Impacts of Natural Organic Matter and Dissolved Solids on Fluoride Retention of Polyelectrolyte Multilayer-Based Hollow Fiber Nanofiltration Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Membrane and Chemicals
2.2. Preparation and Characterization of SMW
2.3. Calculation of Permeability, Retention, and Recovery
2.4. Lab-Scale Performance Experiments Using NF Membranes
2.4.1. Routine Performance Testing
2.4.2. Studying the Effect of Feed Salinity on the Separation Efficiency of dNF40 Membranes
2.4.3. Mini-Plant Filtration Tests Using SMW with NOM at Different Initial Concentrations Employing a Constant Crossflow Velocity
2.4.4. Mini-Plant Filtration Tests Using SMW with NOM at a Constant Initial Concentration Employing Different Crossflow Velocities
2.4.5. Mini-Plant Filtration Tests Using SMW with NOM at a Constant Initial Concentration Employing Constant Crossflow Velocity at Different Recoveries
3. Results and Discussion
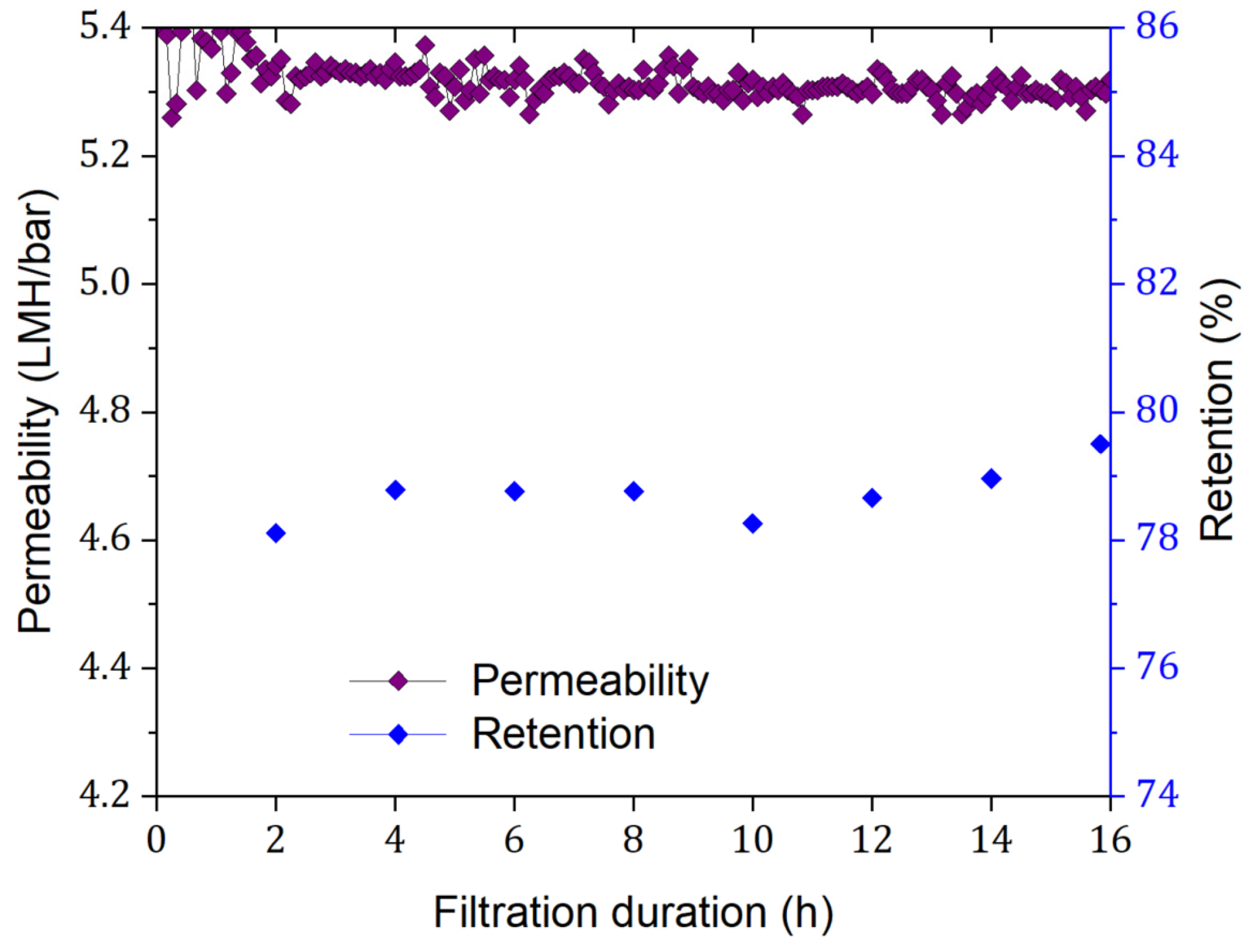
3.1. Routine Membrane Performance Testing
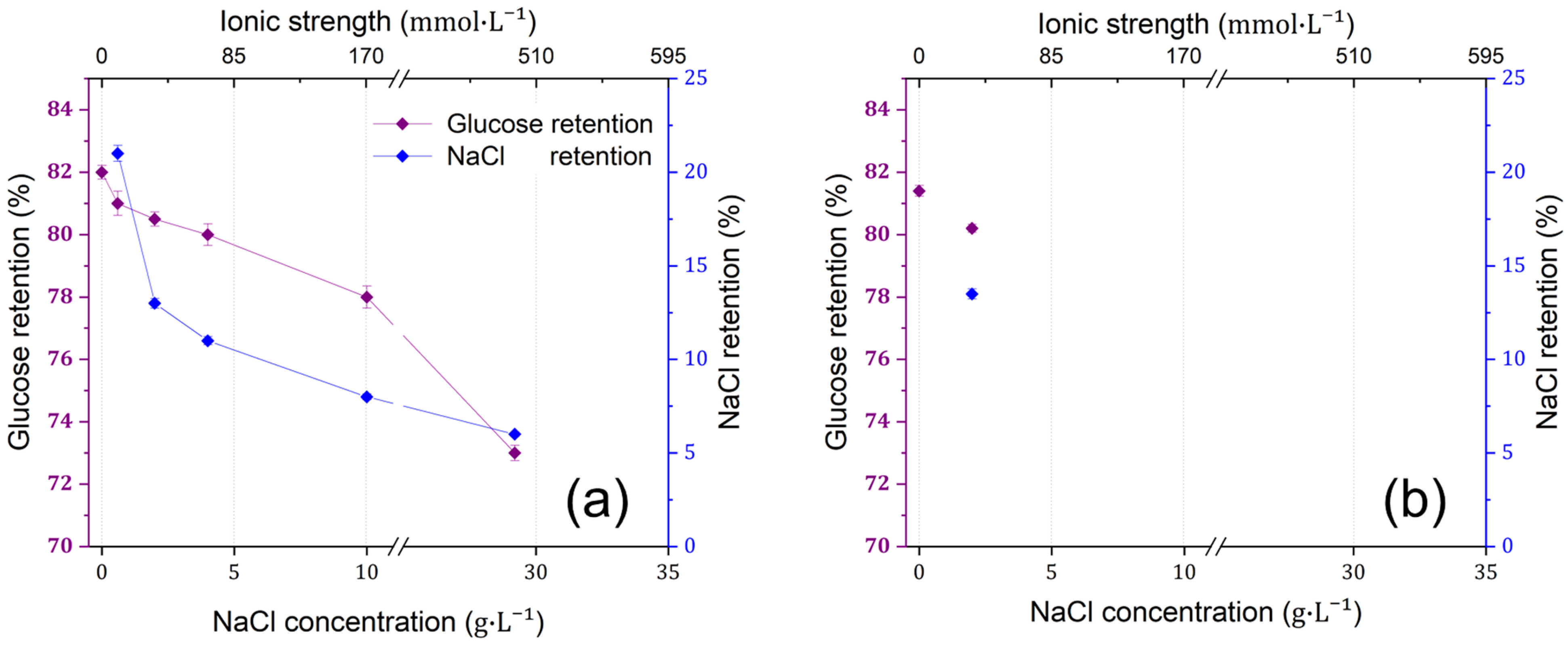
3.2. The Effect of Feed Salinity on the Separation Efficiency of dNF40 Membranes
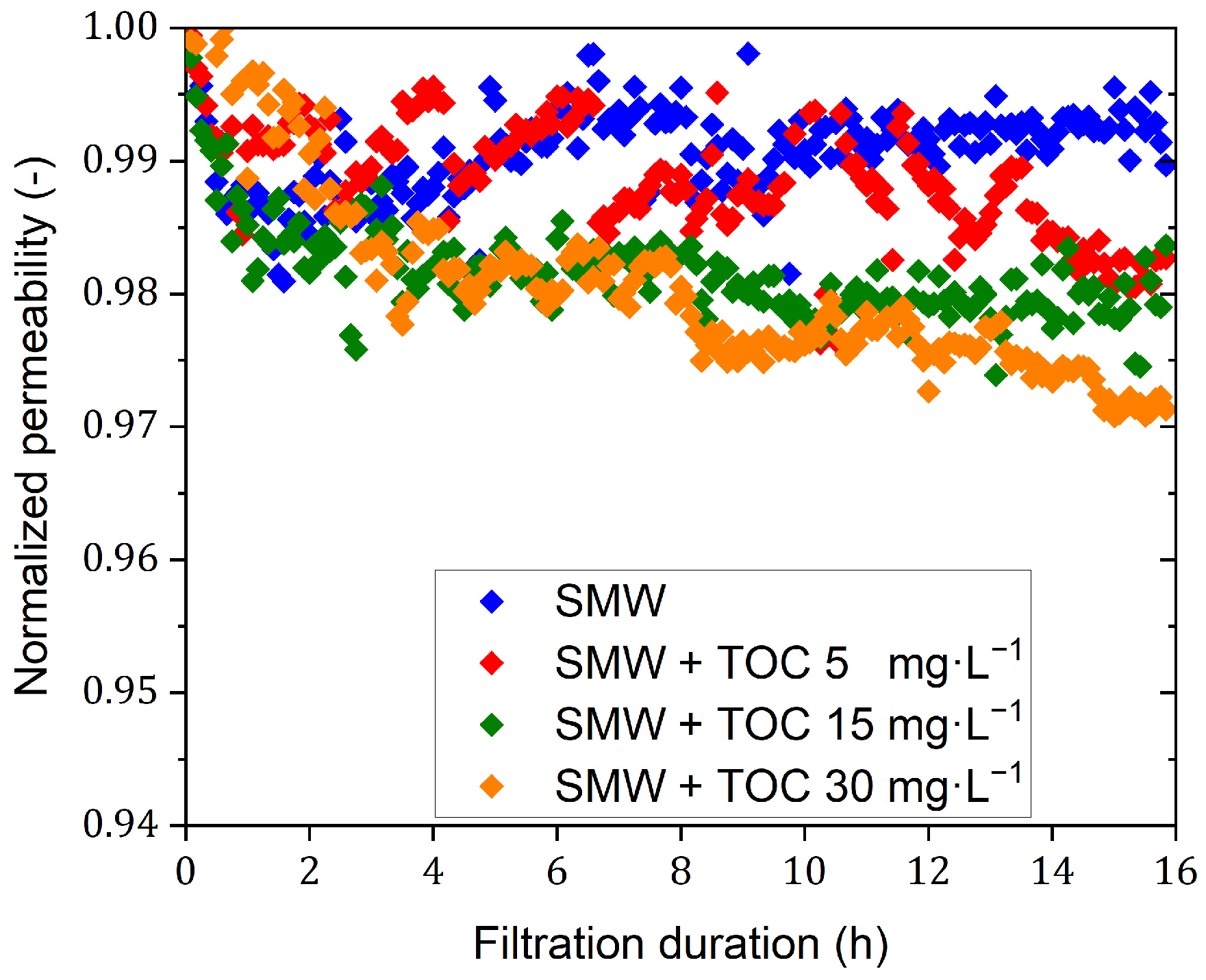
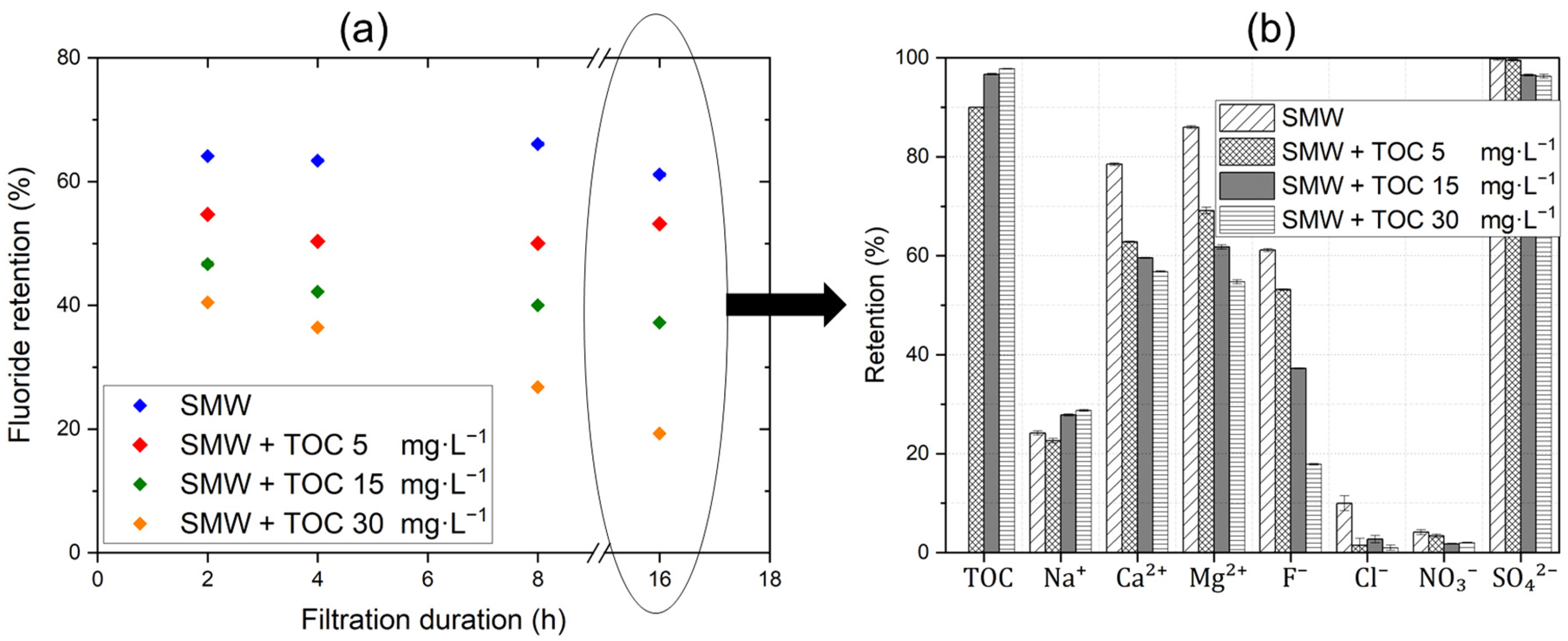
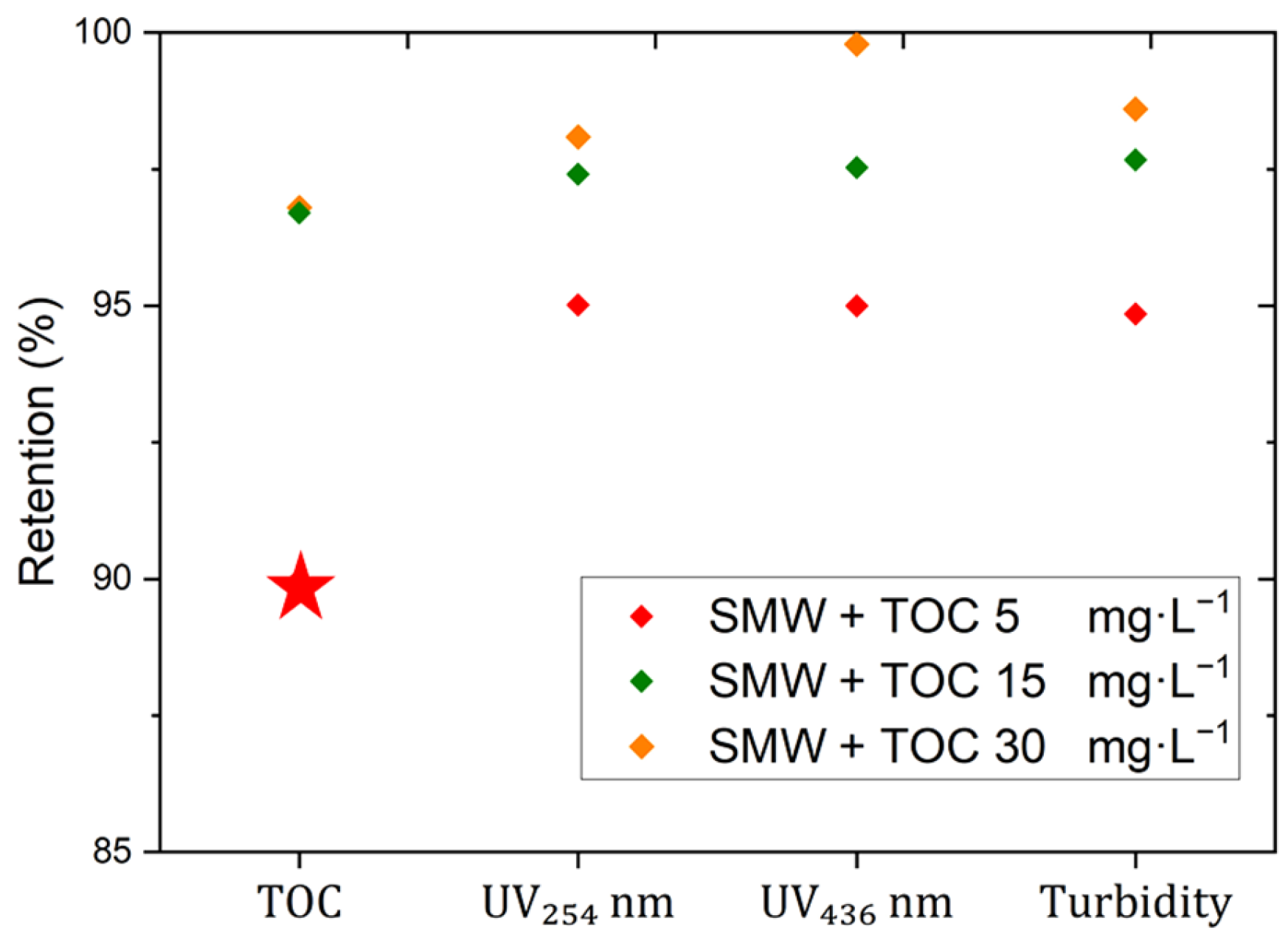
3.3. Mini-Plant Filtration Tests Using SMW with NOM at Different Concentrations Employing a Constant Crossflow Velocity
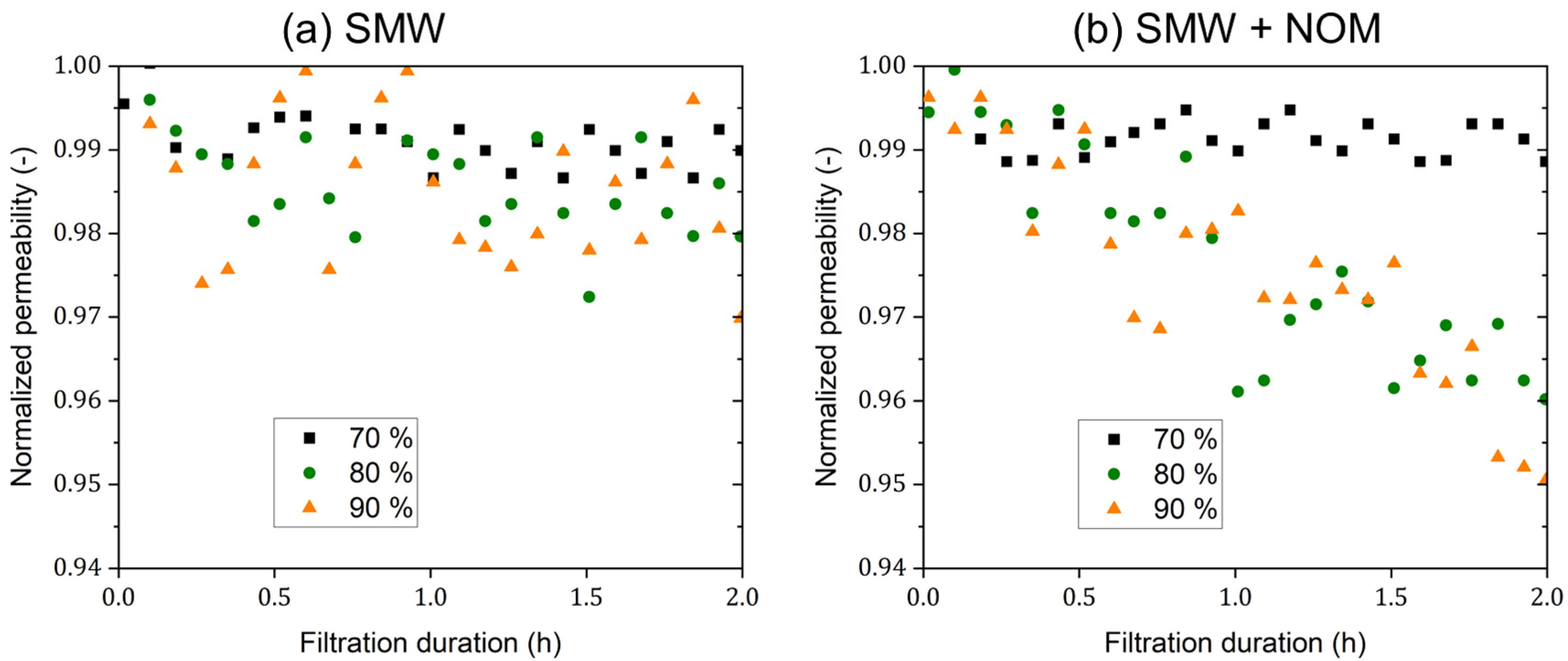
3.4. Mini-Plant Filtration Tests Using SMW with NOM at a Constant Concentration Employing Different Crossflow Velocities
3.5. Mini-Plant Filtration Tests Using SMW and NOM at a Constant Initial Concentration Employing a Constant Crossflow Velocity at Different Recoveries
4. Conclusions and Application Remarks
- dNF40 exhibited an F− retention of >70% during single salt retention experiments (without NOM and competitive ions), which increased with higher membrane flux but decreased with an increasing initial feed concentration.
- For SMW with a high TDS as the feed water, F− retention decreased due to ion competition, reaching values as low as 25% under the most challenging conditions (high recovery and a low CFV).
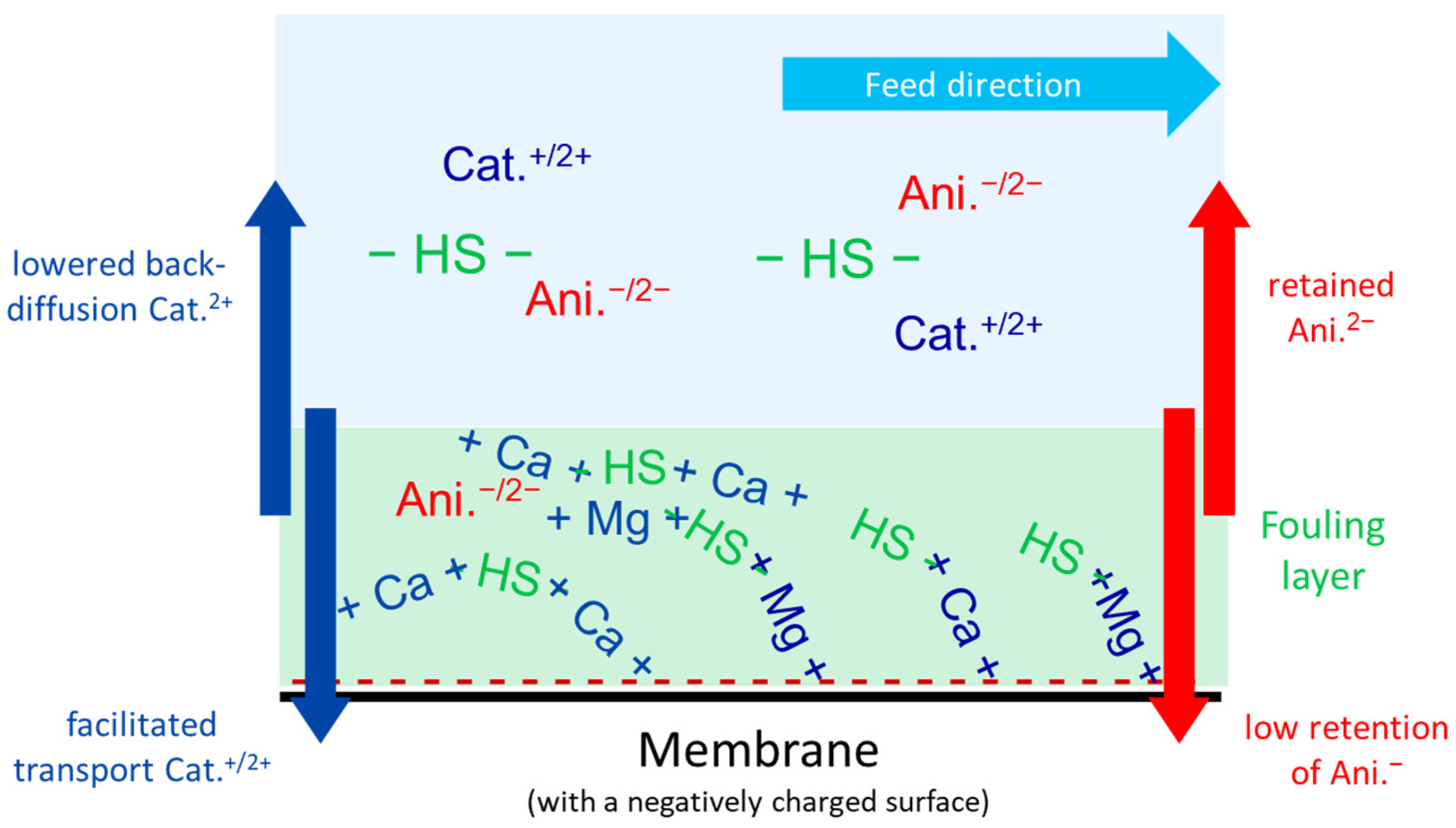
- For SMW with high TDS and NOM contents as the feed water, F− retention further decreased down to about 10% under the most challenging conditions (high recovery, a low CFV, and extended filtration duration) due to fouling effects. Facilitated transport of the divalent cations Ca2+ and Mg2+ could be observed as they accumulated in the organic fouling layer. While SO42− retention remained relatively stable, the retention of monovalent anions (NO3−, Cl−, and F−) decreased dramatically due to drag effects. On the other hand, Na+ retention improved slightly to maintain electroneutrality.
- Increasing the crossflow velocity resulted in a slightly improved separation performance for NOM and all ions. Meanwhile, a crossflow velocity of 0.5 m·s−1 was identified as the threshold value to maintain consistent performance of dNF40 membranes in this study; this also matches the manufacturer’s recommendation.
- The complex interactions between ions of varying charges and sizes, organic substances, and membrane system operating conditions create simultaneous and competing effects, making precise predictions of the retention of individual anions and cations challenging. As a result, only general trends can be identified.
- It is important to consider that the separation performance of polyelectrolyte multilayer membranes decreases with increasing feed salinity due to structural changes in the multilayers, although these changes remain reversible.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vithanage, M.; Bhattacharya, P. Fluoride in Drinking Water: Health Effects and Remediation. In CO2 Sequestration, Biofuels and Depollution; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 105–151. [Google Scholar]
- Diop, S.N.; Dieme, M.M.; Diallo, M.A.; Diawara, C.K. Fluoride excess removal from brackish drinking water in senegal by using KSF and K10 montmorillonite clays. J. Water Resour. Prot. 2022, 14, 21–34. [Google Scholar] [CrossRef]
- Nasri, N.; Souissi, F.; Ben Attia, T.; Ismailia, A.; Smida, O.; Tangour, D.; López Maldonado, E.A.; Souissi, R. Hydro Geochemical Characteristics and Mineralization Process of Groundwater in the Phosphatic Basin of Gafsa, Southwestern Tunisia. Hydrology 2024, 11, 142. [Google Scholar] [CrossRef]
- Fawell, J.; Bailey, K.; Chilton, J.; Dahi, E.; Fewtrell, L.; Magara, Y. Fluoride in Drinking-Water; IWA Publishing: London, UK, 2013. [Google Scholar]
- Guissouma, W.; Tarhouni, J. Fluoride in Tunisian Drinking Tap Water. J. Water Resour. Prot. 2015, 7, 860–870. [Google Scholar] [CrossRef]
- Guissouma, W.; Hakami, O.; Al-Rajab, A.J.; Tarhouni, J. Risk assessment of fluoride exposure in drinking water of Tunisia. Chemosphere 2017, 177, 102–108. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of fluoride removal from water environment by adsorption. J. Environ. Chem. Eng. 2020, 8, 104516. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef]
- Dar, F.A.; Kurella, S. Recent advances in adsorption techniques for fluoride removal—An overview. Groundw. Sustain. Dev. 2023, 23, 101017. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mukhtar, H. Characterisation and prediction of separation performance of nanofiltration membranes. J. Membr. Sci. 1996, 112, 263–274. [Google Scholar] [CrossRef]
- Mohammad, A.W. A modified Donnan–steric-pore model for predicting flux and rejection of dye/NaCl mixture in nanofiltration membranes. Sep. Sci. Technol. 2002, 37, 1009–1029. [Google Scholar] [CrossRef]
- Shen, J.; Schäfer, A. Removal of fluoride and uranium by nanofiltration and reverse osmosis: A review. Chemosphere 2014, 117, 679–691. [Google Scholar] [CrossRef]
- Richards, L.A.; Vuachère, M.; Schäfer, A.I. Impact of pH on the removal of fluoride, nitrate and boron by nanofiltration/reverse osmosis. Desalination 2010, 261, 331–337. [Google Scholar] [CrossRef]
- Owusu-Agyeman, I.; Reinwald, M.; Jeihanipour, A.; Schäfer, A.I. Removal of fluoride and natural organic matter from natural tropical brackish waters by nanofiltration/reverse osmosis with varying water chemistry. Chemosphere 2019, 217, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Tahaikt, M.; El-Ghzizel, S.; Essafi, N.; Hafsi, M.; Taky, M.; Elmidaoui, A. Technical-economic comparison of nanofiltration and reverse osmosis in the reduction of fluoride ions from groundwater: Experimental, modeling, and cost estimate. Desalination Water Treat. 2021, 216, 83–95. [Google Scholar]
- Sehn, P. Fluoride removal with extra low energy reverse osmosis membranes: Three years of large scale field experience in Finland. Desalination 2008, 223, 73–84. [Google Scholar] [CrossRef]
- Tahaikt, M.; El Habbani, R.; Ait Haddou, A.; Achary, I.; Amor, Z.; Taky, M.; Alami, A.; Boughriba, A.; Hafsi, M.; Elmidaoui, A. Fluoride removal from groundwater by nanofiltration. Desalination 2007, 212, 46–53. [Google Scholar] [CrossRef]
- Tahaikt, M.; Ait Haddou, A.; El Habbani, R.; Amor, Z.; Elhannouni, F.; Taky, M.; Kharif, M.; Boughriba, A.; Hafsi, M.; Elmidaoui, A. Comparison of the performances of three commercial membranes in fluoride removal by nanofiltration. Continuous operations. Desalination 2008, 225, 209–219. [Google Scholar] [CrossRef]
- Shen, J.; Schäfer, A.I. Factors affecting fluoride and natural organic matter (NOM) removal from natural waters in Tanzania by nanofiltration/reverse osmosis. Sci. Total Environ. 2015, 527, 520–529. [Google Scholar] [CrossRef]
- Owusu-Agyeman, I.; Jeihanipour, A.; Luxbacher, T.; Schäfer, A.I. Implications of humic acid, inorganic carbon and speciation on fluoride retention mechanisms in nanofiltration and reverse osmosis. J. Membr. Sci. 2017, 528, 82–94. [Google Scholar]
- Matin, A.; Khan, Z.; Zaidi, S.; Boyce, M. Biofouling in reverse osmosis membranes for seawater desalination: Phenomena and prevention. Desalination 2011, 281, 1–16. [Google Scholar] [CrossRef]
- Shen, J.; Mkongo, G.; Abbt-Braun, G.; Ceppi, S.L.; Richards, B.S.; Schäfer, A.I. Renewable energy powered membrane technology: Fluoride removal in a rural community in northern Tanzania. Sep. Purif. Technol. 2015, 149, 349–361. [Google Scholar] [CrossRef]
- Schäfer, A.I.; Fane, A.G.; Waite, T.D. Nanofiltration of natural organic matter: Removal, fouling and the influence of multivalent ions. Desalination 1998, 118, 109–122. [Google Scholar] [CrossRef]
- Nasr, A.B.; Charcosset, C.; Amar, R.B.; Walha, K. Defluoridation of water by nanofiltration. J. Fluor. Chem. 2013, 150, 92–97. [Google Scholar] [CrossRef]
- de Grooth, J.; Haakmeester, B.; Wever, C.; Potreck, J.; de Vos, W.M.; Nijmeijer, K. Long term physical and chemical stability of polyelectrolyte multilayer membranes. J. Membr. Sci. 2015, 489, 153–159. [Google Scholar] [CrossRef]
- Sewerin, T.; Elshof, M.G.; Matencio, S.; Boerrigter, M.; Yu, J.; de Grooth, J. Advances and Applications of Hollow Fiber Nanofiltration Membranes: A Review. Membranes 2021, 11, 890. [Google Scholar] [CrossRef]
- Li, F.Y.; Li, Y.; Chung, T.-S.; Chen, H.; Jean, Y.C.; Kawi, S. Development and positron annihilation spectroscopy (PAS) characterization of polyamide imide (PAI)–polyethersulfone (PES) based defect-free dual-layer hollow fiber membranes with an ultrathin dense-selective layer for gas separation. J. Membr. Sci. 2011, 378, 541–550. [Google Scholar] [CrossRef]
- Urper, G.M.; Sengur-Tasdemir, R.; Turken, T.; Ates Genceli, E.; Tarabara, V.V.; Koyuncu, I. Hollow fiber nanofiltration membranes: A comparative review of interfacial polymerization and phase inversion fabrication methods. Sep. Sci. Technol. 2017, 52, 2120–2136. [Google Scholar] [CrossRef]
- Scheepers, D.; Chatillon, B.; Borneman, Z.; Nijmeijer, K. Influence of charge density and ionic strength on diallyldimethylammonium chloride (DADMAC)-based polyelectrolyte multilayer membrane formation. J. Membr. Sci. 2021, 617, 118619. [Google Scholar] [CrossRef]
- Jonkers, W.A.; Cornelissen, E.R.; de Vos, W.M. Hollow fiber nanofiltration: From lab-scale research to full-scale applications. J. Membr. Sci. 2023, 669, 121234. [Google Scholar] [CrossRef]
- Joseph, N.; Ahmadiannamini, P.; Hoogenboom, R.; Vankelecom, I.F. Layer-by-layer preparation of polyelectrolyte multilayer membranes for separation. Polym. Chem. 2014, 5, 1817–1831. [Google Scholar] [CrossRef]
- Niestroj-Pahl, R.; te Brinke, E.; Roth, H.; Dähne, L.; de Vos, W.M. Symmetric and asymmetric ceramic-supported polyelectrolyte multilayer nanofiltration membranes. J. Membr. Sci. 2025, 713, 123366. [Google Scholar] [CrossRef]
- Sengur-Tasdemir, R.; Urper-Bayram, G.M.; Turken, T.; Ates-Genceli, E.; Tarabara, V.V.; Koyuncu, I. Hollow fiber nanofiltration membranes for surface water treatment: Performance evaluation at the pilot scale. J. Water Process Eng. 2021, 42, 102100. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mukherjee, M.; De, S. Groundwater defluoridation and disinfection using carbonized bone meal impregnated polysulfone mixed matrix hollow-fiber membranes. J. Water Process Eng. 2020, 33, 101002. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Yang, D.; Jian, X. Preparation and characterization of polypiperazine amide/PPESK hollow fiber composite nanofiltration membrane. J. Membr. Sci. 2007, 301, 85–92. [Google Scholar] [CrossRef]
- Haddad, M.; Ohkame, T.; Bérubé, P.R.; Barbeau, B. Performance of thin-film composite hollow fiber nanofiltration for the removal of dissolved Mn, Fe and NOM from domestic groundwater supplies. Water Res. 2018, 145, 408–417. [Google Scholar] [CrossRef]
- Shalmani, A.K.; ElSherbiny, I.M.; Panglisch, S. Application-oriented mini-plant experiments using non-conventional model foulants to evaluate new hollow fiber membrane materials. Sep. Purif. Technol. 2020, 251, 117345. [Google Scholar] [CrossRef]
- Poel, S.v.d. Parting Ways—Removal of Salts and Organic Micropollutants by Direct Nanofiltration Pretreatment of Surface Water for the Production of Dune Infiltration Water. Master’s Thesis, TU Delft Faculty of Civil Engineering and Geosciences, Delft, The Netherlands, 2020. [Google Scholar]
- de Grooth, J.; Oborný, R.; Potreck, J.; Nijmeijer, K.; de Vos, W.M. The role of ionic strength and odd–even effects on the properties of polyelectrolyte multilayer nanofiltration membranes. J. Membr. Sci. 2015, 475, 311–319. [Google Scholar] [CrossRef]
- De Grooth, J.; Elshof, M.G.; Roesink, H.D.W. Polyelectrolyte Multilayer (PEM) Membranes and Their Use. Pantent AU2017412844A1; World Intellectual Property Organization: Geneva, Switzerland, 25 March 2025. [Google Scholar]
- Arun, A. Direct Nanofiltration of Surface Water Investigating the fouling and rejection performance of Low MWCO Hollow fiber Nanofiltration Membranes. Master’s Thesis, TU Delft Faculty of Civil Engineering and Geosciences, Delft, The Netherlands, 2019. [Google Scholar]
- Quilitzsch, M.; Osmond, R.; Krug, M.; Heijnen, M.; Ulbricht, M. Macro-initiator mediated surface selective functionalization of ultrafiltration membranes with anti-fouling hydrogel layers applicable to ready-to-use capillary membrane modules. J. Membr. Sci. 2016, 518, 328–337. [Google Scholar] [CrossRef]
- Modak, S.S. Membrane scaling in reverse osmosis (RO): Comparing conventional plug flow RO with semi-batch RO in pilot plant experiments. Bachelor’s Thesis, University of Duisburg-Essen Faculty of Engineering, Chair of Mechanical Process Engineering/Water Technology, Duisburg, Germany, 2023. [Google Scholar]
- Verliefde, A.R.D.; Cornelissen, E.R.; Heijman, S.G.J.; Verberk, J.Q.J.C.; Amy, G.L.; Van der Bruggen, B.; van Dijk, J.C. Construction and validation of a full-scale model for rejection of organic micropollutants by NF membranes. J. Membr. Sci. 2009, 339, 10–20. [Google Scholar] [CrossRef]
- Junker, M.A.; de Vos, W.M.; Lammertink, R.G.H.; de Grooth, J. Bridging the gap between lab-scale and commercial dimensions of hollow fiber nanofiltration membranes. J. Membr. Sci. 2021, 624, 119100. [Google Scholar] [CrossRef]
- Jährig, J.; Vredenbregt, L.; Wicke, D.; Miehe, U.; Sperlich, A. Capillary Nanofiltration under Anoxic Conditions as Post-Treatment after Bank Filtration. Water 2018, 10, 1599. [Google Scholar] [CrossRef]
- Peeters, J.; Boom, J.; Mulder, M.; Strathmann, H. Retention measurements of nanofiltration membranes with electrolyte solutions. J. Membr. Sci. 1998, 145, 199–209. [Google Scholar] [CrossRef]
- Ramdani, A.; Deratani, A.; Taleb, S.; Drouiche, N.; Lounici, H. Performance of NF90 and NF270 commercial nanofiltration membranes in the defluoridation of Algerian brackish water. Desalination Water Treat. 2021, 212, 286–296. [Google Scholar]
- Dong, C.; He, R.; Xu, S.; He, H.; Chen, H.; Zhang, Y.-B.; He, T. Layer-by-layer (LBL) hollow fiber nanofiltration membranes for seawater treatment: Ion rejection. Desalination 2022, 534, 115793. [Google Scholar] [CrossRef]
- Johnson, D.J.; Hilal, N. Nanocomposite nanofiltration membranes: State of play and recent advances. Desalination 2022, 524, 115480. [Google Scholar] [CrossRef]
- Micari, M.; Diamantidou, D.; Heijman, B.; Moser, M.; Haidari, A.; Spanjers, H.; Bertsch, V. Experimental and theoretical characterization of commercial nanofiltration membranes for the treatment of ion exchange spent regenerant. J. Membr. Sci. 2020, 606, 118117. [Google Scholar] [CrossRef]
- Niewersch, C.; Battaglia Bloch, A.L.; Yüce, S.; Melin, T.; Wessling, M. Nanofiltration for the recovery of phosphorus—Development of a mass transport model. Desalination 2014, 346, 70–78. [Google Scholar] [CrossRef]
- Rutten, S.B.; Junker, M.A.; Leal, L.H.; de Vos, W.M.; Lammertink, R.G.H.; de Grooth, J. Influence of dominant salts on the removal of trace micropollutants by hollow fiber nanofiltration membranes. J. Membr. Sci. 2023, 678, 121625. [Google Scholar] [CrossRef]
- Reid, D.K.; Summers, A.; O’Neal, J.; Kavarthapu, A.V.; Lutkenhaus, J.L. Swelling and Thermal Transitions of Polyelectrolyte Multilayers in the Presence of Divalent Ions. Macromolecules 2016, 49, 5921–5930. [Google Scholar] [CrossRef]
- Fares, H.M.; Wang, Q.; Yang, M.; Schlenoff, J.B. Swelling and Inflation in Polyelectrolyte Complexes. Macromolecules 2019, 52, 610–619. [Google Scholar] [CrossRef]
- Bóna, Á.; Galambos, I.; Nemestóthy, N. Progress towards Stable and High-Performance Polyelectrolyte Multilayer Nanofiltration Membranes for Future Wastewater Treatment Applications. Membranes 2023, 13, 368. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, C.; Tong, T.; Epsztein, R.; Sun, M.; Verduzco, R.; Ma, J.; Elimelech, M. Selective removal of divalent cations by polyelectrolyte multilayer nanofiltration membrane: Role of polyelectrolyte charge, ion size, and ionic strength. J. Membr. Sci. 2018, 559, 98–106. [Google Scholar] [CrossRef]
- Luo, J.; Wan, Y. Effect of highly concentrated salt on retention of organic solutes by nanofiltration polymeric membranes. J. Membr. Sci. 2011, 372, 145–153. [Google Scholar] [CrossRef]
- Chen, J.; Xu, S.; Tang, C.Y.; Hu, B.; Tokay, B.; He, T. Stability of layer-by-layer nanofiltration membranes in highly saline streams. Desalination 2023, 555, 116520. [Google Scholar] [CrossRef]
- de Grooth, J.; Dong, M.; de Vos, W.M.; Nijmeijer, K. Building polyzwitterion-based multilayers for responsive membranes. Langmuir 2014, 30, 5152–5161. [Google Scholar] [CrossRef]
- Ladhari, N.; Hemmerlé, J.; Haikel, Y.; Voegel, J.-C.; Schaaf, P.; Ball, V. Stability of embossed PEI-(PSS–PDADMAC)20 multilayer films versus storage time and versus a change in ionic strength. Appl. Surf. Sci. 2008, 255, 1988–1995. [Google Scholar] [CrossRef]
- Han, L.; Mao, Z.; Wuliyasu, H.; Wu, J.; Gong, X.; Yang, Y.; Gao, C. Modulating the structure and properties of poly(sodium 4-styrenesulfonate)/poly(diallyldimethylammonium chloride) multilayers with concentrated salt solutions. Langmuir 2012, 28, 193–199. [Google Scholar] [CrossRef]
- Cornelissen, E.R.; Siegers, W.G.; Ogier, J.; Beerendonk, E.F. Influence of calcium-NOM complexes on fouling of nanofiltration membranes in drinking water production. Water Supply 2006, 6, 171–178. [Google Scholar] [CrossRef]
- Mahlangu, O.T.; Mamba, B.B.; Verliefde, A.R.D. Effect of multivalent cations on membrane-foulant and foulant-foulant interactions controlling fouling of nanofiltration membranes. Polym. Adv. Technol. 2020, 31, 2588–2600. [Google Scholar] [CrossRef]
- Al-Amoudi, A.S. Factors affecting natural organic matter (NOM) and scaling fouling in NF membranes: A review. Desalination 2010, 259, 1–10. [Google Scholar] [CrossRef]
- Epsztein, R.; Shaulsky, E.; Dizge, N.; Warsinger, D.M.; Elimelech, M. Role of Ionic Charge Density in Donnan Exclusion of Monovalent Anions by Nanofiltration. Environ. Sci. Technol. 2018, 52, 4108–4116. [Google Scholar] [CrossRef]
- Volkov, A.; Paula, S.; Deamer, D. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochemistry Bioenerg. 1997, 42, 153–160. [Google Scholar]
- Marcus, Y. A simple empirical model describing the thermodynamics of hydration of ions of widely varying charges, sizes, and shapes. Biophys. Chem. 1994, 51, 111–127. [Google Scholar]




| Samples | TOC (mg·L−1) | UV254 (cm−1) | VIS436 (cm−1) | Turbidity (FNU) | Conductivity (μS·cm−1) | Ion Concentration (mg·L−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | Ca2+ | Mg2+ | F− | Cl− | NO3− | SO42− | ||||||
| * PS | 30 ± 0.30 | 1.2 ± 0.02 | 1.08 ± 0.01 | 20.6 ± 0.40 | 170 ± 1.30 | 32 | 2 | 2 | <DL | 26 | 1 | 38 |
| ** Real | 8721 ± 1.30 | 1400 | 325 | 234 | 2.5 | 1840 | 11 | 1830 | ||||
| SMW | 8960 ± 1.30 | 1393 | 330 | 235 | 20 | 2004 | 50 | 1827 | ||||
| SMW + NOM (5 mg·L−1) | 5 ± 0.30 | 0.2 ± 0.02 | 0.03 ± 0.01 | 2.9 ± 0.40 | 8992 ± 1.30 | 1404 | 330 | 235 | 20 | 2003 | 52 | 1850 |
| SMW + NOM (15 mg·L−1) | 15 ± 0.30 | 0.7 ± 0.02 | 0.12 ± 0.01 | 9.4 ± 0.40 | 9020 ± 1.30 | 1420 | 330 | 234 | 20 | 2010 | 50 | 1862 |
| SMW + NOM (30 mg·L−1) | 30 ± 0.30 | 1.2 ± 0.02 | 1.08 ± 0.01 | 21.8 ± 0.40 | 9130 ± 1.30 | 1435 | 332 | 236 | 20 | 2225 | 51 | 1892 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuelgasim, H.; Nasri, N.; Futterlieb, M.; Souissi, R.; Souissi, F.; Panglisch, S.; ElSherbiny, I.M.A. Impacts of Natural Organic Matter and Dissolved Solids on Fluoride Retention of Polyelectrolyte Multilayer-Based Hollow Fiber Nanofiltration Membranes. Membranes 2025, 15, 110. https://doi.org/10.3390/membranes15040110
Abuelgasim H, Nasri N, Futterlieb M, Souissi R, Souissi F, Panglisch S, ElSherbiny IMA. Impacts of Natural Organic Matter and Dissolved Solids on Fluoride Retention of Polyelectrolyte Multilayer-Based Hollow Fiber Nanofiltration Membranes. Membranes. 2025; 15(4):110. https://doi.org/10.3390/membranes15040110
Chicago/Turabian StyleAbuelgasim, Hussein, Nada Nasri, Martin Futterlieb, Radhia Souissi, Fouad Souissi, Stefan Panglisch, and Ibrahim M. A. ElSherbiny. 2025. "Impacts of Natural Organic Matter and Dissolved Solids on Fluoride Retention of Polyelectrolyte Multilayer-Based Hollow Fiber Nanofiltration Membranes" Membranes 15, no. 4: 110. https://doi.org/10.3390/membranes15040110
APA StyleAbuelgasim, H., Nasri, N., Futterlieb, M., Souissi, R., Souissi, F., Panglisch, S., & ElSherbiny, I. M. A. (2025). Impacts of Natural Organic Matter and Dissolved Solids on Fluoride Retention of Polyelectrolyte Multilayer-Based Hollow Fiber Nanofiltration Membranes. Membranes, 15(4), 110. https://doi.org/10.3390/membranes15040110








