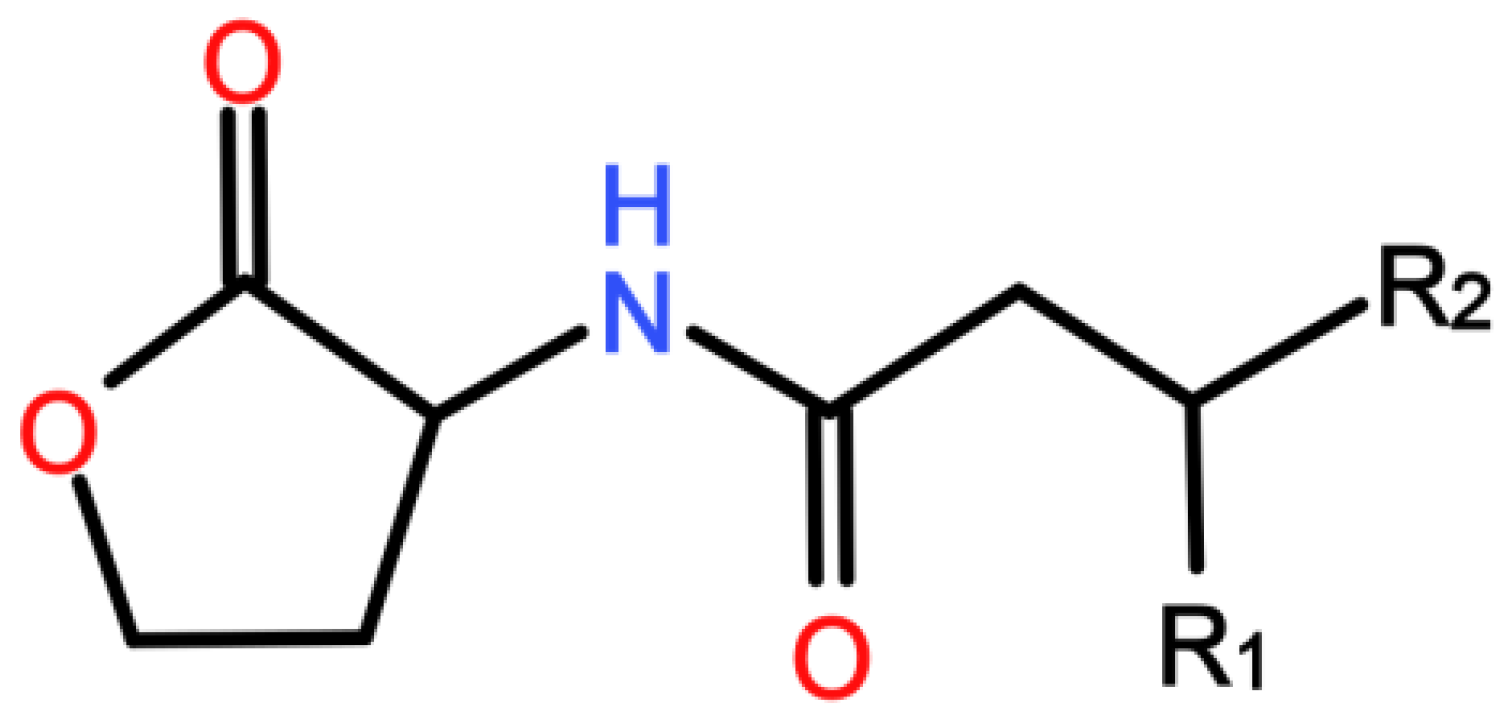
Process-Oriented Review of Bacterial Quorum Quenching for Membrane Biofouling Mitigation in Membrane Bioreactors (MBRs)
Abstract
:1. Introduction
2. History of Bacterial QQ
3. Isolation of QQ Bacteria
4. Roles of Rhodococcus sp. BH4 and Pseudomonas sp. 1A1 as QQ Bacteria
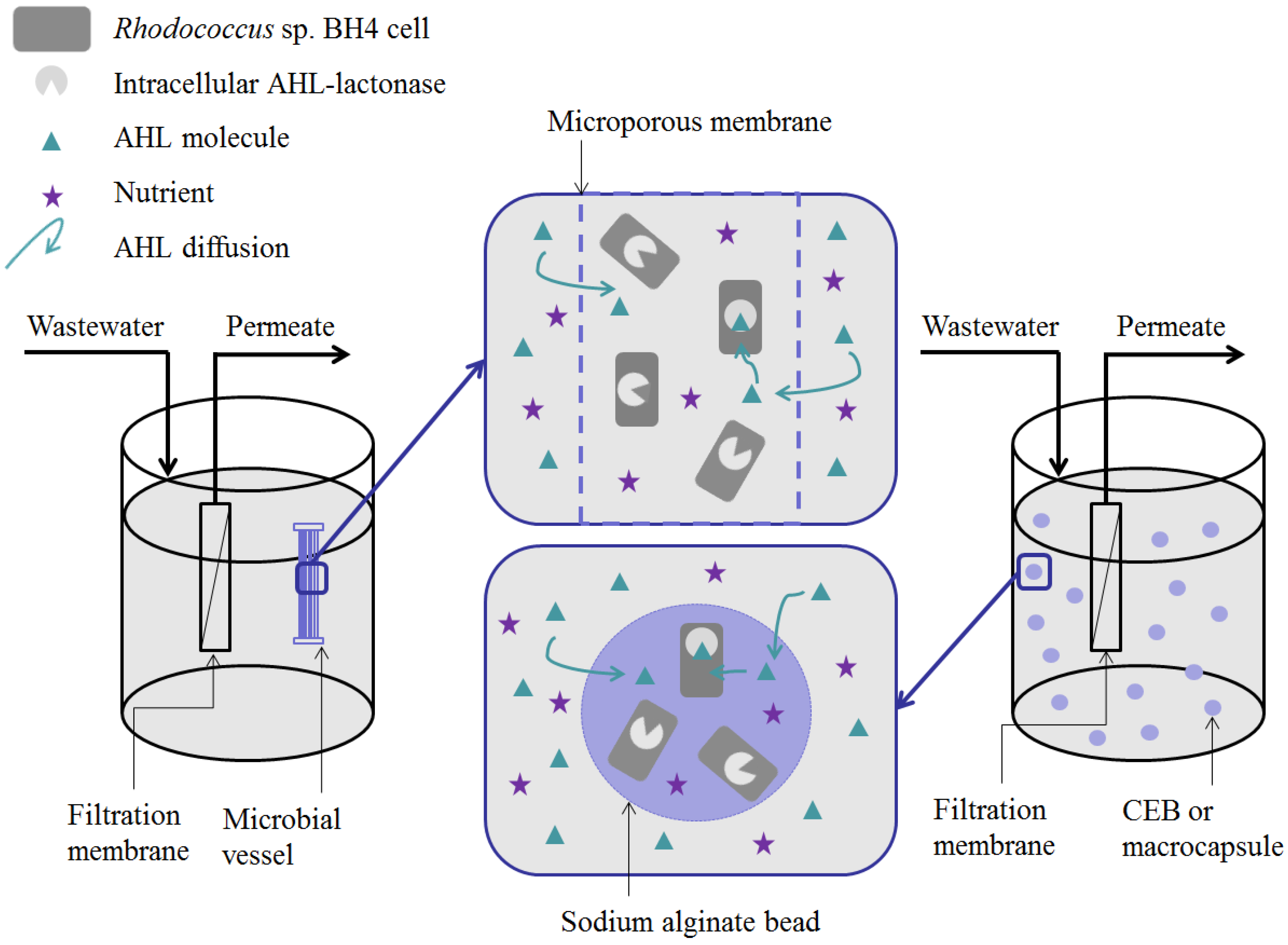
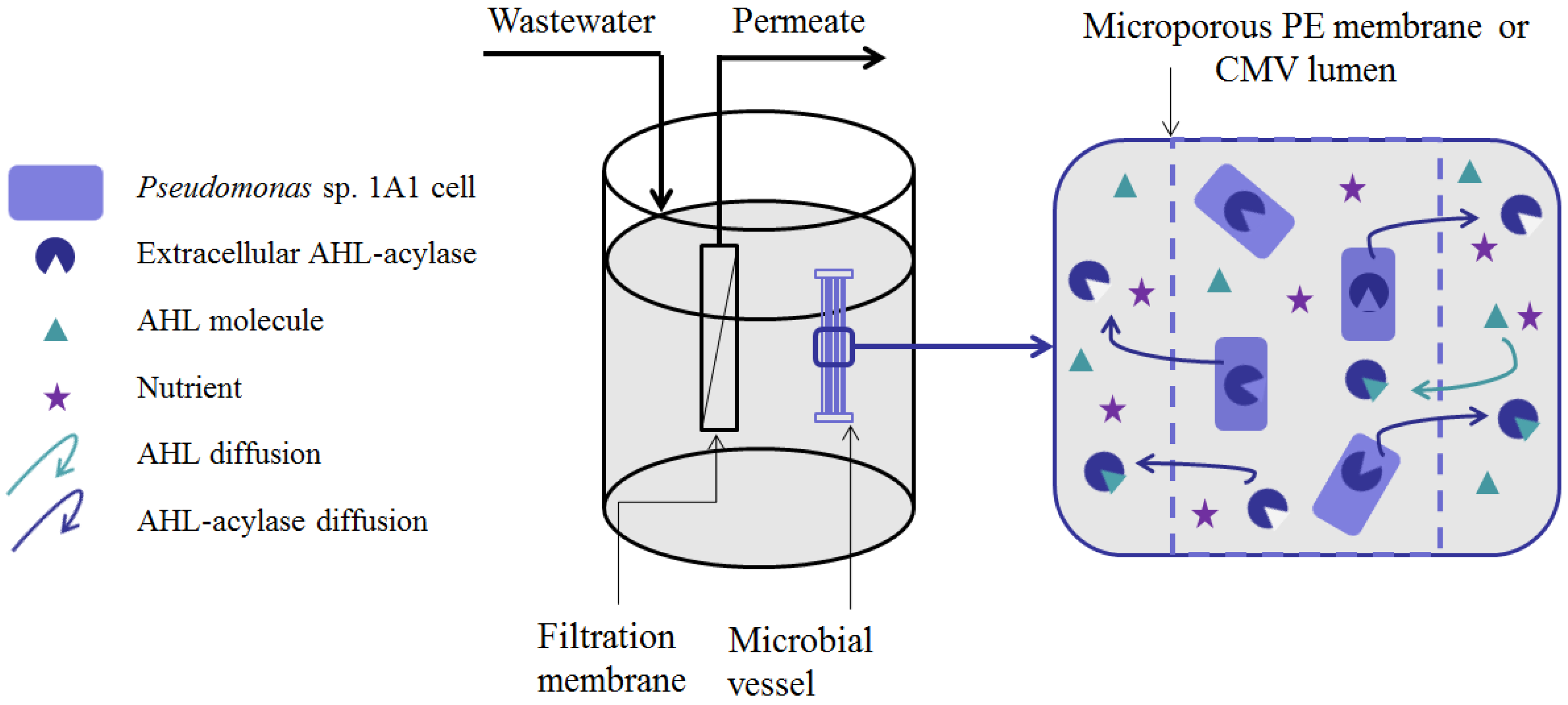
5. Methods for Entrapping QQ Bacteria in MBRs
- (i)
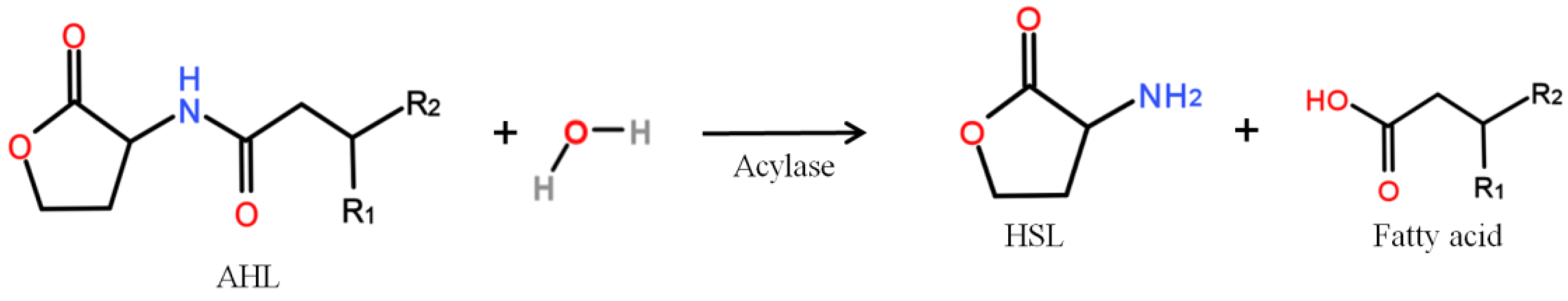
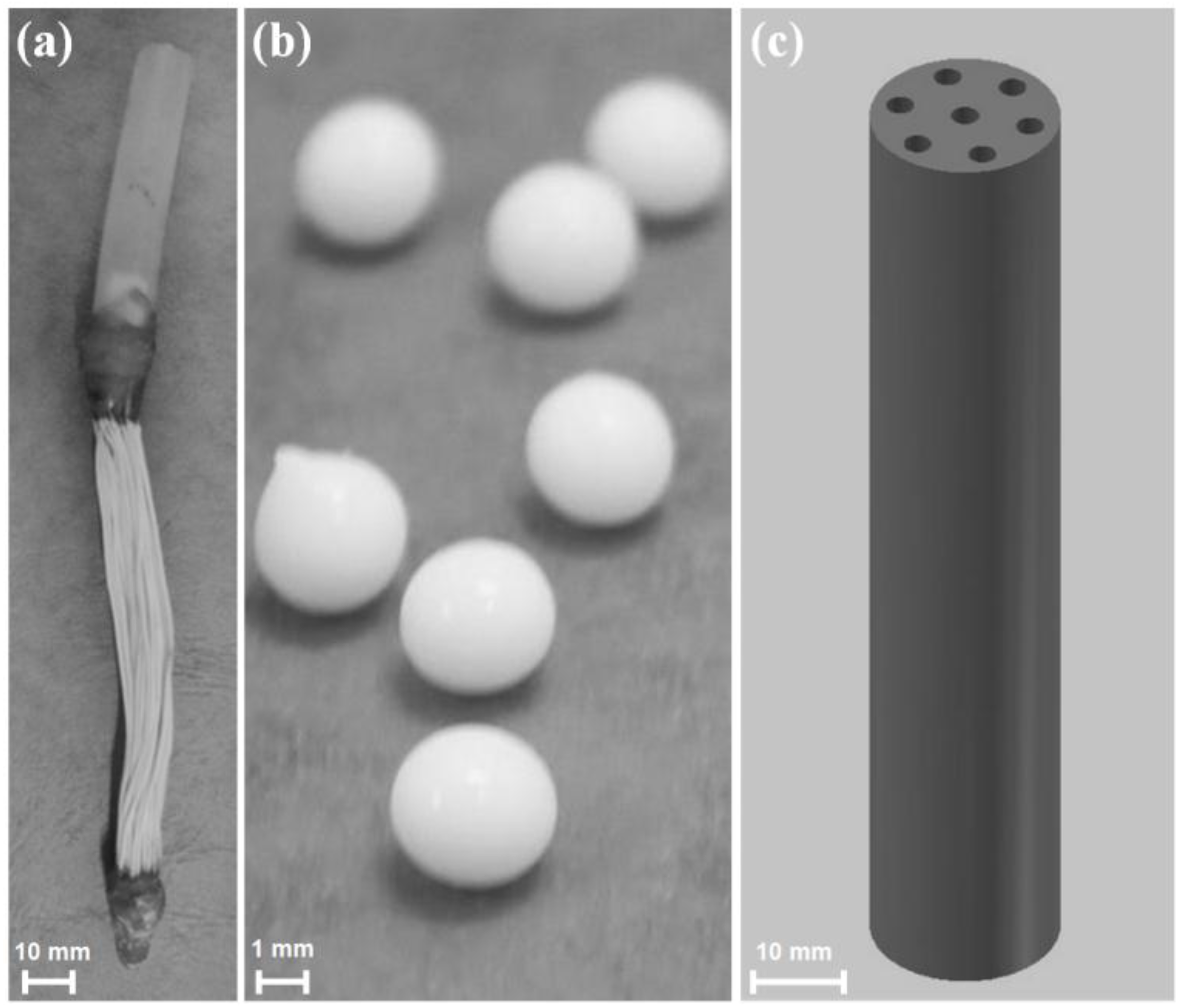
- Microbial vessels were designed to maintain the QQ bacteria immobilized using a porous material permitting the free diffusion of AHLs and nutrients through the vessel. For example, Oh et al. [28] used a module composed of hollow, 10-cm long polyethylene (PE), fibers to encapsulate the Rhodococcus sp. BH4 cells (Figure 4a). The module was then submerged and held in a fixed place in the MBR. The initial amounts of Rhodococcus sp. BH4 cells inserted in microbial vessels ranged from approximately 130 to 450 mg/L of the total working volume [28,40,49]. The volume of the vessel itself represented less than 0.08% of the MBR volume. For Pseudomonas sp. 1A1, two types of materials were used for these vessels: PE and ceramic. As for Rhodococcus sp. BH4, Cheong et al. [38] made PE vessels using microporous HF (0.4 µm). They also designed a ceramic microbial vessel (CMV) consisting of a monolithic microporous module (0.45 µm) with a total length of 10 cm and composed of several lumens into which the cells were injected using a sterile syringe (Figure 4c). The initial quantities of Pseudomonas sp. 1A1 cells tested varied from approximately 200 to 700 mg 1A1/L of the total working volume.
- (ii)
- Sodium alginate beads were only used for entrapping Rhodococcus sp. BH4. They were prepared by dripping a mixture of a Rhodococcus sp. BH4 suspension and a sodium alginate solution through a nozzle into a CaCl2 solution to obtain spherical Cell Entrapping Beads (CEBs) as described by Kim et al. [25] (Figure 4b). In that case, the amount of Rhodococcus sp. BH4 entrapped in the beads was around 6 mg BH4/g sodium alginate. Kim et al. [50] further developed macrocapsules by coating the CEBs with a microporous polymer layer for better resistance to harsh conditions resulting from the use of real WW. Recently, Lee et al. [26] developed QQ beads made of a mixture of polyvinyl alcohol and sodium alginate to reinforce their stability in real WW. The beads were then inserted into the MBR where they could move freely.
- (iii)
- Recently, an RMCF has been developed as a new entrapping technology for Rhodococcus sp. BH4, using a polycarbonate frame and four cubbyholes covered with a polyvinylidene fluoride (PVDF) microfiltration membrane, and packed with a Rhodococcus sp. BH4 suspension using a syringe. The RMCF was then set into the MBR similarly to a mechanical stirring device [51].
6. Localization of the Activity of the QQ Bacteria in the MBR
7. Performance of the Bacterial QQ-MBR
8. Effect of Bacterial QQ on the MBR Performance at Macroscopic Scale
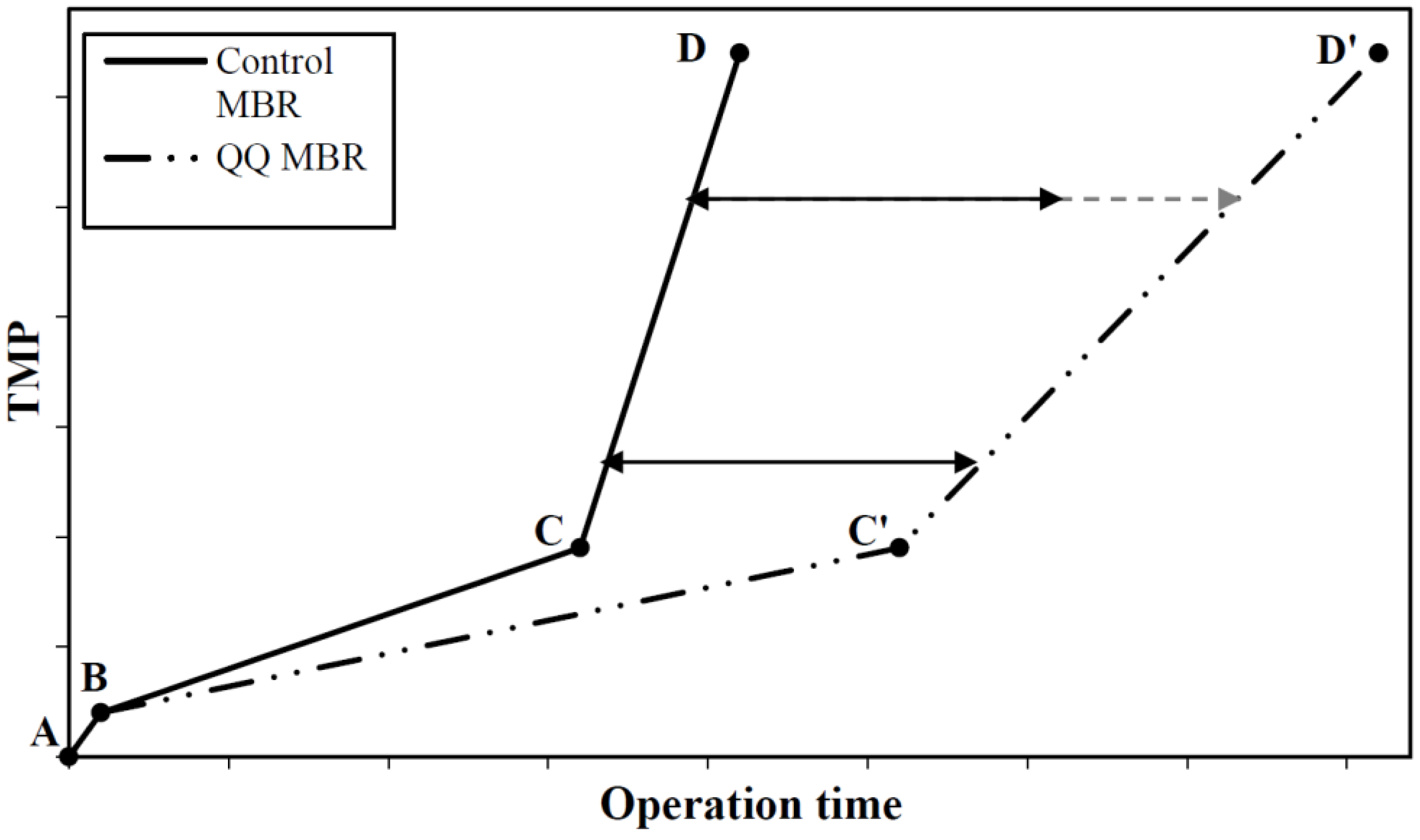
8.1. Effect on the Progressive Biofouling Stage
8.2. Effect on the TMP Jump
8.3. Effect on the Mixed Liquor Characteristics
8.4. Effect on the Biodegradation Efficiencies in the MBR
9. Effect of Bacterial QQ on the MBR Performance at Microscopic Scale
9.1. Effect on the Amount of Biofilm
9.2. Effect on the Biofilm Composition
10. Effect of the Operating Conditions on the Effectiveness of Bacterial QQ
10.1. Effect of the Initial Quantity of QQ Bacteria Inserted into the MBR
10.2. Effect of the Entrapping Method
10.3. Effect of the Materials Used in the QQ Device
10.4. Effect of the Location of the Microbial Vessel in the MBR
10.5. Effect of the Recirculation Rate in a Side-Stream MBR
10.6. Effect of Coupled Physical Cleaning Methods
10.7. Effect of the Permeate Flux
10.8. Effect of the Feeding Mode in Case of CMV for Entrapping Pseudomonas sp. 1A1
11. Discussion and Concluding Remarks
- When studying the effect of the two bacteria on the progressive biofouling stage, it appeared that Pseudomonas sp. 1A1 had a less pronounced effect in terms of TMP reduction. Yet, it has been assumed that the effectiveness of Pseudomonas sp. 1A1 in mitigating membrane biofouling could be expected to be more significant, given that the QQ enzyme that it produces (acylase) is extracellular. Hence, a comparative study with Rhodococcus sp. BH4 under the exact same conditions could help identify the possible differences between these two QQ bacteria.
- In this work, the presentation of the QQ activity localization and the entrapping methods for both the QQ bacteria considered helped underline that the transport of the main molecules involved in the QQ process (AHLs as substrates and lactonases or acylases as QQ enzymes) is not completely understood yet, although some assumptions have been made in that direction. Hence, an in-depth characterization of the transport of these molecules in the MBR should be carried out, given the MBR hydrodynamics and the variety of methods existing for QQ bacteria entrapment.
- The analysis of the bacterial QQ effect on the progressive biofouling stage has given some interesting clues about the time needed for the QQ activity to become significant in the MBRs. It appears that the QQ activity takes place in the early phase of the MBR operation for both Rhodococcus sp. BH4 and Pseudomonas sp. 1A1. Thus, it could be of great interest for both of these QQ strains to investigate which, of QQ enzyme production or QQ enzyme transport, is the limiting step in the QQ process for biofouling mitigation in MBRs.
- In all the studies considered in this review, the TMP jump was successfully delayed with the application of bacterial QQ over the run times investigated. However, bacterial QQ does not completely prevent biofouling; it simply postpones its occurrence. One of the hypotheses to explain this observation could be that other kinds of QS-controlled biofouling become prevalent in the MBRs. Thus, the relationship between biofouling and other kinds of autoinducers present in the MBR (i.e., AIPs and AI-2s) should be investigated, and might lead to a more efficient QQ strategy to mitigate biofouling.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| AHL | N-acyl-l-homoserine lactone |
| AI | Autoinducer |
| AS | Activated Sludge |
| CEB | Cell Entrapping Bead |
| CLSM | Confocal Laser Scanning Microscope |
| CMV | Ceramic Microbial Vessel |
| COD | Chemical Oxygen Demand |
| EPS | Extracellular Polymeric Substances (or Exopolysaccharides) |
| F/M | Food-to-Mass ratio |
| HF | Hollow Fiber |
| HPLC | High Performance Liquid Chromatography |
| HRT | Hydraulic Retention Time |
| HSL | Homoserine Lactone |
| LB (biofilm) | Loosely Bound |
| LB (medium) | Luria-Bertani |
| MBR | Membrane Bioreactor |
| MEC | Magnetic Enzyme Carrier |
| MLSS | Mixed Liquor Suspended Solids |
| PBE | Piper Betle Extract |
| PE | Polyethylene |
| PESM | Modified Poly Ether Sulfone |
| PVDF | Polyvinylidene fluoride |
| Quorum Quenching | |
| QS | Quorum Sensing |
| QSI | Quorum Sensing Inhibitors |
| SMP | Soluble Microbial Product |
| SRT | Solid Retention Time |
| SVI | Sludge Volume Index |
| TAB | Total Attached Biomass |
| TB | Tightly Bound |
| TMP | Transmembrane Pressure |
| TKN | Total Kjeldahl Nitrogen |
| WW | Wastewater |
| WWT | Wastewater Treatment |
References
- Branda, S.S.; Vik, S.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Schaule, G.; Griebe, T.; Schmitt, J.; Tamachkiarowa, A. Biofouling—The Achilles heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Lade, H.; Pual, D.; Kweon, J.H. N-Acyl Homoserine Lactone-Mediated Quorum Sensing with Special Reference to Use of Quorum Quenching Bacteria in Membrane Biofouling Control. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Lade, H.; Paul, D.; Kweon, J.H. Quorum Quenching Mediated Approaches for Control of Membrane Biofouling. Int. J. Biol. Sci. 2014, 10, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.F.; Rzechowicz, M.; Harvey, W.; Zularisam, A.W.; Anthony, G.F. Quorum sensing based membrane biofouling control for water treatment: A review. J. Water Process Eng. 2015, 7, 112–122. [Google Scholar] [CrossRef]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, S.; Yeon, K.-M.; Sang, B.-I.; Chun, J.; Lee, C.-H. Correlation between microbial community structure and biofouling in a laboratory scale membrane bioreactor with synthetic wastewater. Desalination 2012, 287, 209–215. [Google Scholar] [CrossRef]
- Luxmy, N.; Nakajima, F.; Yamamoto, K. Analysis of Bacterial Community in Membrane-Separation Bioreactors Byfluorescent in Situ Hybridization (FISH) and Denaturing Gradient Gelelectrophoresis (DGGE) Techniques. Available online: http://www.iwaponline.com/wst/04110/wst041100259.htm (accessed on 15 October 2015).
- Waheed, H.; Hashmi, I.; Khan, S.J.; Kim, S.R.; Arshad, M.; Nasir, H. Microbial population dynamics and profiling of quorum sensing agents in membrane bioreactor. Int. Biodeterior. Biodegrad. 2015, 113, 66–73. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Greenberg, E.P. Acyl-homoserine lactone quorum sensing in Gram-negative bacteria: A signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 2000, 97, 8789–8793. [Google Scholar] [CrossRef] [PubMed]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, N.A.; Barnard, A.M.L.; Slater, H.; Simpson, N.J.L.; Salmond, G.P.C. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 2001, 25, 365–404. [Google Scholar] [CrossRef] [PubMed]
- Williams, P. Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 2007, 153, 3923–3938. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The Involvement of Cell-to-Cell Signals in the Development of a Bacterial Biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Stoodley, P. Developmental regulation of microbial biofilms. Curr. Opin. Biotechnol. 2002, 13, 228–233. [Google Scholar] [CrossRef]
- Hammer, B.K.; Bassler, B.L. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 2003, 50, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Labbate, M.; Queck, S.Y.; Koh, K.S.; Rice, S.A.; Givskov, M.; Kjelleberg, S. Quorum Sensing-Controlled Biofilm Development in Serratia liquefaciens MG1. J. Bacteriol. 2004, 186, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Greenberg, E.P. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005, 13, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.A.; Koh, K.S.; Queck, S.Y.; Labbate, M.; Lam, K.W.; Kjelleberg, S. Biofilm Formation and Sloughing in Serratia Marcescens Are Controlled by Quorum Sensing and Nutrient Cues. J. Bacteriol. 2005, 187, 3477–3485. [Google Scholar] [CrossRef] [PubMed]
- Shrout, J.D.; Chopp, D.L.; Just, C.L.; Hentzer, M.; Givskov, M.; Parsek, M.R. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006, 62, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Waters, C.M.; Lu, W.; Rabinowitz, J.D.; Bassler, B.L. Quorum Sensing Controls Biofilm Formation in Vibrio cholerae through Modulation of Cyclic Di-GMP Levels and Repression of vpsT. J. Bacteriol. 2008, 190, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhou, L.; Zhang, Z.; Li, J. Influence and mechanism of N-(3-oxooxtanoyl)-l-homoserine lactone (C8-oxo-HSL) on biofilm behaviors at early stage. J. Environ. Sci. 2012, 24, 2035–2040. [Google Scholar] [CrossRef]
- Yeon, K.-M.; Cheong, W.-S.; Oh, H.-S.; Lee, W.-N.; Hwang, B.-K.; Lee, C.-H.; Beyenal, H.; Lewandowski, Z. Quorum Sensing: A New Biofouling Control Paradigm in a Membrane Bioreactor for Advanced Wastewater Treatment. Environ. Sci. Technol. 2009, 43, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-R.; Oh, H.-S.; Jo, S.-J.; Yeon, K.-M.; Lee, C.-H.; Lim, D.-J.; Lee, C.-H.; Lee, J.-K. Biofouling Control with Bead-Entrapped Quorum Quenching Bacteria in Membrane Bioreactors: Physical and Biological Effects. Environ. Sci. Technol. 2013, 47, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, S.-K.; Kwon, H.; Lee, S.H.; Lee, K.; Nahm, C.H.; Jo, S.J.; Oh, H.-S.; Park, P.-K.; Choo, K.-H.; et al. Crossing the Border between Laboratory and Field: Bacterial Quorum Quenching for Anti-Biofouling Strategy in an MBR. Environ. Sci. Technol. 2016, 50, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, T.; Khan, S.J.; Waheed, H.; Lee, C.-H.; Hashmi, I.; Iqbal, H. Membrane biofouling retardation and improved sludge characteristics using quorum quenching bacteria in submerged membrane bioreactor. J. Membr. Sci. 2015, 483, 75–83. [Google Scholar] [CrossRef]
- Oh, H.-S.; Yeon, K.-M.; Yang, C.-S.; Kim, S.-R.; Lee, C.-H.; Park, S.Y.; Han, J.Y.; Lee, J.-K. Control of Membrane Biofouling in MBR for Wastewater Treatment by Quorum Quenching Bacteria Encapsulated in Microporous Membrane. Environ. Sci. Technol. 2012, 46, 4877–4884. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.F.; Sakinah, M.; Singh, L.; Zularisam, A.W. Targeting N-acyl-homoserine-lactones to mitigate membrane biofouling based on quorum sensing using a biofouling reducer. J. Biotechnol. 2012, 161, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Weerasekara, N.A.; Choo, K.-H.; Lee, C.-H. Hybridization of physical cleaning and quorum quenching to minimize membrane biofouling and energy consumption in a membrane bioreactor. Water Res. 2014, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-H.; Wang, L.-H.; Zhang, L.-H. Quorum-Quenching Microbial Infections: Mechanisms and Implications. Philos. Trans. Biol. Sci. 2007, 362, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.B.; Givskov, M. Quorum sensing inhibitors: A bargain of effects. Microbiology 2006, 152, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.F.; Sakinah, M.; Ismail, A.F.; Matsuura, T.; Zularisam, A.W. The anti-biofouling effect of Piper betle extract against Pseudomonas aeruginosa and bacterial consortium. Desalination 2012, 288, 24–30. [Google Scholar] [CrossRef]
- Jiang, W.; Xia, S.; Liang, J.; Zhang, Z.; Hermanowicz, S.W. Effect of quorum quenching on the reactor performance, biofouling and biomass characteristics in membrane bioreactors. Water Res. 2013, 47, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Oh, H.-S.; Kim, S.-R.; Lee, K.-B.; Yeon, K.-M.; Lee, C.-H.; Kim, S.; Lee, J.-K. Microbial population dynamics and proteomics in membrane bioreactors with enzymatic quorum quenching. Appl. Microbiol. Biotechnol. 2012, 97, 4665–4675. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Choi, D.-C.; Yeon, K.-M.; Kim, S.-R.; Lee, C.-H. Enzyme-Immobilized Nanofiltration Membrane To Mitigate Biofouling Based on Quorum Quenching. Environ. Sci. Technol. 2011, 45, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Yeon, K.-M.; Lee, C.-H.; Kim, J. Magnetic Enzyme Carrier for Effective Biofouling Control in the Membrane Bioreactor Based on Enzymatic Quorum Quenching. Environ. Sci. Technol. 2009, 43, 7403–7409. [Google Scholar] [CrossRef] [PubMed]
- Cheong, W.-S.; Lee, C.-H.; Moon, Y.-H.; Oh, H.-S.; Kim, S.-R.; Lee, S.H.; Lee, C.-H.; Lee, J.-K. Isolation and Identification of Indigenous Quorum Quenching Bacteria, Pseudomonas sp. 1A1, for Biofouling Control in MBR. Ind. Eng. Chem. Res. 2013, 52, 10554–10560. [Google Scholar] [CrossRef]
- Czajkowski, R.; Jafra, S. Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim. Pol. 2009, 56, 1–16. [Google Scholar] [PubMed]
- Oh, H.-S.; Kim, S.-R.; Cheong, W.-S.; Lee, C.-H.; Lee, J.-K. Biofouling inhibition in MBR by Rhodococcus sp. BH4 isolated from real MBR plant. Appl. Microbiol. Biotechnol. 2013, 97, 10223–10231. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Hwang, B.-J.; Shin, M.-H.; Kim, J.-A.; Kim, H.-K.; Lee, J.-K. N-acylhomoserine lactonase producing Rhodococcus spp. with different AHL-degrading activities. FEMS Microbiol. Lett. 2006, 261, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; Oger, P.M.; Chapelle, E.; Adeline, M.-T.; Faure, D.; Dessaux, Y. A Rhodococcus qsdA-Encoded Enzyme Defines a Novel Class of Large-Spectrum Quorum-Quenching Lactonases. Appl. Environ. Microbiol. 2008, 74, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; Chhabra, S.R.; Camara, M.; Williams, P.; Oger, P.; Dessaux, Y. N-Acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology 2005, 151, 3313–3322. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Weng, L.-X.; Dong, Y.-H.; Zhang, L.-H. Specificity and Enzyme Kinetics of the Quorum-quenching N-Acyl Homoserine Lactone Lactonase (AHL-lactonase). J. Biol. Chem. 2004, 279, 13645–13651. [Google Scholar] [CrossRef] [PubMed]
- Sakr, M.M.; Anwar Aboshanab, K.M.; Aboulwafa, M.M.; Hassouna, N.A. Characterization and Complete Sequence of Lactonase Enzyme from Bacillus weihenstephanensis Isolate P65 with Potential Activity against Acyl Homoserine Lactone Signal Molecules. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Sio, C.F.; Otten, L.G.; Cool, R.H.; Diggle, S.P.; Braun, P.G.; Bos, R.; Daykin, M.; Cámara, M.; Williams, P.; Quax, W.J. Quorum Quenching by an N-Acyl-Homoserine Lactone Acylase from Pseudomonas aeruginosa PAO1. Infect. Immun. 2006, 74, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Diggle, S.P.; Heeb, S.; Cámara, M.; Otero, A. Quorum quenching activity in Anabaena sp. PCC 7120: Identification of AiiC, a novel AHL-acylase. FEMS Microbiol. Lett. 2008, 280, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Byun, T.; Dussen, H.-J.; Duke, K.R. Degradation of N-acylhomoserine lactones, the bacterial quorum-sensing molecules, by acylase. J. Biotechnol. 2003, 101, 89–96. [Google Scholar] [CrossRef]
- Jahangir, D.; Oh, H.-S.; Kim, S.-R.; Park, P.-K.; Lee, C.-H.; Lee, J.-K. Specific location of encapsulated quorum quenching bacteria for biofouling control in an external submerged membrane bioreactor. J. Membr. Sci. 2012, 411–412, 130–136. [Google Scholar] [CrossRef]
- Kim, S.-R.; Lee, K.-B.; Kim, J.-E.; Won, Y.-J.; Yeon, K.-M.; Lee, C.-H.; Lim, D.-J. Macroencapsulation of quorum quenching bacteria by polymeric membrane layer and its application to MBR for biofouling control. J. Membr. Sci. 2015, 473, 109–117. [Google Scholar] [CrossRef]
- Köse-Mutlu, B.; Ergön-Can, T.; Koyuncu, İ.; Lee, C.-H. Quorum quenching MBR operations for biofouling control under different operation conditions and using different immobilization media. Desalination Water Treat. 2016, 57, 17696–17706. [Google Scholar] [CrossRef]
- Cheong, W.-S.; Kim, S.-R.; Oh, H.-S.; Lee, S.H.; Yeon, K.-M.; Lee, C.-H.; Lee, J.-K. Design of Quorum Quenching Microbial Vessel to Enhance Cell Viability for Biofouling Control in Membrane Bioreactor. J. Microbiol. Biotechnol. 2014, 24, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Chae, S.-R.; Drews, A.; Kraume, M.; Shin, H.-S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef] [PubMed]
- Pollice, A.; Brookes, A.; Jefferson, B.; Judd, S. Sub-critical flux fouling in membrane bioreactors—A review of recent literature. Desalination 2005, 174, 221–230. [Google Scholar] [CrossRef]
- Zhang, J.; Chua, H.C.; Zhou, J.; Fane, A.G. Factors affecting the membrane performance in submerged membrane bioreactors. J. Membr. Sci. 2006, 284, 54–66. [Google Scholar] [CrossRef]
- Cho, B.D.; Fane, A.G. Fouling transients in nominally sub-critical flux operation of a membrane bioreactor. J. Membr. Sci. 2002, 209, 391–403. [Google Scholar] [CrossRef]
- Hwang, B.-K.; Lee, W.-N.; Yeon, K.-M.; Park, P.-K.; Lee, C.-H.; Chang, In-S.; Drews, A.; Kraume, M. Correlating TMP Increases with Microbial Characteristics in the Bio-Cake on the Membrane Surface in a Membrane Bioreactor. Environ. Sci. Technol. 2008, 42, 3963–3968. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cabassud, C.; Guigui, C. Effects of carbamazepine in peak injection on fouling propensity of activated sludge from a MBR treating municipal wastewater. J. Membr. Sci. 2015, 475, 122–130. [Google Scholar] [CrossRef]
- Li, C.; Cabassud, C.; Reboul, B.; Guigui, C. Effects of pharmaceutical micropollutants on the membrane fouling of a submerged MBR treating municipal wastewater: Case of continuous pollution by carbamazepine. Water Res. 2015, 69, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Massé, A.; Spérandio, M.; Cabassud, C. Comparison of sludge characteristics and performance of a submerged membrane bioreactor and an activated sludge process at high solids retention time. Water Res. 2006, 40, 2405–2415. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, L.; Rodríguez, M.; Prats, D. Effect of different extraction methods on bound EPS from MBR sludges. Part I: Influence of extraction methods over three-dimensional EEM fluorescence spectroscopy fingerprint. Desalination 2010, 261, 19–26. [Google Scholar] [CrossRef]
- Bouhabila, E.H.; Ben Aim, R.; Buisson, H. Fouling characterisation in membrane bioreactors. Sep. Purif. Technol. 2001, 22–23, 123–132. [Google Scholar] [CrossRef]
- Teychene, B.; Guigui, C.; Cabassud, C.; Amy, G. Toward a better identification of foulant species in MBR processes. Desalination 2008, 231, 27–34. [Google Scholar] [CrossRef]
- Brelles-Mariño, G.; Bedmar, E.J. Detection, purification and characterisation of quorum-sensing signal molecules in plant-associated bacteria. J. Biotechnol. 2001, 91, 197–209. [Google Scholar] [CrossRef]
- Li, X.; Fekete, A.; Englmann, M.; Götz, C.; Rothballer, M.; Frommberger, M.; Buddrus, K.; Fekete, J.; Cai, C.; Schröder, P.; et al. Development and application of a method for the analysis of N-acylhomoserine lactones by solid-phase extraction and ultra high pressure liquid chromatography. J. Chromatogr. A 2006, 1134, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Jahn, A.; Nielsen, P.H. Extraction of extracellular polymeric substances (EPS) from biofilms using a cation exchange resin. Water Sci. Technol. 1995, 32, 157–164. [Google Scholar] [CrossRef]
- Teychene, B.; Guigui, C.; Cabassud, C. Engineering of an MBR supernatant fouling layer by fine particles addition: A possible way to control cake compressibility. Water Res. 2011, 45, 2060–2072. [Google Scholar] [CrossRef] [PubMed]
- Weerasekara, N.A.; Choo, K.-H.; Lee, C.-H. Biofouling control: Bacterial quorum quenching versus chlorination in membrane bioreactors. Water Res. 2016, 103, 293–301. [Google Scholar] [CrossRef] [PubMed]
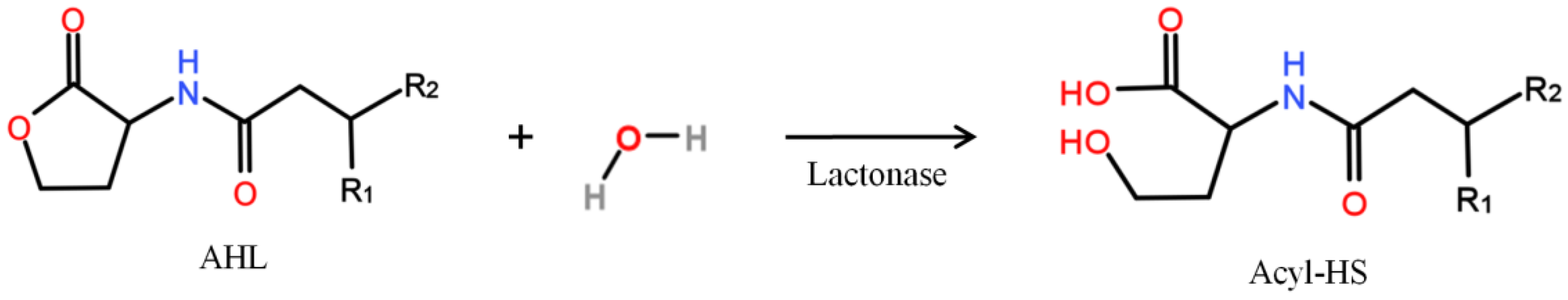
| QQ MBR Design | MBR Operation | Resulting QQ Effect Expressed in% of the Control MBR | Ref. | N° | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reactor | Membrane | Rhodococcus sp. BH4 | ||||||||||||||||||||||||||||||
| Geometry | Working Volume (L) | Nature | Geometry | Configuration | Area (cm2) | Pore Size (µm) | Entrapping Method | Inserted Quantity of BH4 Cells in the Reactor (mg/L) | F/M Ratio | MLSS (mg/L) | HRT (h) | SRT (d) | Permeate Flux (L/m2 h) | Air Supply (m3/h) | Filtration/Relaxation | Run Time (days) | Number of Cycles | TMP | Time for TMP to Reach | TAB in Biofilm | SMP in Mixed Liquor | EPS in Biofilm | COD removal Efficiency | |||||||||
| For Control MBR | For QQ MBR | After 1 Day of Operation | At the End of the 1st cycle | At the End of Operation | The Breaking Point | 25 kPa | 40 kPa | Proteins | Polysaccharides | Proteins | Polysaccharides | |||||||||||||||||||||
| Synthetic wastewater | ||||||||||||||||||||||||||||||||
| ○ | 1.2 | PVDF | HF | Submerged | 86 | 0.04 | PE microbial vessels (2/reactor) | 450 | n.a. | 4500–5000 | 12 | 40 | 18 | n.a. | - | 3.75 | 2 | 2 | −68% | −51% | −61% | - | +148% | - | −49 w % after 1.7 day | - | - | - | - | - | [28] | 1 |
| ○ | 2.8 (2 L bioreactor + 0.8 L membrane tank) | PVDF | HF | Side-stream (external submerged) | 120 | 0.04 | PE microbial vessel (1 in the bioreactor) | 128.6 | 0.22 | n.a. | 12 | 50 | 30 | 0.09 in the bioreactor and 0.06 in the membrane tank | 60 min/1 min | 1.4 | 1 | 1 | −23% | −35% | −35% | - | +110% | - | - | - | - | - | - | - | [49] | 2 |
| ○ | 2.8 (2 L bioreactor + 0.8 L membrane tank) | PVDF | HF | Side-stream (external submerged) | 120 | 0.04 | PE microbial vessel (1 in the membrane tank) | 128.6 | 0.22 | n.a. | 12 | 50 | 30 | 0.09 in the bioreactor and 0.06 in the membrane tank | 60 min/1 min | 1.75 | 1 | 1 | −50% | −77% | −77% | +240% | +160% | - | - | - | - | - | - | - | [49] | 3 |
| n.a. | 1.2 | PVDF | HF | Submerged | 86 | 0.04 | PE microbial vessels (2/reactor) | 167 | 0.17–0.2 | 7000–7500 | 10 | 40 | 35 | n.a. | - | 5 | 1 | 1 | −18% | −68% | −68% | +300% | +216% (+2.8 day) | +205%(+3.1 day) | - | - | - | - | - | - | [40] | 4 |
| ○ | 1.6 | PVDF | HF | Submerged | 13.4 | 0.04 | CEBs (40/reactor) | ~8 | n.a. | 12,500–13,000 | 5.3 | 25 | 28.7 | n.a. | - | 17 | 4 | 1 | −10% | −93% | −94% | +672% | +548% (+14.3 day) | +504% (+14.2 day) | −69 w % after 3.1 days | - | - | −81% after 3.1 days * | +0.2% | [25] | 5 | |
| □ | 35 | PVDF | HF | Submerged | 7000 | 0.1 | CEBs (1860/reactor) | n.c. | n.a. | 10,000 | 4 | 20 | 15 | 1.08 | 8 min/2 min | 90 | 7 | 1 | −24% | −88% | −37% | +550% | +724% | - | - | −89% after 80 days | - | - | −0.3% | [27] | 6 | |
| ○ | 5 | n.a. | HF | Submerged | 100 | n.a. | CEBs | n.c. | n.a. | 12,000–13,000 | 13 | 30 | 20 | n.a. | - | 3.8 | 1 | 1 | −85% | −93% | −93% | - | - | - | - | - | - | - | - | +1.6% | [51] | 7 |
| ○ | 5 | n.a. | HF | Submerged | 100 | n.a. | CEBs | n.c. | n.a. | 12,000–13,000 | 13 | 30 | 20 | n.a. | - | 2.4 | 1 | 1 | −98% | −99.6% | −99.6% | - | - | - | - | - | - | - | - | +2.5% | [51] | 8 |
| ○ | 5 | n.a. | HF | Submerged | 100 | n.a. | CEBs | n.c. | n.a. | 12,000–13,000 | 13 | 30 | 30 | n.a. | - | 3.5 | 1 | 1 | −95% | −97% | −97% | - | - | - | - | - | - | - | - | ~0% | [51] | 9 |
| ○ | 5 | n.a. | HF | Submerged | 100 | n.a. | CEBs | n.c. | n.a. | 12,000–13,000 | 13 | 30 | 50 | n.a. | - | 1.7 | 1 | 1 | −90% | −14% | −14% | - | +295% | - | - | - | - | - | - | ~0% | [51] | 10 |
| ○ | 5 | n.a. | HF | Submerged | 100 | n.a. | PVDF microbial vessel (1/reactor) | n.c. | n.a. | 12,000–13,000 | 13 | 30 | 50 | n.a. | - | 1.9 | 1 | 1 | −49% | −40% | −40% | - | +138% | - | - | - | - | - | - | +2% | [51] | 11 |
| ○ | 5 | n.a. | HF | Submerged | 100 | n.a. | RMCF | n.c. | n.a. | 12,000–13,000 | 13 | 30 | 50 | n.a. | - | 1.9 | 1 | 1 | −59% | −63% | −63% | - | - | - | - | - | - | - | - | ~0% | [51] | 12 |
| Real wastewater | ||||||||||||||||||||||||||||||||
| □ | 2.5 | PVDF | HF | Submerged | 155 | 0.04 | Macrocapsules (500/reactor) | n.c. | 0.1–0.2 | 5300–5700 | 8 | 30 | 30 | 0.06 | - | 22 | 3 | 1 | −23% | −87% | −21% | +1135% | +750% (+ 19 day) | +667% (+19.7 day) | −48 w % after a 9 days cycle | - | - | −53% | −88% | +0.5% | [50] | 13 |
| n.a. | 80 | C-PVC | FS | Submerged | 9000 | 0.4 | QQ beads (~19000/reactor) | ~4000 | n.a. | 10,000–13,000 | 5.2 | 25 | 20 | 0.27 | 10 min/ 2 min | 14 | 2 | 1 | n.c. | −83% | −81% | +590% | +470% | - | −86% after 14 days | −52% | −85% | −21% | - | - | [26] | 14 |
| MBR Design | MBR Operation | Resulting QQ Effect Expressed in% of the Control MBR | Ref. | N° | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reactor | Membrane | Pseudomonas sp. 1A1 | |||||||||||||||||||||||||
| Geometry | Working Volume (L) | Configuration | Area (cm2) | Pore Size (µm) | Entrapping Method | Inserted Quantity of 1A1 Cells in the Reactor (mg/L) | F/M Ratio | MLSS (mg/L) | HRT (h) | SRT (d) | Permeate Flux (L/m2 h) | Run Time (days) | Number of Cycles | TMP | Time for TMP to Reach | TAB in Biofilm | SMP in Mixed Liquor | EPS in Biofilm | |||||||||
| For Control MBR | For QQ MBR | After 1 Day of Operation | At the End of the 1st Cycle | At the End of Operation | The Breaking Point | 25 kPa | 40 kPa | Proteins | Polysaccharides | Proteins | Polysaccharides | ||||||||||||||||
| ○ | 2.5 | Submerged | 155.2 | 0.04 | PE vessels (4/reactor) | 192 | 0.20–0.22 | 7600–8000 | 8 | 30 | 25 | 7.8 | 2 | 2 | −15% | −71% | −79% | +180% | +134% (+2.6 day) | +150% (+3.1 day) | - | - | - | - | - | [38] | 1 |
| ○ | 3.0 | Submerged | 210 | 0.04 | CMV under inner flow feeding mode (1/reactor) | 706.8 | 0.12–0.21 | 11,000–13,000 | 6 | 60 | 30 | 2.1 | 1 | 1 | −15% | −77% | −77% | - | - | - | −63 w% | - | - | - | - | [52] | 2 |
| ○ | 3.0 | Submerged | 210 | 0.04 | CMV under inner flow feeding mode (1/reactor) | 706.8 | 0.12–0.21 | 11,000–13,000 | 6 | 60 | 25 | 9.4 | 1 | 1 | −22% | −76% | −76% | - | - | - | - | - | - | - | - | [52] | 3 |
| ○ | 3.0 | Submerged | 210 | 0.04 | CMV under inner flow feeding mode (1/reactor) | 266.4 | 0.12–0.21 | 11,000–13,000 | 6 | 60 | 35 | 6 | 2 | 2 | −25% | −60% | −56% | - | - | - | - | −6% | −62% | −77% | −37% | [52] | 4 |
| ○ | 3.0 | Submerged | 210 | 0.04 | CMV under normal feeding mode (1/reactor) | 266.4 | 0.12–0.21 | 11,000–13,000 | 6 | 60 | 35 | 6 | 2 | 2 | −5% | −44% | −25% | - | −3% | - | - | −2% | −51% | −31% | −36% | [52] | 5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouayed, N.; Dietrich, N.; Lafforgue, C.; Lee, C.-H.; Guigui, C. Process-Oriented Review of Bacterial Quorum Quenching for Membrane Biofouling Mitigation in Membrane Bioreactors (MBRs). Membranes 2016, 6, 52. https://doi.org/10.3390/membranes6040052
Bouayed N, Dietrich N, Lafforgue C, Lee C-H, Guigui C. Process-Oriented Review of Bacterial Quorum Quenching for Membrane Biofouling Mitigation in Membrane Bioreactors (MBRs). Membranes. 2016; 6(4):52. https://doi.org/10.3390/membranes6040052
Chicago/Turabian StyleBouayed, Naila, Nicolas Dietrich, Christine Lafforgue, Chung-Hak Lee, and Christelle Guigui. 2016. "Process-Oriented Review of Bacterial Quorum Quenching for Membrane Biofouling Mitigation in Membrane Bioreactors (MBRs)" Membranes 6, no. 4: 52. https://doi.org/10.3390/membranes6040052
APA StyleBouayed, N., Dietrich, N., Lafforgue, C., Lee, C.-H., & Guigui, C. (2016). Process-Oriented Review of Bacterial Quorum Quenching for Membrane Biofouling Mitigation in Membrane Bioreactors (MBRs). Membranes, 6(4), 52. https://doi.org/10.3390/membranes6040052





