A Conceptual Approach to Reduce the Product Gas Crossover in Alkaline Electrolyzers
Abstract
1. Introduction
2. Crossover in Alkaline Electrolyzers
3. Reducing the Amount of Crossover
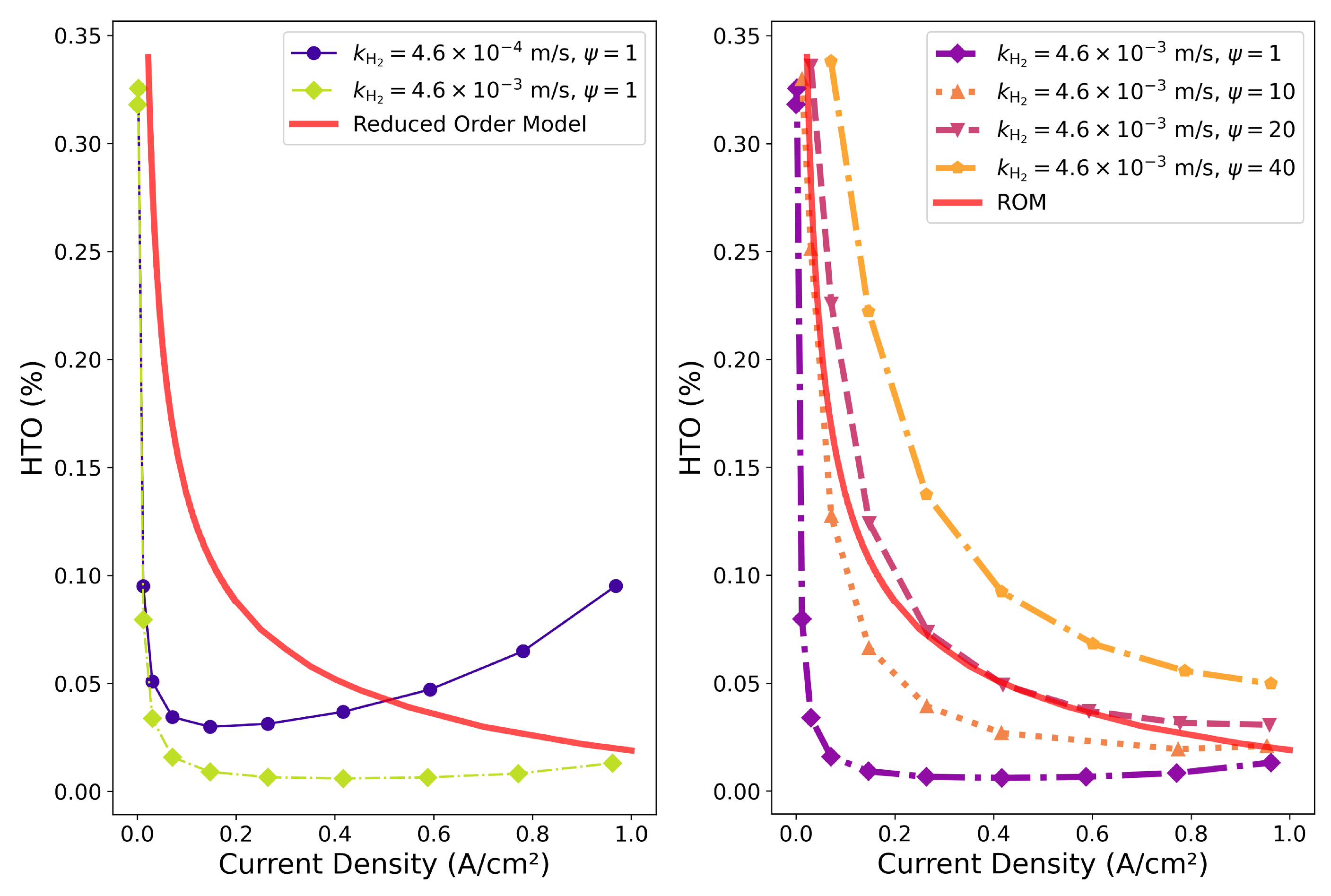
4. Effect of the Bubble Sizes
5. Conclusions
- In order to increase the operating current density in alkaline electrolyzer cells, the Zirfon-like diaphragm has to be carefully optimized, taking into account parameters like the thickness, porosity, permeability, and the bubble point. A high porosity and ensuing permeability of the hydroxides lead to a low ohmic resistance, but also to a higher amount of hydrogen and oxygen crossover, which severely limits the operational window of such electrolyzers.
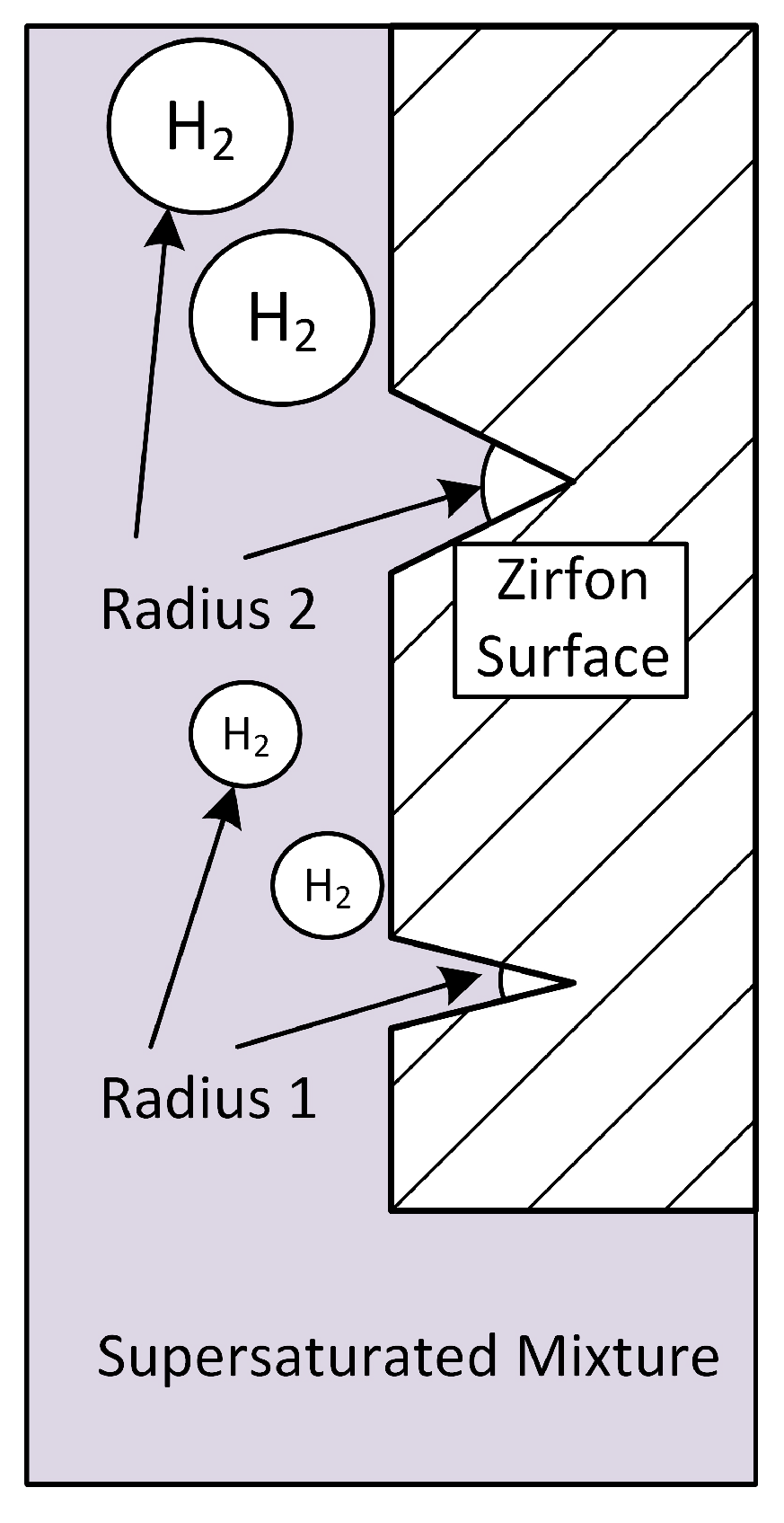
- The underling reason for the high amounts of hydrogen and oxygen crossover is the oversaturation of the liquid electrolyte phase with these species, leading to high Fickean diffusion. Conceptually, hydrogen and oxygen gas bubbles can be created at the Zirfon surface by manufacturing micro-cracks in it. Ideally, the size of the bubbles created corresponds to the size of the cracks. Typical bubble sizes in alkaline electrolyzers are in the range of 50 microns–200 microns.
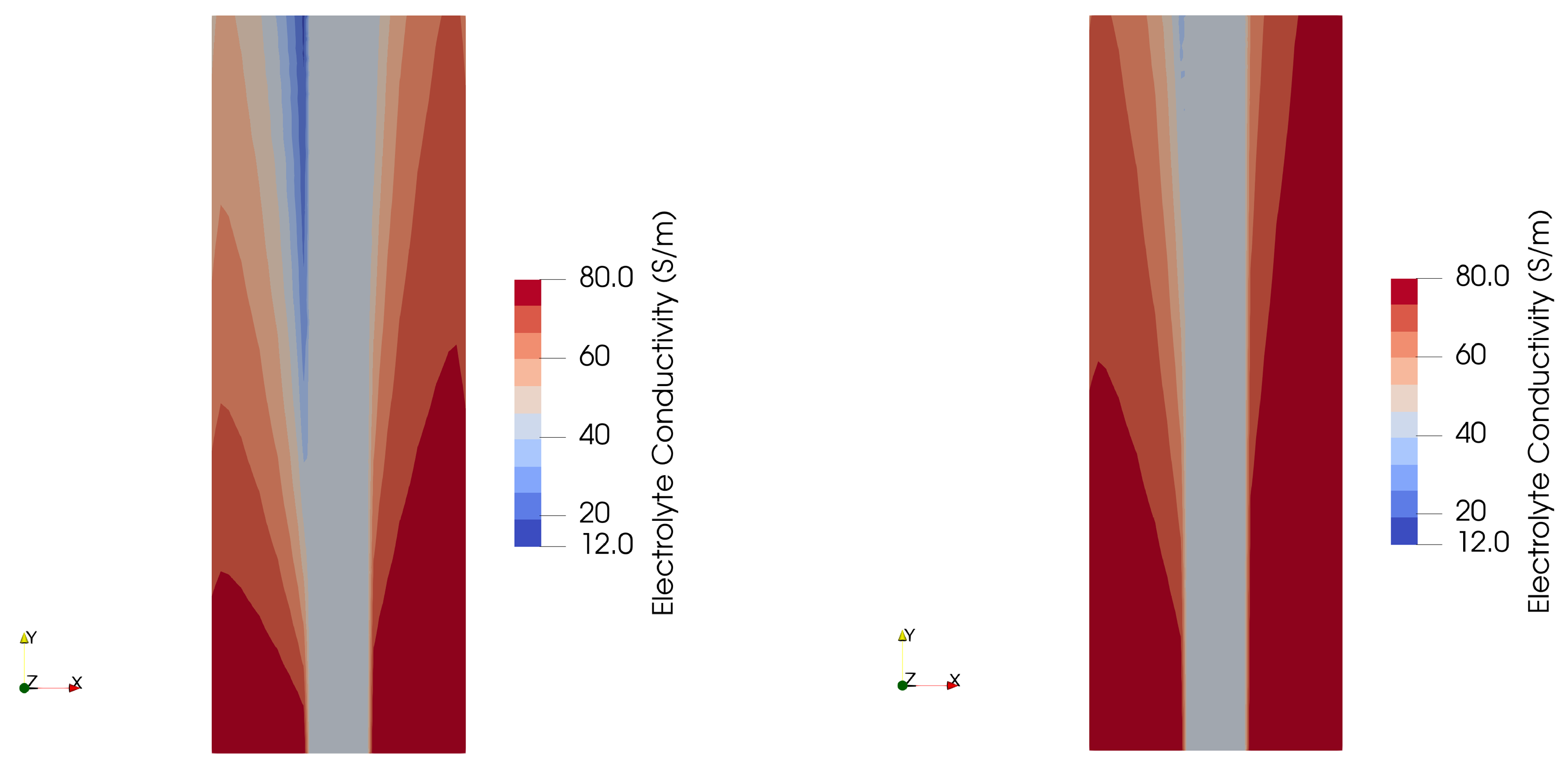
- From computational fluid dynamics, we can conclude that larger bubbles lead to a lower ohmic resistance inside the electrolyte phase, and therefore are preferred. The predicted current density at a cell voltage of 2.1 V is 1.06 A/cm2 for 150-micron bubbles versus 0.96 A/cm2 for 50-micron bubbles.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feng, Q.; Yuan, X.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Cummins. HyLYZER Water Electrolyzers. 2025. Available online: https://mart.cummins.com/imagelibrary/data/assetfiles/0070328.pdf (accessed on 25 April 2025).
- Loureiro Martinho, D. Three-Dimensional Computational Analysis of Transport Phenomena in an Alkaline Electrolyzer Cell. Ph.D. Thesis, Aalborg University, Aalborg, Denmark, 2025. [Google Scholar]
- Luo, X.; Xu, N.; Zhou, Y.; Yang, X.; Yang, W.; Liu, G.; Lee, J.K.; Qiao, J. Porous PVA skin-covered thin Zirfon-type separator as a new approach boosting high-rate alkaline water electrolysis beyond 1000 hours’ lifespan. eScience 2024, 4, 100290. [Google Scholar] [CrossRef]
- Vermeiren, P.; Adriansens, W.; Moreels, J.; Leysen, R. Evaluation of the Zirfon® separator for use in alkaline water electrolysis and Ni-H2 batteries. Int. J. Hydrogen Energy 1998, 23, 321–324. [Google Scholar] [CrossRef]
- Kim, S.; Han, J.H.; Yuk, J.; Kim, S.; Song, Y.; So, S.; Lee, K.T.; Kim, T.H. Highly selective porous separator with thin skin layer for alkaline water electrolysis. J. Power Sources 2022, 524, 231059. [Google Scholar] [CrossRef]
- Brauns, J.; Schönebeck, J.; Kraglund, M.R.; Aili, D.; Hnát, J.; Žitka, J.; Mues, W.; Jensen, J.O.; Bouzek, K.; Turek, T. Evaluation of Diaphragms and Membranes as Separators for Alkaline Water Electrolysis. J. Electrochem. Soc. 2021, 168, 014510. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Trinke, P. Experimental and Model-Based Investigations on Gas Crossover in Polymer Electrolyte Membrane Water Electrolyzers. Ph.D. Thesis, Gottfried Wilhelm Leibniz University at Hannover, Hannover, Germany, 2021. [Google Scholar]
- Yodwong, B.; Guilbert, D.; Phattanasak, M.; Kaewmanee, W.; Hinaje, M.; Vitale, G. Faraday’s Efficiency Modeling of a Proton Exchange Membrane Electrolyzer Based on Experimental Data. Energies 2020, 13, 4792. [Google Scholar] [CrossRef]
- Trinke, P.; Bensmann, B.; Hanke-Rauschenbach, R. Current density effect on hydrogen permeation in PEM water electrolyzers. Int. J. Hydrogen Energy 2017, 42, 14355–14366. [Google Scholar] [CrossRef]
- Trinke, P.; Haug, P.; Brauns, J.; Bensmann, B.; Hanke-Rauschenbach, R.; Turek, T. Hydrogen Crossover in PEM and Alkaline Water Electrolysis: Mechanisms, Direct Comparison and Mitigation Strategies. J. Electrochem. Soc. 2018, 165, F502. [Google Scholar] [CrossRef]
- Lira Garcia Barros, R.; Kraakman, J.T.; Sebregts, C.; van der Schaaf, J.; de Groot, M.T. Impact of an electrode-diaphragm gap on diffusive hydrogen crossover in alkaline water electrolysis. Int. J. Hydrogen Energy 2024, 49, 886–896. [Google Scholar] [CrossRef]
- Martinho, D.L.; Abdollahzadehsangroudi, M.; Araya, S.S.; Berning, T. Eulerian-Eulerian CFD Modeling of Multiphase Flow and Heat Transfer in Alkaline Electrolysis Cells. J. Electrochem. Soc. 2025, 172, 064501. [Google Scholar] [CrossRef]
- Appelhaus, S.; Ritz, L.; Pape, S.V.; Lohmann-Richters, F.; Kraglund, M.R.; Jensen, J.O.; Massari, F.; Boroomandnia, M.; Romanò, M.; Albers, J.; et al. Benchmarking performance: A round-robin testing for liquid alkaline electrolysis. Int. J. Hydrogen Energy 2024, 95, 1004–1010. [Google Scholar] [CrossRef]
- Jones, S.; Evans, G.; Galvin, K. Bubble nucleation from gas cavities—A review. Adv. Colloid Interface Sci. 1999, 80, 27–50. [Google Scholar] [CrossRef]
- Henkensmeier, D.; Cho, W.C.; Jannasch, P.; Stojadinovic, J.; Li, Q.; Aili, D.; Jensen, J.O. Separators and membranes for advanced alkaline water electrolysis. Chem. Rev. 2024, 124, 6393–6443. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinho, D.L.; Berning, T. A Conceptual Approach to Reduce the Product Gas Crossover in Alkaline Electrolyzers. Membranes 2025, 15, 206. https://doi.org/10.3390/membranes15070206
Martinho DL, Berning T. A Conceptual Approach to Reduce the Product Gas Crossover in Alkaline Electrolyzers. Membranes. 2025; 15(7):206. https://doi.org/10.3390/membranes15070206
Chicago/Turabian StyleMartinho, Diogo Loureiro, and Torsten Berning. 2025. "A Conceptual Approach to Reduce the Product Gas Crossover in Alkaline Electrolyzers" Membranes 15, no. 7: 206. https://doi.org/10.3390/membranes15070206
APA StyleMartinho, D. L., & Berning, T. (2025). A Conceptual Approach to Reduce the Product Gas Crossover in Alkaline Electrolyzers. Membranes, 15(7), 206. https://doi.org/10.3390/membranes15070206





