Membrane Technologies for Bioengineering Microalgae: Sustainable Applications in Biomass Production, Carbon Capture, and Industrial Wastewater Valorization
Abstract
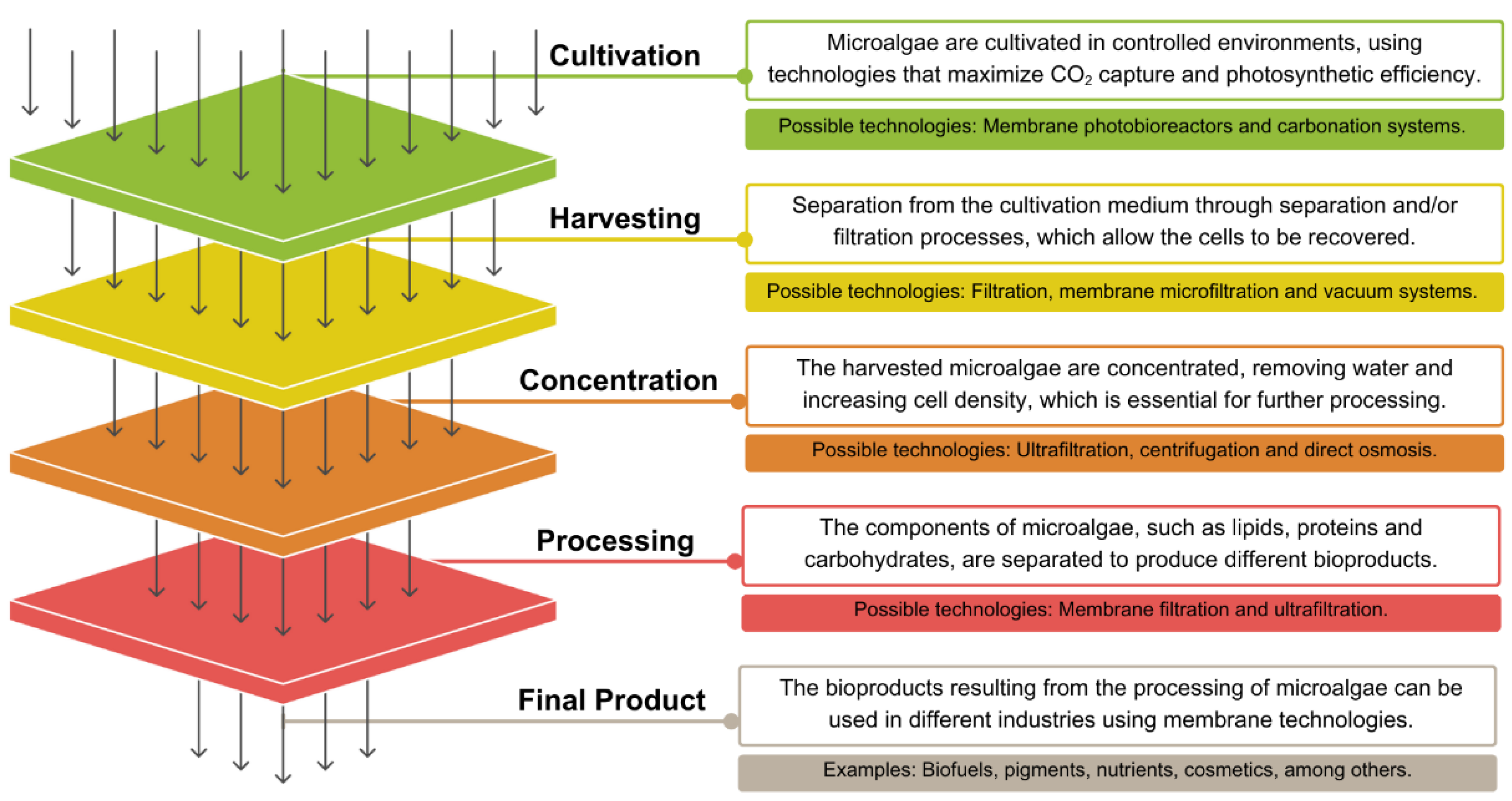
1. Introduction
2. Membrane Technologies for Microalgae-Based Systems
2.1. Membrane Photobioreactors (MPBRs)
2.2. Biomass Retention Mechanisms
2.3. Biofilm-Based Systems
3. Carbon Capture via Membrane-Based Systems
3.1. Direct CO2 Transfer via Membranes in Microalgae Cultures
3.2. Selective Separation of CO2 from Flue Gases
3.3. Selective Bicarbonate Supply and Ionic Regulation with Electrolysis Membranes
4. Integration of Membrane-Based Systems for Wastewater Treatment and Circular Resource Recovery
4.1. Membrane Photobioreactors in Wastewater Treatment: Nutrient Recovery and Biomass Production
4.2. Integrated Membrane Photobioreactors for Carbon Capture, Wastewater Treatment, and Biomass Valorization
5. Process Optimization and Technological Innovations
5.1. Innovations in Membrane Materials and Surface Modifications
5.2. Strategies for Fouling Mitigation
5.3. Control and Automation Strategies
6. Environmental and Industrial Implications
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costa, J.A.V.; Freitas, B.C.B.; Moraes, L.; Zaparoli, M.; Morais, M.G. Progress in the Physicochemical Treatment of Microalgae Biomass for Value-Added Product Recovery. Bioresour. Technol. 2020, 301, 122727. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Xia, C.; Alqahtani, A.; Sharma, A.; Pugazhendhi, A. A Review on Optimistic Biorefinery Products: Biofuel and Bioproducts from Algae Biomass. Fuel 2023, 338, 127378. [Google Scholar] [CrossRef]
- Saha, S.; Xaxa, D.S.; Ghosh, S.K.; Maiti, M.K. Enhanced Accumulation of Important Bioproducts in Chlorella vulgaris through AGPase Gene Silencing Coupled with Polyethylene Glycol Treatment. J. Biotechnol. 2025, 403, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Ezhumalai, G.; Arun, M.; Manavalan, A.; Rajkumar, R.; Heese, K. A Holistic Approach to Circular Bioeconomy Through the Sustainable Utilization of Microalgal Biomass for Biofuel and Other Value-Added Products. Microb. Ecol. 2024, 87, 61. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.S.; Ejeromedoghene, O.; Okoye, C.O.; Ezeorba, T.P.C.; Nyaruaba, R.; Ikechukwu, C.K.; Oladipo, A.; Orege, J.I. Microalgae Biorefinery: An Integrated Route for the Sustainable Production of High-Value-Added Products. Energy Convers. Manag. X 2022, 16, 100323. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Inorganic Algal Nutrition. In Handbook of Microalgal Culture; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 123–133. ISBN 9781118567166. [Google Scholar]
- Esteves, A.F.; Pires, J.C.M.; Gonçalves, A.L. Current Utilization of Microalgae in the Food Industry beyond Direct Human Consumption. In Cultured Microalgae for the Food Industry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 199–248. [Google Scholar]
- Zhao, Z.; Muylaert, K.; Vankelecom, I.F.J. Applying Membrane Technology in Microalgae Industry: A Comprehensive Review. Renew. Sustain. Energy Rev. 2023, 172, 113041. [Google Scholar] [CrossRef]
- Gerardo, M.L.; Aljohani, N.H.M.; Oatley-Radcliffe, D.L.; Lovitt, R.W. Moving towards Sustainable Resources: Recovery and Fractionation of Nutrients from Dairy Manure Digestate Using Membranes. Water Res. 2015, 80, 80–89. [Google Scholar] [CrossRef]
- François, M.; Lin, K.-S.; Rachmadona, N. Microalgae-Based Membrane Bioreactor for Wastewater Treatment, Biogas Production, and Sustainable Energy: A Review. Environ. Res. 2025, 268, 120802. [Google Scholar] [CrossRef]
- Chanquia, S.N.; Vernet, G.; Kara, S. Photobioreactors for Cultivation and Synthesis: Specifications, Challenges, and Perspectives. Eng. Life Sci. 2022, 22, 712–724. [Google Scholar] [CrossRef]
- Nasser, T.; Emamshoushtari, M.M.; Helchi, S.; Saeidi, A.; Pajoum Shariati, F. Mitigating Membrane Fouling in an Internal Loop Airlift Membrane Photobioreactor Containing Spirulina platensis: Effects of Riser Cross-Sectional Area and Hydrophilic Baffles. Prep. Biochem. Biotechnol. 2024, 54, 779–787. [Google Scholar] [CrossRef]
- Bilad, M.R.; Arafat, H.A.; Vankelecom, I.F.J. Membrane Technology in Microalgae Cultivation and Harvesting: A Review. Biotechnol. Adv. 2014, 32, 1283–1300. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.H.; Zhang, Y.T.; Zhang, L.; Chen, H.L. Evaluation of a Membrane-Sparged Helical Tubular Photobioreactor for Carbon Dioxide Biofixation by Chlorella vulgaris. J. Memb. Sci. 2008, 325, 336–345. [Google Scholar] [CrossRef]
- Lai, Y.S.; Eustance, E.; Shesh, T.; Rittmann, B.E. Enhanced Carbon-Transfer and -Utilization Efficiencies Achieved Using Membrane Carbonation with Gas Sources Having a Range of CO2 Concentrations. Algal Res. 2020, 52, 102098. [Google Scholar] [CrossRef]
- Kim, H.W.; Marcus, A.K.; Shin, J.H.; Rittmann, B.E. Advanced Control for Photoautotrophic Growth and CO2-Utilization Efficiency Using a Membrane Carbonation Photobioreactor (MCPBR). Environ. Sci. Technol. 2011, 45, 5032–5038. [Google Scholar] [CrossRef]
- Bilad, M.R.; Discart, V.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F.J. Coupled Cultivation and Pre-Harvesting of Microalgae in a Membrane Photobioreactor (MPBR). Bioresour. Technol. 2014, 155, 410–417. [Google Scholar] [CrossRef]
- Marbelia, L.; Bilad, M.R.; Passaris, I.; Discart, V.; Vandamme, D.; Beuckels, A.; Muylaert, K.; Vankelecom, I.F.J. Membrane Photobioreactors for Integrated Microalgae Cultivation and Nutrient Remediation of Membrane Bioreactors Effluent. Bioresour. Technol. 2014, 163, 228–235. [Google Scholar] [CrossRef]
- Bilad, M.R.; Discart, V.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F.J. Harvesting Microalgal Biomass Using a Magnetically Induced Membrane Vibration (MMV) System: Filtration Performance and Energy Consumption. Bioresour. Technol. 2013, 138, 329–338. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Hu, Q.; Cheng, P.; Ji, B.; Liu, J.; Chen, Y.; Zhang, W.; Chen, X.; Chen, L.; et al. Attached Cultivation Technology of Microalgae for Efficient Biomass Feedstock Production. Bioresour. Technol. 2013, 127, 216–222. [Google Scholar] [CrossRef]
- Goh, P.S.; Ahmad, N.A.; Lim, J.W.; Liang, Y.Y.; Kang, H.S.; Ismail, A.F.; Arthanareeswaran, G. Microalgae-Enabled Wastewater Remediation and Nutrient Recovery through Membrane Photobioreactors: Recent Achievements and Future Perspective. Membranes 2022, 12, 1094. [Google Scholar] [CrossRef]
- González-Camejo, J.; Aparicio, S.; Jiménez-Benítez, A.; Pachés, M.; Ruano, M.V.; Borrás, L.; Barat, R.; Seco, A. Improving Membrane Photobioreactor Performance by Reducing Light Path: Operating Conditions and Key Performance Indicators. Water Res. 2020, 172, 115518. [Google Scholar] [CrossRef]
- Theepharaksapan, S.; Lerkmahalikit, Y.; Namyuang, C.; Ittisupornrat, S. Performance of Membrane Photobioreactor for Integrated Spirulina Strain Cultivation and Nutrient Removal of Membrane Bioreactor Effluent. J. Environ. Chem. Eng. 2023, 11, 110579. [Google Scholar] [CrossRef]
- Novoa, A.F.; Vrouwenvelder, J.S.; Fortunato, L. Membrane Fouling in Algal Separation Processes: A Review of Influencing Factors and Mechanisms. Front. Chem. Eng. 2021, 3, 687422. [Google Scholar] [CrossRef]
- Mora-Sánchez, J.F.; Ribes, J.; González-Camejo, J.; Seco, A.; Ruano, M.V. Towards Optimisation of Microalgae Cultivation through Monitoring and Control in Membrane Photobioreactor Systems. Water 2024, 16, 155. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Z.; Wang, A.; Nopens, I.; Hu, H.; Chen, H. High Biomass Yields of Chlorella protinosa with Efficient Nitrogen Removal from Secondary Effluent in a Membrane Photobioreactor. J. Environ. Sci. 2024, 146, 272–282. [Google Scholar] [CrossRef]
- Honda, R.; Teraoka, Y.; Noguchi, M.; Yang, S. Optimization of Hydraulic Retention Time and Biomass Concentration in Microalgae Biomass Production from Treated Sewage with a Membrane Photobioreactor. J. Water Environ. Technol. 2017, 15, 1–11. [Google Scholar] [CrossRef]
- Liu, S.; Rouquié, C.; Lavenant, L.; Frappart, M.; Couallier, E. Coupling Bead-Milling and Microfiltration for the Recovery of Lipids and Proteins from Parachlorella kessleri: Impact of the Cell Disruption Conditions on the Separation Performances. Sep. Purif. Technol. 2022, 287, 120570. [Google Scholar] [CrossRef]
- Gkotsis, P.K.; Zouboulis, A.I. Biomass Characteristics and Their Effect on Membrane Bioreactor Fouling. Molecules 2019, 24, 2867. [Google Scholar] [CrossRef]
- Penloglou, G.; Pavlou, A.; Kiparissides, C. Recent Advancements in Photo-Bioreactors for Microalgae Cultivation: A Brief Overview. Processes 2024, 12, 1104. [Google Scholar] [CrossRef]
- Senatore, V.; Buonerba, A.; Zarra, T.; Oliva, G.; Belgiorno, V.; Boguniewicz-Zablocka, J.; Naddeo, V. Innovative Membrane Photobioreactor for Sustainable CO2 Capture and Utilization. Chemosphere 2021, 273, 129682. [Google Scholar] [CrossRef]
- Onur, A.; Ng, A.; Batchelor, W.; Garnier, G. Multi-Layer Filters: Adsorption and Filtration Mechanisms for Improved Separation. Front. Chem. 2018, 6, 417. [Google Scholar] [CrossRef]
- Deng, E.; Chen, X.; Rub, D.; Tran, T.N.; Lin, H. Energy-Efficient Membranes for Microalgae Dewatering: Fouling Challenges and Mitigation Strategies. Sep. Purif. Technol. 2022, 296, 121382. [Google Scholar] [CrossRef]
- Zarra, T.; Senatore, V.; Zorpas, A.A.; Oliva, G.; Voukkali, I.; Belgiorno, V.; Naddeo, V. Advanced Membrane Photobioreactors in Algal CO2 Biofixation and Valuable Biomass Production: Integrative Life Cycle Assessment and Sustainability Analysis. Sustain. Chem. Pharm. 2024, 40, 101658. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Bui, X.-T.; Ngo, H.H.; Nguyen, T.-T.-D.; Nguyen, K.-Q.; Nguyen, H.-H.; Huynh, K.-P.-H.; Némery, J.; Fujioka, T.; Duong, C.H.; et al. Nutrient Recovery and Microalgae Biomass Production from Urine by Membrane Photobioreactor at Low Biomass Retention Times. Sci. Total Environ. 2021, 785, 147423. [Google Scholar] [CrossRef]
- Luo, Y.; Le-Clech, P.; Henderson, R.K. Assessment of Membrane Photobioreactor (MPBR) Performance Parameters and Operating Conditions. Water Res. 2018, 138, 169–180. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, A.; Leyva-Diaz, J.C.; Gonzalez-Lopez, J.; Poyatos, J.M. Membrane Bioreactor and Hybrid Moving Bed Biofilm Reactor-Membrane Bioreactor for the Treatment of Variable Salinity Wastewater: Influence of Biomass Concentration and Hydraulic Retention Time. Chem. Eng. J. 2018, 336, 102–111. [Google Scholar] [CrossRef]
- Gholizadeh, S.; Allahyari, Z.; Carter, R.N.; Delgadillo, L.F.; Blaquiere, M.; Nouguier-Morin, F.; Marchi, N.; Gaborski, T.R. Robust and Gradient Thickness Porous Membranes for In Vitro Modeling of Physiological Barriers. Adv. Mater. Technol. 2020, 5, 2000474. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, R.; Chu, H.; Zhou, X.; Yao, T.; Zhang, Y. Evaluation of the Performance of Different Membrane Materials for Microalgae Cultivation on Attached Biofilm Reactors. RSC Adv. 2022, 12, 1451–1459. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Verma, P.; Lavecchia, R.; Zuorro, A. Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries. Energies 2021, 14, 2282. [Google Scholar] [CrossRef]
- Meng, Y.; Li, A.; Li, H.; Shen, Z.; Ma, T.; Liu, J.; Zhou, Z.; Feng, Q.; Sun, Y. Effect of Membrane Blocking on Attached Cultivation of Microalgae. J. Clean. Prod. 2021, 284, 124695. [Google Scholar] [CrossRef]
- Wang, R.-L.; Li, M.-J.; Martin, G.J.O.; Kentish, S.E. Enhancing Direct Air Carbon Capture into Microalgae: A Membrane Sparger Design with Carbonic Anhydrase Integration. Algal Res. 2025, 85, 103875. [Google Scholar] [CrossRef]
- Xu, X.; Martin, G.J.O.; Kentish, S.E. Enhanced CO2 Bio-Utilization with a Liquid-Liquid Membrane Contactor in a Bench-Scale Microalgae Raceway Pond. J. CO2 Util. 2019, 34, 207–214. [Google Scholar] [CrossRef]
- Kerner, M.; Wolff, T.; Brinkmann, T. Efficient Supply with Carbon Dioxide from Flue Gas during Large Scale Production of Microalgae: A Novel Approach for Bioenergy Facades. Bioresour. Technol. 2024, 391, 129917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yao, L.; Maleki, E.; Liao, B.-Q.; Lin, H. Membrane Technologies for Microalgal Cultivation and Dewatering: Recent Progress and Challenges. Algal Res. 2019, 44, 101686. [Google Scholar] [CrossRef]
- Zheng, Q.; Martin, G.J.O.; Kentish, S.E. The Effects of Medium Salinity on the Delivery of Carbon Dioxide to Microalgae from Capture Solvents Using a Polymeric Membrane System. J. Appl. Phycol. 2019, 31, 1615–1622. [Google Scholar] [CrossRef]
- Moraes, L.; Rosa, G.M.; Santos, L.O.; Costa, J.A.V. Innovative Development of Membrane Sparger for Carbon Dioxide Supply in Microalgae Cultures. Biotechnol. Prog. 2020, 36, e2987. [Google Scholar] [CrossRef]
- Pasichnyk, M.; Stanovsky, P.; Polezhaev, P.; Zach, B.; Šyc, M.; Bobák, M.; Jansen, J.C.; Přibyl, M.; Bara, J.E.; Friess, K.; et al. Membrane Technology for Challenging Separations: Removal of CO2, SO2 and NOx from Flue and Waste Gases. Sep. Purif. Technol. 2023, 323, 124436. [Google Scholar] [CrossRef]
- Gkotsis, P.; Peleka, E.; Zouboulis, A. Membrane-Based Technologies for Post-Combustion CO2 Capture from Flue Gases: Recent Progress in Commonly Employed Membrane Materials. Membranes 2023, 13, 898. [Google Scholar] [CrossRef]
- He, X. Polyvinylamine-Based Facilitated Transport Membranes for Post-Combustion CO2 Capture: Challenges and Perspectives from Materials to Processes. Engineering 2021, 7, 124–131. [Google Scholar] [CrossRef]
- Han, Y.; Yang, Y.; Winston Ho, W.S. Recent Progress in the Engineering of Polymeric Membranes for CO2 Capture from Flue Gas. Membranes 2020, 10, 365. [Google Scholar] [CrossRef]
- Klinthong, W.; Yang, Y.-H.; Huang, C.-H.; Tan, C.-S. A Review: Microalgae and Their Applications in CO2 Capture and Renewable Energy. Aerosol Air Qual. Res. 2015, 15, 712–742. [Google Scholar] [CrossRef]
- Costa, J.A.V.; de Morais, M.G.; Radmann, E.M.; Santana, F.B.; Camerini, F.; de Souza, M.d.R.A.Z.; Henrard, A.A.; da Rosa, A.P.C.; Brusch, L. Biofixation of Carbon Dioxide from Coal Station Flue Gas Using Spirulina sp. LEB 18 and Scenedesmus obliquus LEB 22. African J. Microbiol. Res. 2015, 9, 2202–2208. [Google Scholar] [CrossRef]
- Duarte, J.H.; Fanka, L.S.; Costa, J.A.V. Utilization of Simulated Flue Gas Containing CO2, SO2, NO and Ash for Chlorella fusca Cultivation. Bioresour. Technol. 2016, 214, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Han, T.; Wu, R.; Liu, Z.; Ma, Y.; Guo, Z.; Hao, N.; Wang, W.; Ji, X.; Zhu, Z.; et al. A Novel System Integrating Electrolysis and Ionic Membranes (EIMs) Enables Artificial Carbon Concentration and Alleviation of Metal Cation Stress in Microalgae Cultivation. Green Chem. 2023, 25, 7273–7282. [Google Scholar] [CrossRef]
- Kim, G.Y.; Heo, J.; Kim, K.; Chung, J.; Han, J.I. Electrochemical pH Control and Carbon Supply for Microalgae Cultivation. Chem. Eng. J. 2021, 426, 131796. [Google Scholar] [CrossRef]
- Luo, Y.; Le-Clech, P.; Henderson, R.K. Simultaneous Microalgae Cultivation and Wastewater Treatment in Submerged Membrane Photobioreactors: A Review. Algal Res. 2017, 24, 425–437. [Google Scholar] [CrossRef]
- Zou, H.; Rutta, N.C.; Chen, S.; Zhang, M.; Lin, H.; Liao, B. Membrane Photobioreactor Applied for Municipal Wastewater Treatment at a High Solids Retention Time: Effects of Microalgae Decay on Treatment Performance and Biomass Properties. Membranes 2022, 12, 564. [Google Scholar] [CrossRef]
- Segredo-Morales, E.; González, E.; González-Martín, C.; Vera, L. Secondary Wastewater Effluent Treatment by Microalgal-Bacterial Membrane Photobioreactor at Long Solid Retention Times. J. Water Process Eng. 2022, 49, 103200. [Google Scholar] [CrossRef]
- Luo, Y.; Le-Clech, P.; Henderson, R.K. Assessing the Performance of Membrane Photobioreactors (MPBR) for Polishing Effluents Containing Different Types of Nitrogen. Algal Res. 2020, 50, 102013. [Google Scholar] [CrossRef]
- Soomro, R.R.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Lin, L.; Danquah, M.K. Development of a Two-Stage Microalgae Dewatering Process—A Life Cycle Assessment Approach. Front. Plant Sci. 2016, 7, 113. [Google Scholar] [CrossRef]
- Kumar, A.; Yuan, X.; Sahu, A.K.; Dewulf, J.; Ergas, S.J.; Van Langenhove, H. A Hollow Fiber Membrane Photo-Bioreactor for CO2 Sequestration from Combustion Gas Coupled with Wastewater Treatment: A Process Engineering Approach. J. Chem. Technol. Biotechnol. 2010, 85, 387–394. [Google Scholar] [CrossRef]
- Borges, J.A.; Rosa, G.M.; Meza, L.H.R.; Henrard, A.A.; Souza, M.R.A.Z.; Costa, J.A.V. Spirulina sp. LEB-18 Culture Using Effluent from the Anaerobic Digestion. Braz. J. Chem. Eng. 2013, 30, 277–288. [Google Scholar] [CrossRef]
- Takabe, Y.; Himeno, S.; Okayasu, Y.; Minamiyama, M.; Komatsu, T.; Nanjo, K.; Yamasaki, Y.; Uematsu, R. Feasibility of Microalgae Cultivation System Using Membrane-Separated CO2 Derived from Biogas in Wastewater Treatment Plants. Biomass and Bioenergy 2017, 106, 191–198. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Cai, C.; Zhou, Y. Membrane Photobioreactor for Biogas Capture and Conversion—Enhanced Microbial Interaction in Biofilm. Bioresour. Technol. 2025, 418, 131999. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-L.; Li, M.-J.; Li, D.; Yang, Y.-W. The Synergy of Light/Fluid Flow and Membrane Modification of a Novel Membrane Microalgal Photobioreactor for Direct Air Carbon Capture. Appl. Energy 2022, 328, 120133. [Google Scholar] [CrossRef]
- Zhao, Z.; Muylaert, K.; Vankelecom, I.F.J. Combining Patterned Membrane Filtration and Flocculation for Economical Microalgae Harvesting. Water Res. 2021, 198, 117181. [Google Scholar] [CrossRef]
- Razak, N.N.A.N.; Rahmawati, R.; Bilad, M.R.; Pratiwi, A.E.; Elma, M.; Nawi, N.I.M.; Jaafar, J.; Lam, M.K. Finned Spacer for Enhancing the Impact of Air Bubbles for Membrane Fouling Control in Chlorella vulgaris Filtration. Bioresour. Technol. Rep. 2020, 11, 100429. [Google Scholar] [CrossRef]
- Zheng, M.; Yang, Y.; Qiao, S.; Zhou, J.; Quan, X. A Porous Carbon-Based Electro-Fenton Hollow Fiber Membrane with Good Antifouling Property for Microalgae Harvesting. J. Memb. Sci. 2021, 626, 119189. [Google Scholar] [CrossRef]
- Mkpuma, V.O.; Moheimani, N.R.; Ennaceri, H. Microalgal Dewatering with Focus on Filtration and Antifouling Strategies: A Review. Algal Res. 2022, 61, 102588. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; García-Depraect, O. Membrane-Based Harvesting Processes for Microalgae and Their Valuable-Related Molecules: A Review. Membranes 2021, 11, 585. [Google Scholar] [CrossRef]
- Liao, Y.; Bokhary, A.; Maleki, E.; Liao, B. A Review of Membrane Fouling and Its Control in Algal-Related Membrane Processes. Bioresour. Technol. 2018, 264, 343–358. [Google Scholar] [CrossRef]
- Marchese, A.; Lima, S.; Cosenza, A.; Giambalvo, F.; Scargiali, F. Effects of Light Quality Adjustment in Microalgal Cultivation: Flashing Light and Wavelength Shifts in Photobioreactor Design. Processes 2025, 13, 1159. [Google Scholar] [CrossRef]
- Wang, L.; Pan, B.; Gao, Y.; Li, C.; Ye, J.; Yang, L.; Chen, Y.; Hu, Q.; Zhang, X. Efficient Membrane Microalgal Harvesting: Pilot-Scale Performance and Techno-Economic Analysis. J. Clean. Prod. 2019, 218, 83–95. [Google Scholar] [CrossRef]
- Monte, J.; Sá, M.; Galinha, C.F.; Costa, L.; Hoekstra, H.; Brazinha, C.; Crespo, J.G. Harvesting of Dunaliella salina by Membrane Filtration at Pilot Scale. Sep. Purif. Technol. 2018, 190, 252–260. [Google Scholar] [CrossRef]
- Monte, J.; Bernardo, J.; Sá, M.; Parreira, C.; Galinha, C.F.; Costa, L.; Casanovas, C.; Brazinha, C.; Crespo, J.G. Development of an Integrated Process of Membrane Filtration for Harvesting Carotenoid-Rich Dunaliella salina at Laboratory and Pilot Scales. Sep. Purif. Technol. 2020, 233, 116021. [Google Scholar] [CrossRef]
- Ennaceri, H.; Fischer, K.; Schulze, A.; Moheimani, N.R. Membrane Fouling Control for Sustainable Microalgal Biodiesel Production: A Review. Renew. Sustain. Energy Rev. 2022, 161, 112335. [Google Scholar] [CrossRef]
- Zhao, Z.; Blockx, J.; Muylaert, K.; Thielemans, W.; Szymczyk, A.; Vankelecom, I.F.J. Exploiting Flocculation and Membrane Filtration Synergies for Highly Energy-Efficient, High-Yield Microalgae Harvesting. Sep. Purif. Technol. 2022, 296, 121386. [Google Scholar] [CrossRef]
- Moser, P.B.; dos Anjos Silva, G.R.; Lima, L.S.F.; Moreira, V.R.; Lebron, Y.A.R.; de Paula, E.C.; Amaral, M.C.S. Effect of Organic and Inorganic Draw Solution on Recalcitrant Compounds Build up in a Hybrid Ultrafiltration-Osmotic Membrane Reactor Treating Refinery Effluent. Chem. Eng. J. 2021, 403, 126374. [Google Scholar] [CrossRef]
- Fulazzaky, M.; Setiadi, T.; Fulazzaky, M.A. An Evaluation of the Oilfield-Produced Water Treatment by the Membrane Bioreactor. J. Environ. Chem. Eng. 2020, 8, 104417. [Google Scholar] [CrossRef]
- Huang, D.; Li, M.-J.; Wang, R.-L.; Yang, Y.-W.; Tao, W.-Q. Advanced Carbon Sequestration by the Hybrid System of Photobioreactor and Microbial Fuel Cell with Novel Photocatalytic Porous Framework. Bioresour. Technol. 2021, 333, 125182. [Google Scholar] [CrossRef]
- Merlo, S.; Gabarrell Durany, X.; Pedroso Tonon, A.; Rossi, S. Marine Microalgae Contribution to Sustainable Development. Water 2021, 13, 1373. [Google Scholar] [CrossRef]
- Sarker, N.K.; Kaparaju, P. Microalgal Bioeconomy: A Green Economy Approach Towards Achieving Sustainable Development Goals. Sustainability 2024, 16, 11218. [Google Scholar] [CrossRef]
- Sutherland, D.L.; McCauley, J.; Labeeuw, L.; Ray, P.; Kuzhiumparambil, U.; Hall, C.; Doblin, M.; Nguyen, L.N.; Ralph, P.J. How Microalgal Biotechnology Can Assist with the UN Sustainable Development Goals for Natural Resource Management. Curr. Res. Environ. Sustain. 2021, 3, 100050. [Google Scholar] [CrossRef]
- Agarwalla, A.; Mohanty, K. A Critical Review on the Application of Membrane Technology in Microalgal Harvesting and Extraction of Value-Added Products. Sep. Purif. Technol. 2024, 344, 127180. [Google Scholar] [CrossRef]

| System | Biomass Concentration (g·L−1) | Volumetric Productivity (g·m−2·d−1) | Energy Consumption (kWh·m−3) | Operating Costs | Water Consumption | Reference |
|---|---|---|---|---|---|---|
| MPBR | 0.6–1.0 | 5.08 | 0.84–0.91 | Economically viable | Reduction of up to 77% | [17] |
| MPBR with MMV | 0.7–1.4 | - | 0.77–0.84 | Economically attractive | Similar to MPBR | [19] |
| Adhered cultivation | Up to 10× higher than suspended system | 50–80 | - | Reduction in cultivation costs | Minimum water requirement | [20] |
| AMBR | Up to 5.0 | - | - | Reduction in nutrient and treatment costs | Reuse of wastewater | [10,21] |
| Species | Wastewater Type | HRT (d) | SRT (d) | Membrane Type/Material | Aeration Rate (L min−1) | Permeate Flux (L m−2 h−1) | Fouling Effect | Control Strategy | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| C. vulgaris | Synthetic municipal sewage | 1 to 5 | 5 to 25 | Flat-sheet, chlorinated PE | 0.003 | 2.6–13 | Flux-induced fouling at short HRT | Air scouring for fouling control | [18] |
| C. vulgaris (CPCC 90) | Synthetic municipal wastewater | 2.9 | 50 | Flat sheet, PVDF (0.1 µm) | 7.5 | 7.3 | ** SMP induced fouling mitigated by larger flocs and reduced EPS | Biomass harvesting, flocculation, and SMP control | [58] |
| C. vulgaris (CS-42) | Synthetic municipal wastewater (secondary effluents) | 1 | 18 | Hollow fiber, PVDF (0.04 µm) | 2.0 | 4.5 | Transmembrane pressure rise due to low filterability and biopolymer-induced biocake | Reduced SRT and culture control to limit biopolymer production | [60] |
| Indigenous microalgae–bacteria consortia (Chlorella, Scenedesmus, Nitzschia, Navicula) | Domestic secondary effluent (activated sludge system) | 0.75, 2, and 5 | 20, 40, and 80 | Hollow fiber PVDF (0.04 μm) | 6 (5 L/min intermittent + 1 L/min constant) | 10 | *** TMP rise from protein-rich * BPCs and biocake under high SRT/HRT (40 d/0.75 d) conditions | SRT extension (80 d) and moderate HRT (2–5 d) to reduce BPCs; physical cleaning and aeration to control biocake | [59] |
| Spirulina sp. TISTR 8875 | MBR-treated municipal wastewater | 6.4 to 9.7 | 20, 40, 60, 80 d, and infinite (no sludge removal) | Submerged flat-sheet microfiltration membranes (0.4 μm) | 4–6 | ~0.83 | Stable operation with controlled fouling | Passive control via high SRT, aeration, and lighting conditions. | [23] |
| System | Strain | Substrate or Application | Biomass Production (g/L) | Removal | Energy Demand | Reference | |
|---|---|---|---|---|---|---|---|
| CO2 | Nitrogen | ||||||
| MPBR | C. vulgaris | Gas stream (15% CO2) | – | 80% | – | – | [31] |
| SFDM–MPBR | C. vulgaris | Synthetic wastewater | – | – | 100% (nitrate) | – | [31] |
| HFMPB | S. platensis | 2% CO2 + wastewater | 2.1 | 85% | 68% | Low pressure | [62] |
| MPBR + biofilm | Mixed culture | Biogas (CH4/CO2) | – | +12% CO2 fix. | – | – | [65] |
| Integrated Application | Related SDGs | Contribution of Membrane—Microalgae Systems | Reference |
|---|---|---|---|
| Bioenergy and Biofuels | 7, 13 | CO2 biofixation and biomass valorization for renewable fuel production | [82,83] |
| Wastewater Treatment | 6, 12, 14 | Nutrient removal, water reuse, and effluent polishing Via MPBRs | [8,10] |
| Food and Feed Applications | 2, 12 | Biomass for feed/supplements; limited food applications | [83,84,85] |
| Circular Economy and Resource Recovery | 9, 12 | CO2 capture Integration, nutrient recycling, and waste minimization | [8,83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, M.G.; Rosa, G.M.; Moraes, L.; Lopes, L.C.; Costa, J.A.V. Membrane Technologies for Bioengineering Microalgae: Sustainable Applications in Biomass Production, Carbon Capture, and Industrial Wastewater Valorization. Membranes 2025, 15, 205. https://doi.org/10.3390/membranes15070205
Morais MG, Rosa GM, Moraes L, Lopes LC, Costa JAV. Membrane Technologies for Bioengineering Microalgae: Sustainable Applications in Biomass Production, Carbon Capture, and Industrial Wastewater Valorization. Membranes. 2025; 15(7):205. https://doi.org/10.3390/membranes15070205
Chicago/Turabian StyleMorais, Michele Greque, Gabriel Martins Rosa, Luiza Moraes, Larissa Chivanski Lopes, and Jorge Alberto Vieira Costa. 2025. "Membrane Technologies for Bioengineering Microalgae: Sustainable Applications in Biomass Production, Carbon Capture, and Industrial Wastewater Valorization" Membranes 15, no. 7: 205. https://doi.org/10.3390/membranes15070205
APA StyleMorais, M. G., Rosa, G. M., Moraes, L., Lopes, L. C., & Costa, J. A. V. (2025). Membrane Technologies for Bioengineering Microalgae: Sustainable Applications in Biomass Production, Carbon Capture, and Industrial Wastewater Valorization. Membranes, 15(7), 205. https://doi.org/10.3390/membranes15070205






