Electrocatalytic PANI-Encapsulated Aluminum Silicate/Ceramic Membranes for Efficient and Energy-Saving Removal of 4-Chlorophenol in Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.1.1. Synthesis of Polyaniline (PANI)
2.1.2. Pretreatment of Aluminum Silicate Powder
2.1.3. Synthesis of Polyaniline-Coated Aluminum Silicate (ASP)
2.2. Fabrication and Characterization of ASP/CM
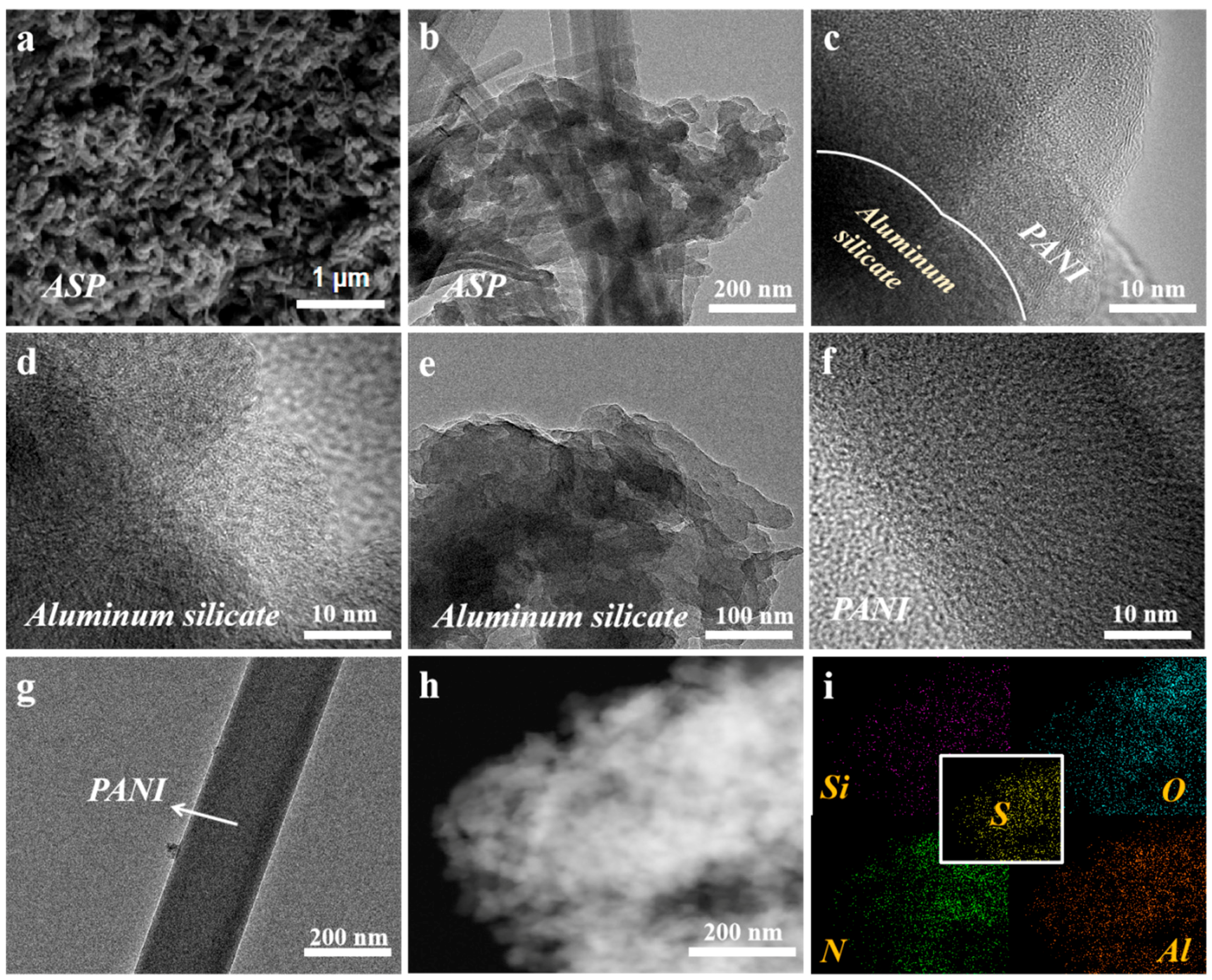
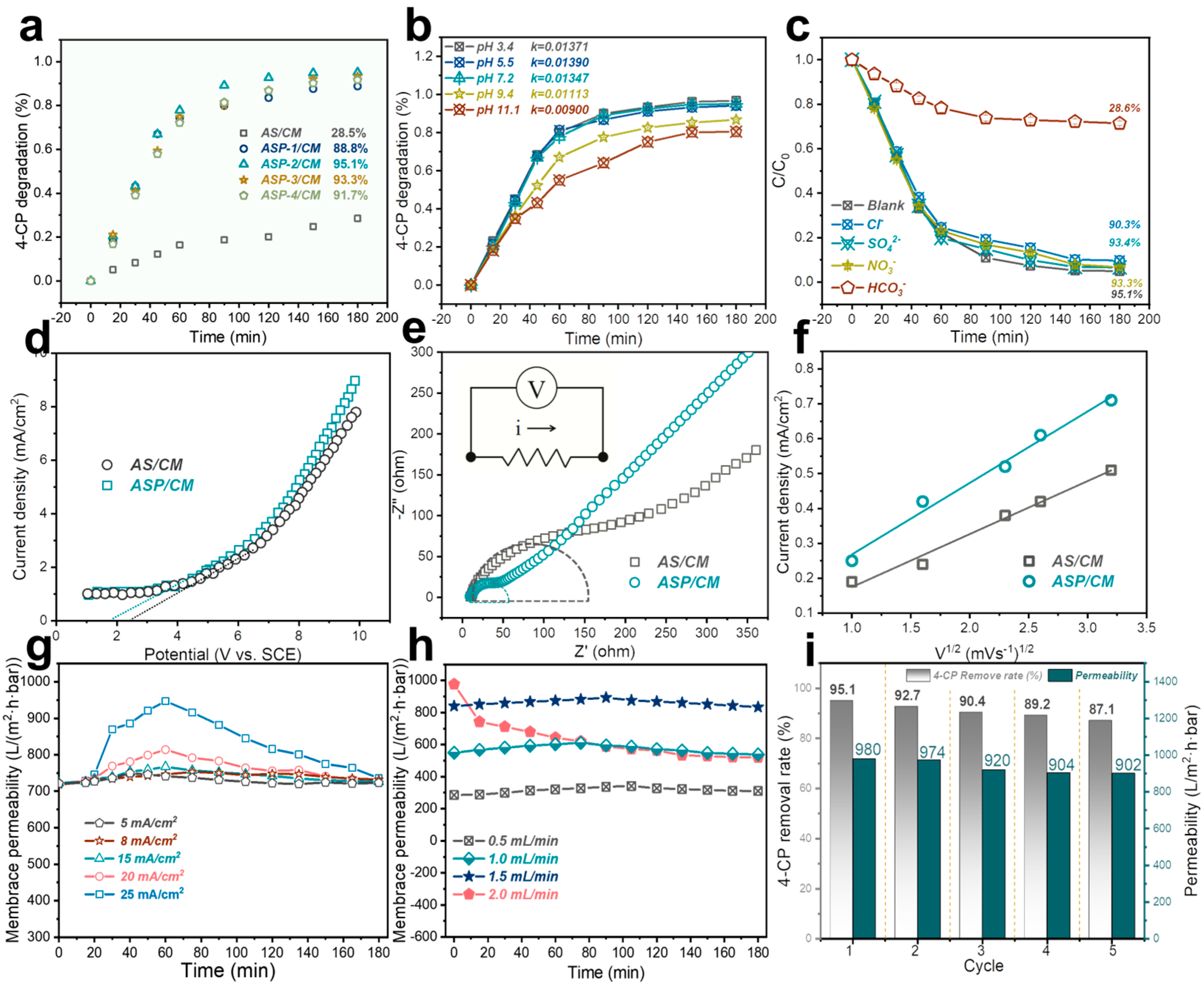
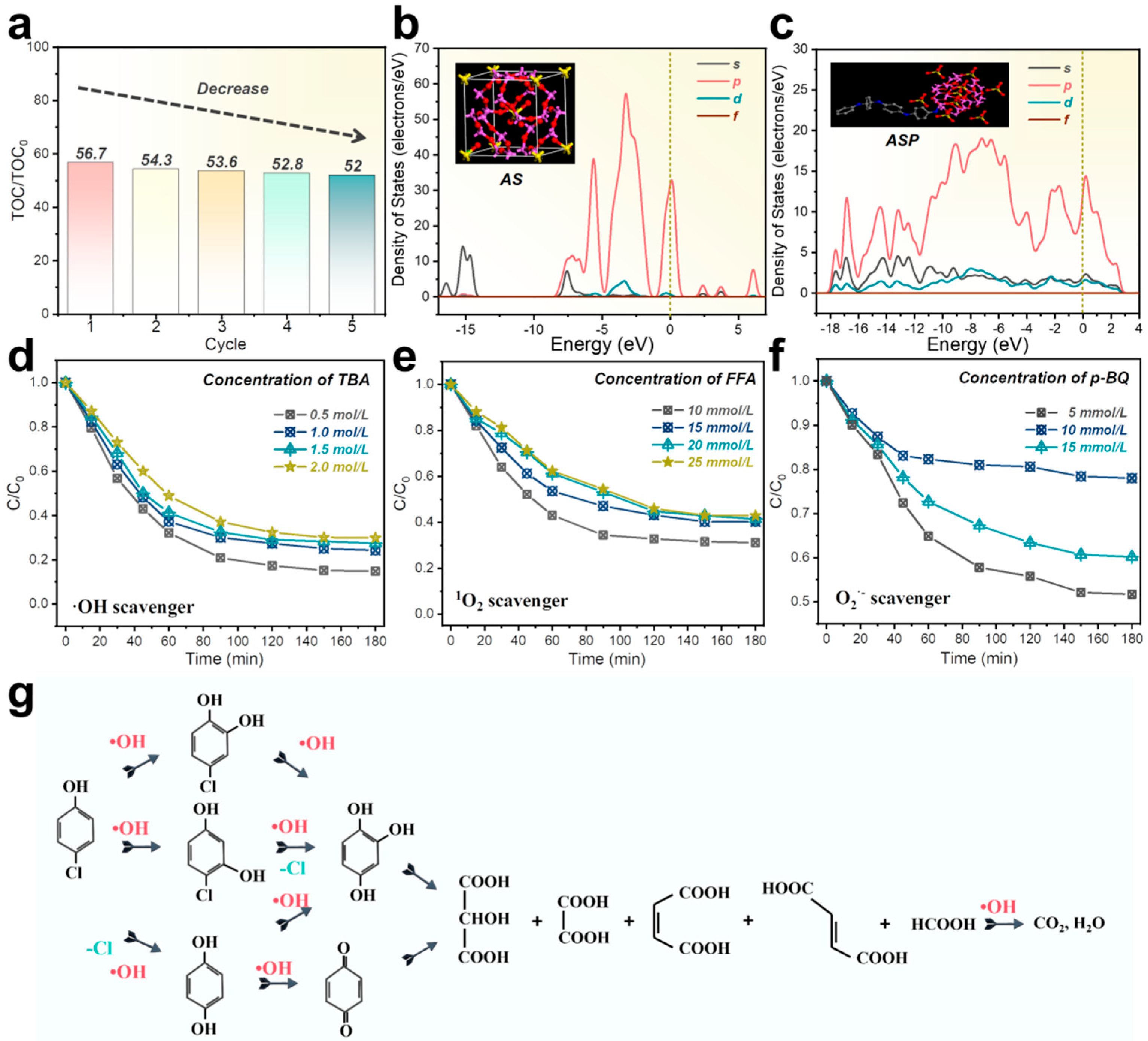
3. Results and Discussion
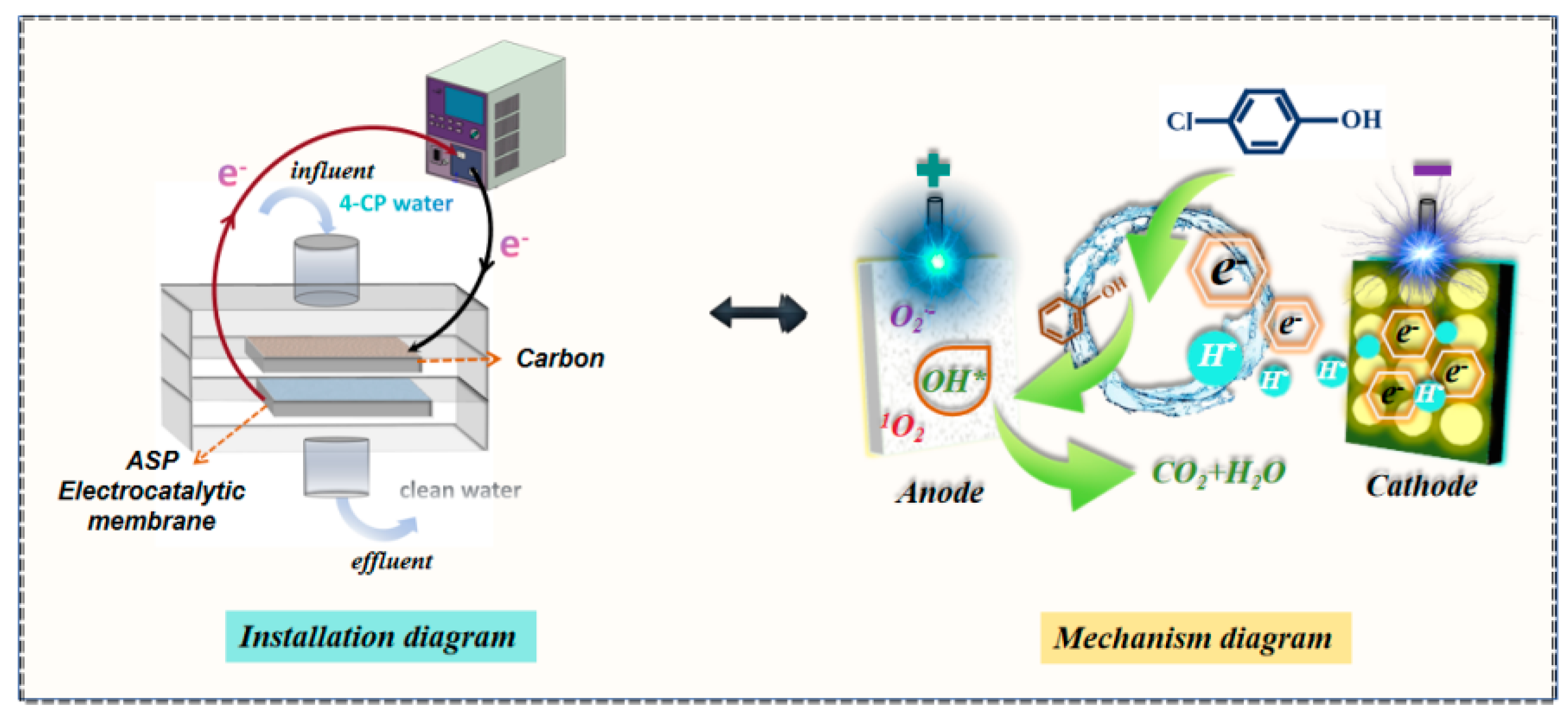
Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.L.; Fang, G.D.; Liu, P.; Zhou, D.M.; Ma, C.; Zhang, D.J.; Zhan, J.H. Fe3O4@β-CD nanocomposite as heterogeneous fenton-like catalyst for enhanced degradation of 4-chlorophenol (4-CP). Appl. Catal. B Environ. 2016, 188, 113–122. [Google Scholar] [CrossRef]
- Du, Y.X.; Zhou, M.H.; Lei, L.C. Novel photocatalysis oxidation system UV/Fe2+/air to degrade 4-CP wastewater. Sci. Bull. 2013, 50, 2118–2120. [Google Scholar]
- Wei, K.; Li, K.X.; Zeng, Z.X.; Dai, Y.H.; Yan, L.S.; Guo, H.Q.; Luo, X.B. Synergistic photocatalytic effect of porous g-C3N4 in a Cr(VI)/4-chlorophenol composite pollution system. Chin. J. Catal. 2017, 38, 1804–1811. [Google Scholar] [CrossRef]
- Liu, X.L.; Mo, J.L.; Wu, W.H.; Song, H.N.; Nie, S.X. Triboelectric pulsed direct-current enhanced radical generation for efficient degradation of organic pollutants in wastewater. Appl. Catal. B Environ. 2022, 312, 121422. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, S.Y. Synergetic multiple free radicals lower the organohalide conversion barrier and potentiate effective contaminant mineralization. Appl. Catal. B Environ. 2024, 343, 123554. [Google Scholar] [CrossRef]
- Shi, C.F.; Nie, L.Y.; Hu, K.; Zheng, C.; Xu, C.M.; Song, H.; Wang, G.X. New insights into peroxydisulfate activation by nanostructured and bulky carbons. Appl. Catal. B Environ. 2023, 325, 122371. [Google Scholar] [CrossRef]
- You, Y. Chemical tools for the generation and detection of singlet oxygen. Org. Biomol. Chem. 2018, 16, 4044–4060. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar]
- Ma, B.; Lu, B.; Tang, H.; Wang, H.; Bian, Z. Square-wave pulsed potential driven electrocatalytic degradation of 4-chlorophenol using Fe-Ni/rGO/PPy@NF three dimensional electrode. J. Hazard. Mater. 2024, 480, 136054. [Google Scholar]
- Gao, F.; Nebel, C.E. Electrically conductive diamond membrane for electrochemical separation processes. ACS Appl. Mater. Interfaces 2016, 8, 18640–18646. [Google Scholar]
- Liu, Z.M.; Zhu, M.; Zhao, L.; Deng, C.; Ma, J.; Wang, Z.; Liu, H.B.; Wang, H. Aqueous tetracycline degradation by coal-based carbon electrocatalytic filtration membrane: Effect of nano antimony-doped tin dioxide coating. Chem. Eng. J. 2017, 314, 59–68. [Google Scholar]
- Sun, M.; Li, J. Graphene oxide membranes: Functional structures, preparation and environmental applications. Nano Today 2018, 20, 121–137. [Google Scholar] [CrossRef]
- Fei, W.Q.; Zhang, C.M.; Feng, J.J.; Li, Q.; Wan, Z.H.; Sun, X.F. Interface-induced modulation of electrocatalytic mechanisms in electrochemical membrane systems. J. Membr. Sci. 2025, 717, 123634. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, J.; Wang, Z.; Yang, Z.; Yang, W.; Yin, Z. Defective MOFs-based electrocatalytic self-cleaning membrane for wastewater reclamation: Enhanced antibiotics removal, membrane fouling control and mechanisms. Water Res. 2022, 220, 118635. [Google Scholar]
- Yin, Z.; Liu, Y.; Zhou, S.; Yang, Z.; Yang, W. Constructing zirconium based metal-organic frameworks based electrically-driven self-cleaning membrane for removal of tetracycline: Effect of ligand substitution. Chem. Eng. J. 2022, 450, 138100. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Winter, L.R.; Zhao, Y.; Ma, W.; Hedtke, T.; Kim, J.; Elimelech, M. Electrified membranes for water treatment applications. ACS EST Engg. 2021, 1, 725–752. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Zhu, Y.; Chen, X.; Gui, S.; Ma, A.; Li, J. Electrochemical reactor with carbon membrane electrodes for efficient phenol removal via anode and cathode synergism. npj Clean Water 2025, 8, 2. [Google Scholar]
- Tognia, M.; Feng, G.; Pan, Z.; Fan, X.; Stephane, M.N.; Song, C.; Wang, T. Prospects of modeling and simulations in membrane-electrodes coupled with electrochemical advanced oxidation processes for organic wastewater treatment. Sep. Purif. 2023, 323, 124372. [Google Scholar] [CrossRef]
- Luo, H.; Fan, X.; Tu, J.; He, J.; Li, X.; Xue, J.; Ye, F.; Cheng, L. Dual-spectrum bands compatible Ti-Si-O film prepared by magnetron co-sputtering. Appl. Surf. Sci. 2023, 609, 155284. [Google Scholar]
- Yin, Y.; Zhu, Y.; Liao, P.; Yuan, X.; Jia, J.; Lan, C.; Li, C. Co-sputtering construction of Gd-doped WO3 nano-stalagmites film for bi-funcional electrochromic and energy storage applications. Chem. Eng. J. 2024, 487, 150615. [Google Scholar] [CrossRef]
- Wei, B.; Tang, G.; Liang, H.; Qi, Z.; Zhang, D.; Hu, W.; Shen, H.; Wang, Z. Bimetallic vanadium-molybdenum nitrides using magnetron co-sputtering as alkaline hydrogen evolution catalyst. Electrochem. Commun. 2018, 93, 166–170. [Google Scholar]
- Bai, S.; Sun, C.; Wan, P.; Wang, C.; Luo, R.; Li, Y.; Liu, J.; Sun, X. Transparent conducting films of hierarchically nanostructured polyaniline networks on flexible substrates for high-performance gas sensors. Small 2015, 11, 306–310. [Google Scholar] [PubMed]
- Abdel Moamen, O.A.; Murad, G.A.; Hassan, H.S. Experimental and theoretical study for the sorption of Ni2+ and Cd2+ onto phosphoryl functionalized aluminum silicate composite. Sep. Purif. Technol. 2024, 344, 127186. [Google Scholar]
- Ju, Z.; Jin, C.; Yuan, H.; Yang, T.; Sheng, O.; Liu, T.; Liu, Y.; Wang, Y.; Ma, F.; Zhang, W.; et al. A fast-ion conducting interface enabled by aluminum silicate fibers for stable Li metal batteries. Chem. Eng. J. 2021, 408, 128016. [Google Scholar]
- Jo, H.; Kim, H.; Yoon, S. Synthesis and characterization of mesoporous aluminum silicate and its adsorption for Pb (II) ions and methylene blue in aqueous solution. Materials 2022, 15, 3562. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Feng, J.; Wei, T.; Ren, Y.; Ma, J. In-situ polymerization of polyaniline modified by phosphotungstic acid on the surface of hollow carbon for two-way efficient reduction of nitrate in water. Chem. Eng. J. 2022, 430, 133175. [Google Scholar]
- Wang, S.; Zhao, Y.; Feng, J.; Wei, T.; Ma, J.; Ren, Y.; Bai, C. ZnS quantum dots implanted polyaniline-wrapped corn straw catalysts for efficient photocatalytic nitrate reduction without external addition of hole scavengers. Chem. Eng. J. 2023, 455, 140787. [Google Scholar]
- Golovakhin, V.; Kim, E.Y.; Novgorodtseva, O.N.; Maksimovskiy, E.A.; Ukhina, A.V.; Ishchenko, A.V.; Bannov, A.G. Treatment of multi-walled carbon nanotubes with dichromic acid: Oxidation and appearance of intercalation. Membranes 2023, 13, 729. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Yin, Z.; Yang, Z.; Chen, Z.; Han, H.; Yu, Q.; Du, M. Hydrogen flux inhibition of Pd-Ru membranes under exposure to NH3. Membranes 2024, 14, 59. [Google Scholar] [CrossRef]
- Low, J.X.; Jiang, C.J.; Cheng, B.; Wageh, W.S.; Al-Ghamdi, A.A.; Yu, J.G. A review of direct Z-scheme photocatalysts. Small Methods 2017, 1, 170080. [Google Scholar]
- Jin, J.; Yu, J.G.; Guo, D.P.; Cui, C.; Ho, W.K. A hierarchical Z-scheme CdS-WO3 photocatalyst with enhanced CO2 reduction activity. Small 2015, 11, 5262–5271. [Google Scholar]
- Yang, J.; Hao, J.; Xu, S.; Dai, J.; Wang, Y.; Pang, X. Visible-light-driven photocatalytic degradation of 4-CP and the synergistic reduction of Cr(VI) on one-pot synthesized amorphous Nb2O5 nanorods/graphene heterostructured composites. Chem. Eng. J. 2018, 353, 100–114. [Google Scholar]
- Liu, Y.; Liu, Y.; Yang, Z.; Wang, J. Fenton degradation of 4-chlorophenol using H2O2 in situ generated by Zn-CNTs/O2 system. RSC Adv. 2017, 7, 49985–49994. [Google Scholar]
- García-Fernández, M.J.; Pastor-Blasa, M.M.; Epron, F.; Sepúlveda-Escribano, A. Proposed mechanisms for the removal of nitrate from water by platinum catalysts supported on polyaniline and polypyrrole. Appl. Catal. B Environ. 2018, 225, 162–171. [Google Scholar]
- Li, X.; Yu, L.; Zhao, W.; Shi, Y.; Yu, L.; Dong, Y.; Zhu, Y.; Fu, Y.; Liu, X.; Fu, F. Prism-shaped hollow carbon decorated with polyaniline for microwave absorption. Chem. Eng. J. 2020, 379, 122393. [Google Scholar]
- Qiao, Q.; Singh, S.; Lo, S.; Jin, J.; Yu, Y.C.; Wang, L. Effect of current density and pH on the electrochemically generated active chloro species for the rapid mineralization of p-substituted phenol. Chemosphere 2021, 275, 129848. [Google Scholar]
- Gong, J.; Lee, C.; Kim, E.; Chang, Y.; Chang, Y. Enhancing the reactivity of bimetallic Bi/Fe(0) by citric acid for remediation of polluted water. J. Hazard. Mater. 2016, 310, 135–142. [Google Scholar]
- Murugan, K.P.; Sabarinathan, S.; Prabhakaran, N.; Swarnalatha, S. Valorization of hazardous chrome tanned leather buffing waste for the production of Cr2O3/carbon/TiO2 composite semiconductors with the removal of chlorophenol from its wastewater. Chem. Eng. J. 2023, 468, 143547. [Google Scholar]
- Yan, X.; Zhu, T.; Fan, X.; Sun, Y. Removal of p-chlorophenol in mist by DC corona discharge plasma. Chem. Eng. J. 2014, 245, 41–46. [Google Scholar]
- Kang, C.; Gao, H.; Guo, P.; Zhang, G.; Tang, X.; Peng, F.; Liu, X. Kinetics and mechanism of para-chlorophenol photoconversion with the presence of nitrite in ice. J. Hazard. Mater. 2009, 170, 163–168. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Huang, T.; Ma, H.; Liu, Z.; Xia, H.; Sun, Z.; Ma, J.; Zhao, Y. Electrocatalytic PANI-Encapsulated Aluminum Silicate/Ceramic Membranes for Efficient and Energy-Saving Removal of 4-Chlorophenol in Wastewater. Membranes 2025, 15, 114. https://doi.org/10.3390/membranes15040114
Wang S, Huang T, Ma H, Liu Z, Xia H, Sun Z, Ma J, Zhao Y. Electrocatalytic PANI-Encapsulated Aluminum Silicate/Ceramic Membranes for Efficient and Energy-Saving Removal of 4-Chlorophenol in Wastewater. Membranes. 2025; 15(4):114. https://doi.org/10.3390/membranes15040114
Chicago/Turabian StyleWang, Shuo, Tianhao Huang, Haoran Ma, Zihan Liu, Houbing Xia, Zhiqiang Sun, Jun Ma, and Ying Zhao. 2025. "Electrocatalytic PANI-Encapsulated Aluminum Silicate/Ceramic Membranes for Efficient and Energy-Saving Removal of 4-Chlorophenol in Wastewater" Membranes 15, no. 4: 114. https://doi.org/10.3390/membranes15040114
APA StyleWang, S., Huang, T., Ma, H., Liu, Z., Xia, H., Sun, Z., Ma, J., & Zhao, Y. (2025). Electrocatalytic PANI-Encapsulated Aluminum Silicate/Ceramic Membranes for Efficient and Energy-Saving Removal of 4-Chlorophenol in Wastewater. Membranes, 15(4), 114. https://doi.org/10.3390/membranes15040114







