Suitability of Electrodialysis with Monovalent Selective Anion-Exchange Membranes for Fractionation of Aqueous Mixture Containing Reactive Dye and Mineral Salt
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Solutions
2.2. Membranes and ED Stack
- -
- Immersion in HCl for 2 h and then flushing with water for the Cl− ions to disappear;
- -
- Immersion in NaOH for 2 h and then flushing with water for the pH to decrease to <8.3;
- -
- Immersion in HCl for 2 h and then flushing with water for the Cl− ions to disappear.
| Parameter | Membrane | |
|---|---|---|
| Cation-Exchange PC-SK | Monovalent Selective Anion-Exchange PC-MVA | |
| Producer | PCA GmbH (Germany) | |
| Thickness, µm | 90–130 | 110 |
| Ion-exchange capacity, mmol/g | Approx. 1.2 | Approx. 1.0 |
| Electrical resistance, Ω cm2 | 1–3 | 20 |
| Thermal stability, °C | Maximal 60 | Maximal 40 |
| Chemical stability (pH range) | 0–9 | 0–7 |
| Burst strength, MPa | 0.4 | N/A |
| Permselectivity (transfer number) | ||
| t-K+ | >0.95 | |
| t-Cl− | >0.97 | |
| Ionic form | Na+ | Cl− |
| Functional group | ||
2.3. Methodology
- Ctd—the actual dye concentration in the diluate (mg/L);
- Cid—the initial dye concentration in the diluate (mg/L).
- Cs—the actual diluate conductivity (mS/cm);
- Ci—the initial diluate conductivity (mS/cm).
- —the limiting current density (A/m2);
- k—the mass transfer coefficient (L/m2 s);
- —the concentration of cations (Na+) in the diluate after desalination (eq/L);
- F—the Faraday constant (96,500 As/eq);
- —the transfer number of cations in the membrane (0.95);
- —the transfer number of cations in the solution (0.45).
- EC—the energy demand for the MASED process (kWh);
- I—the current intensity (A);
- U—the voltage (V);
- t—the time of the process (h);
- EV—the specific electrical energy consumption (kWh/m3);
- Vd—the volume of the treated solution (diluate, 2 L).
- M—the mass of the dye accumulated on/in the IEMs (mg);
- Vid, Vfd—the initial and final volume of the diluate, respectively (2 L);
- Vic, Vfc—the initial and final volume of the concentrate, respectively (2 L);
- Cid, Cfd—the initial and final concentration of the dye in the diluate, respectively (mg/L);
- Cic, Cfc—the initial and final concentration of the dye in the concentrate, respectively (mg/L).
3. Results and Discussion
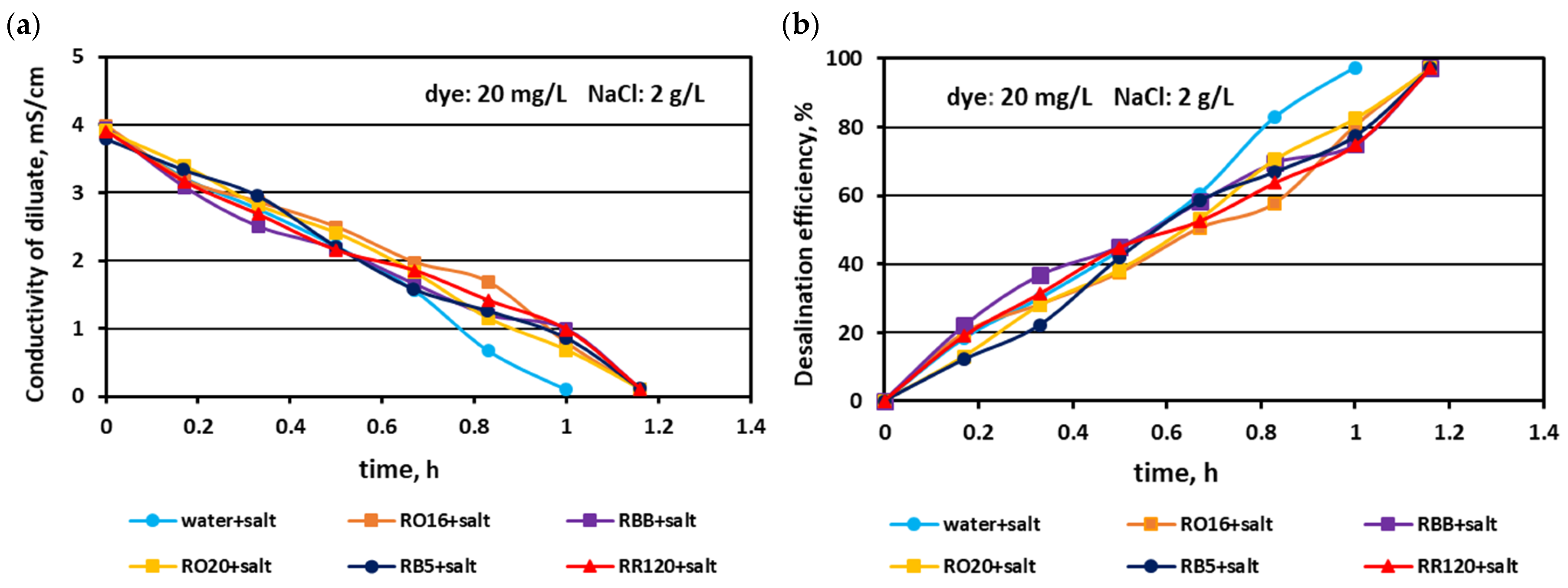
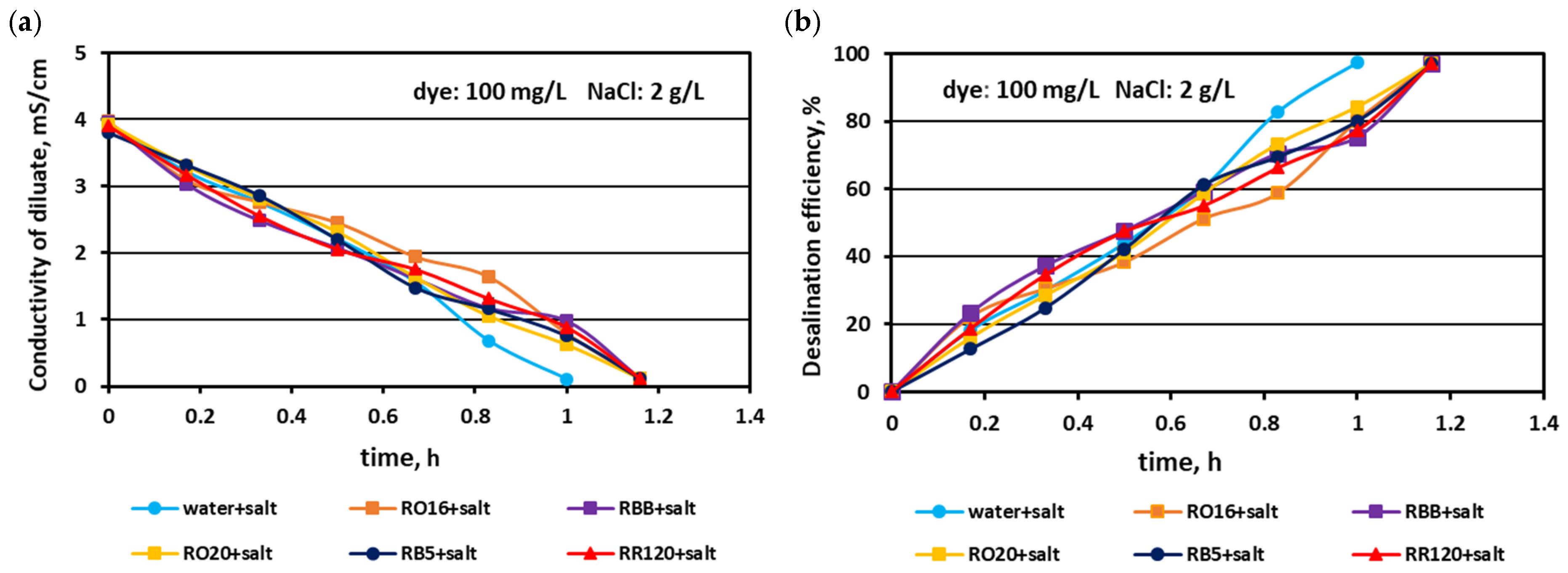
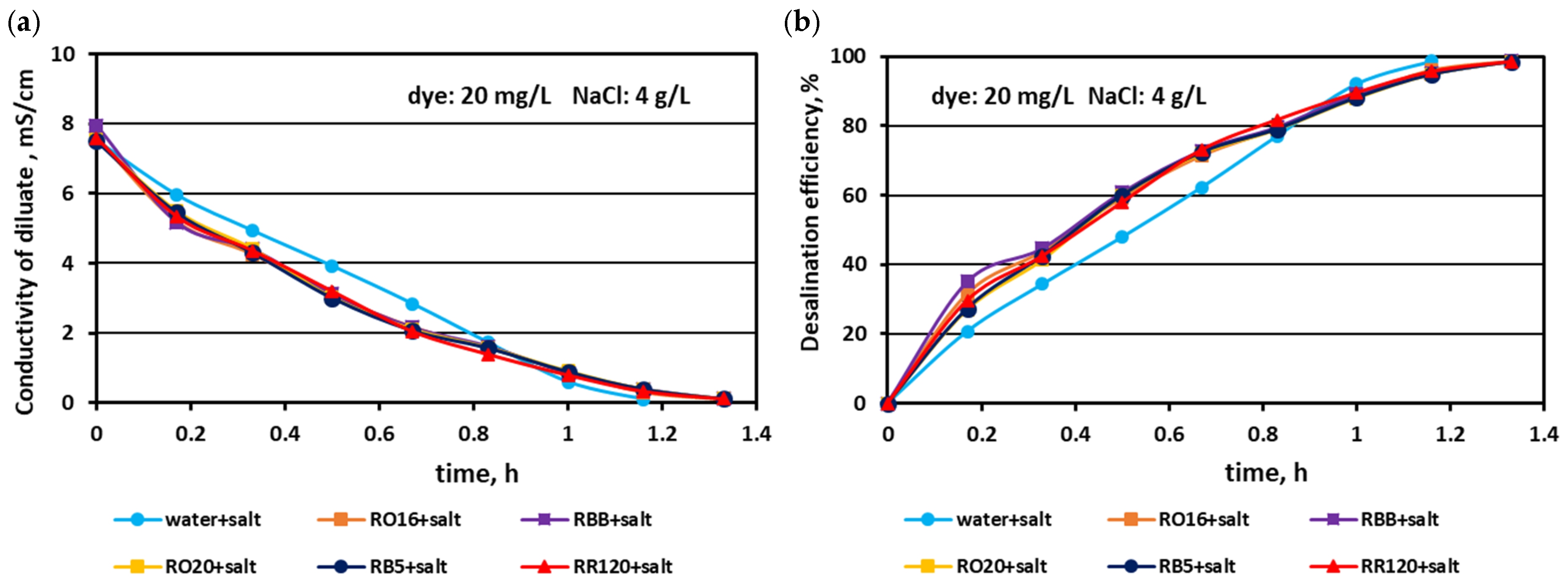
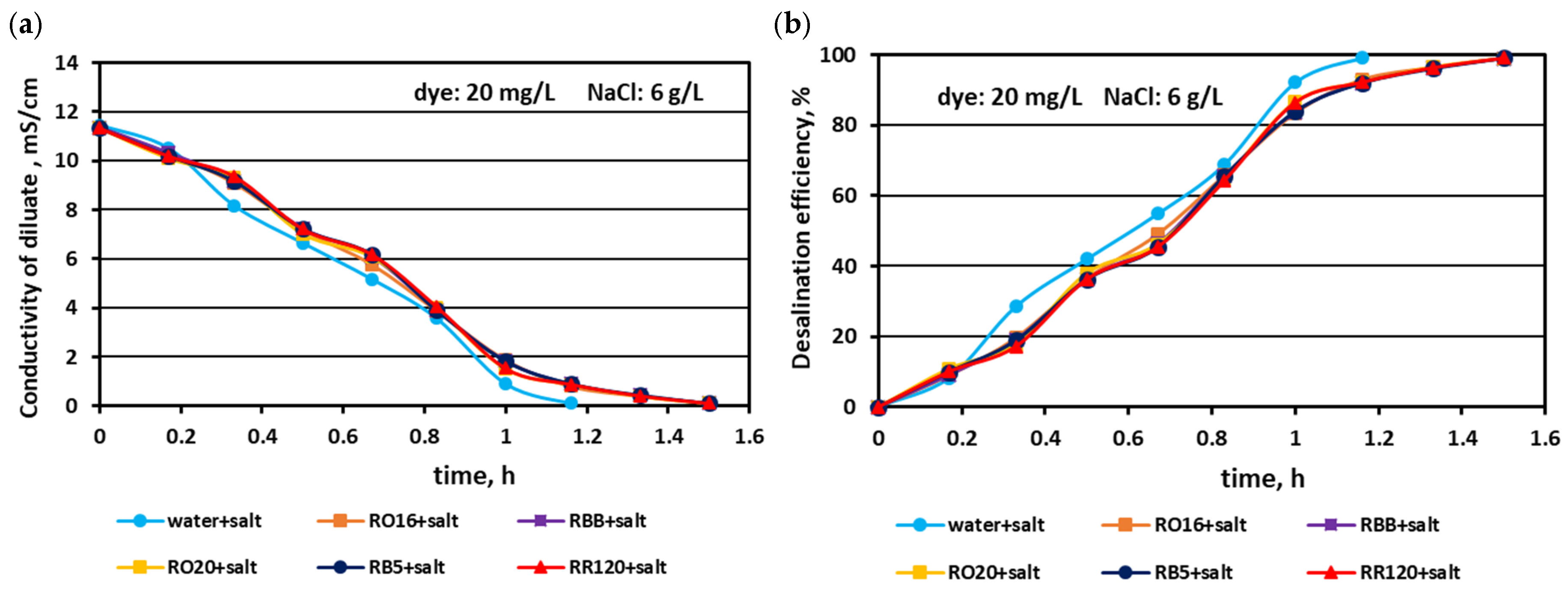
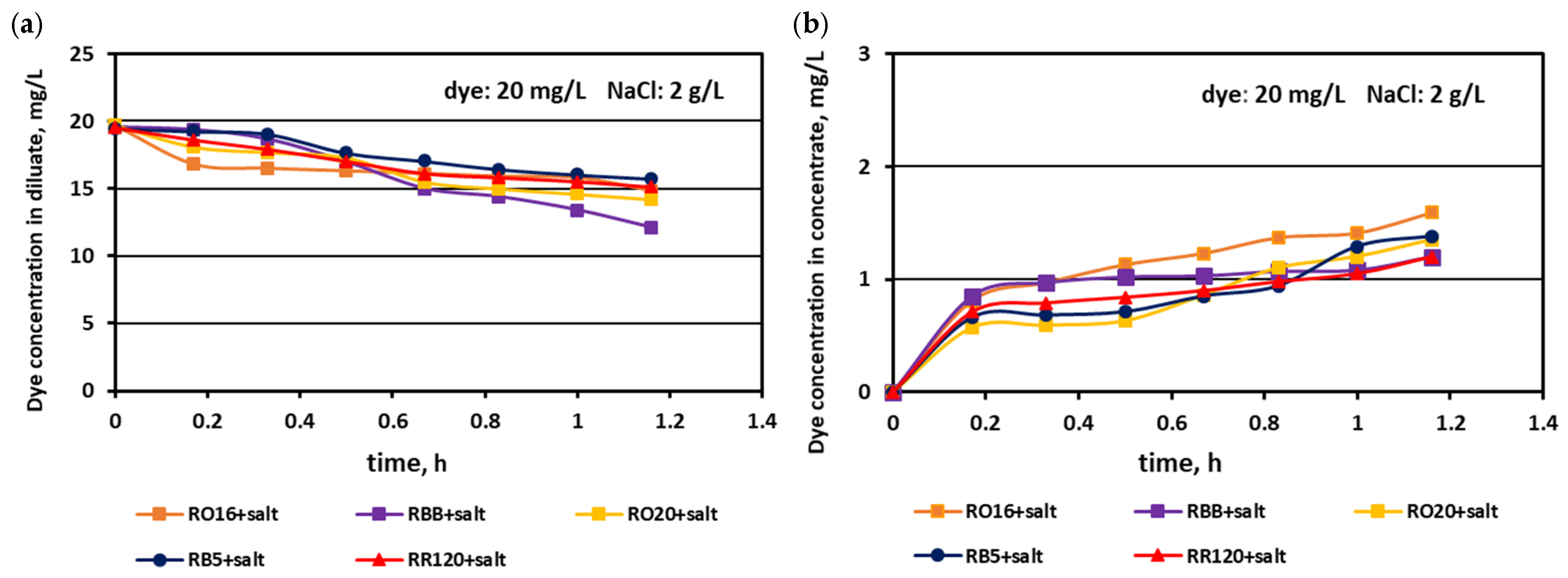
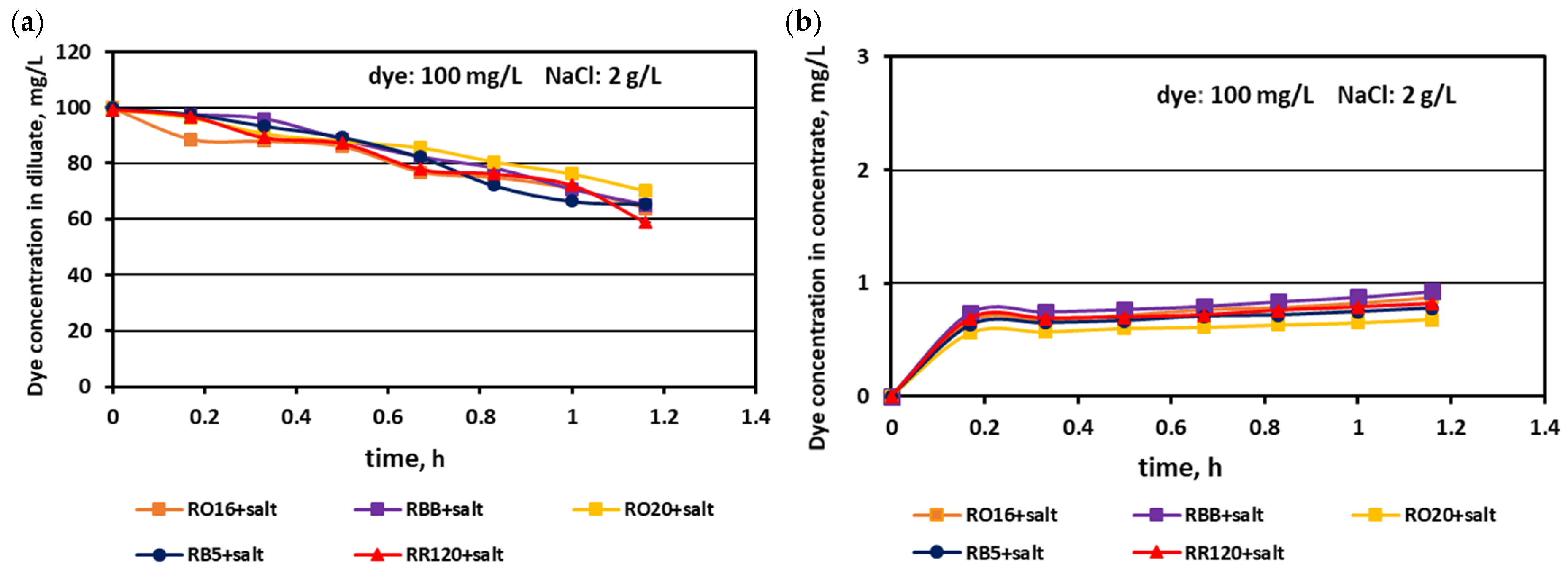
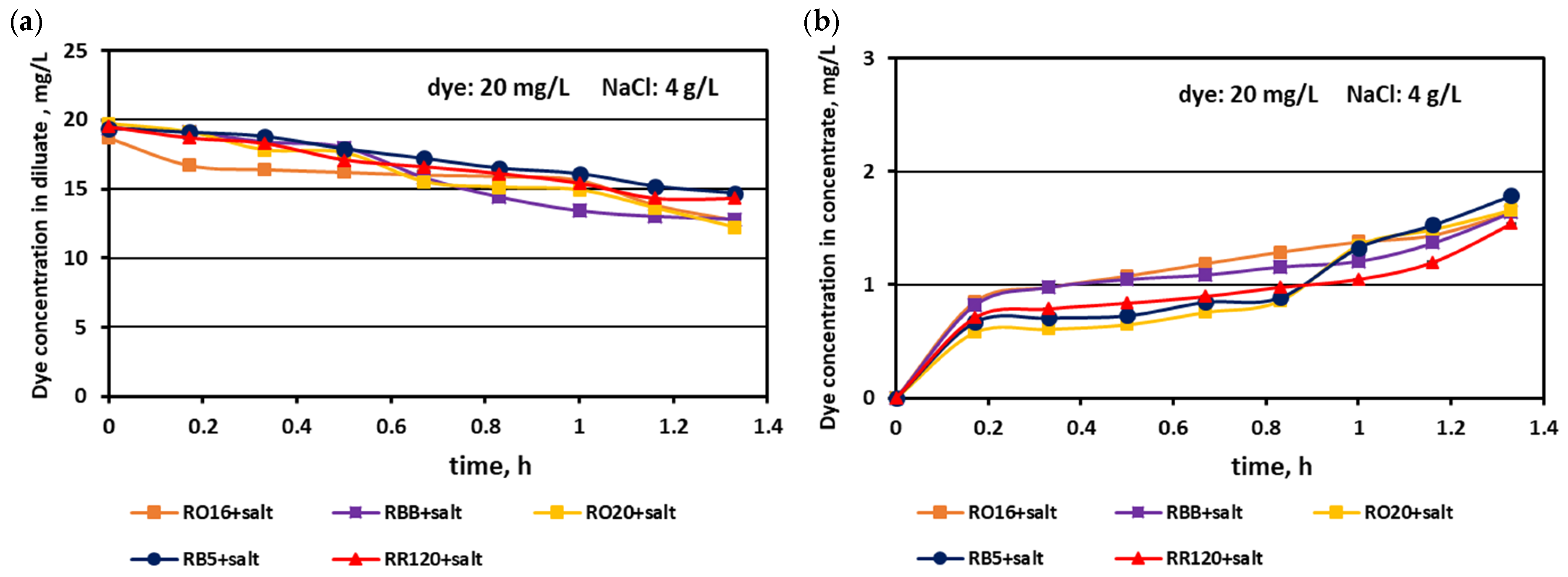
3.1. Fractionation of Binary Mixtures Containing Dye and Salt by MASED
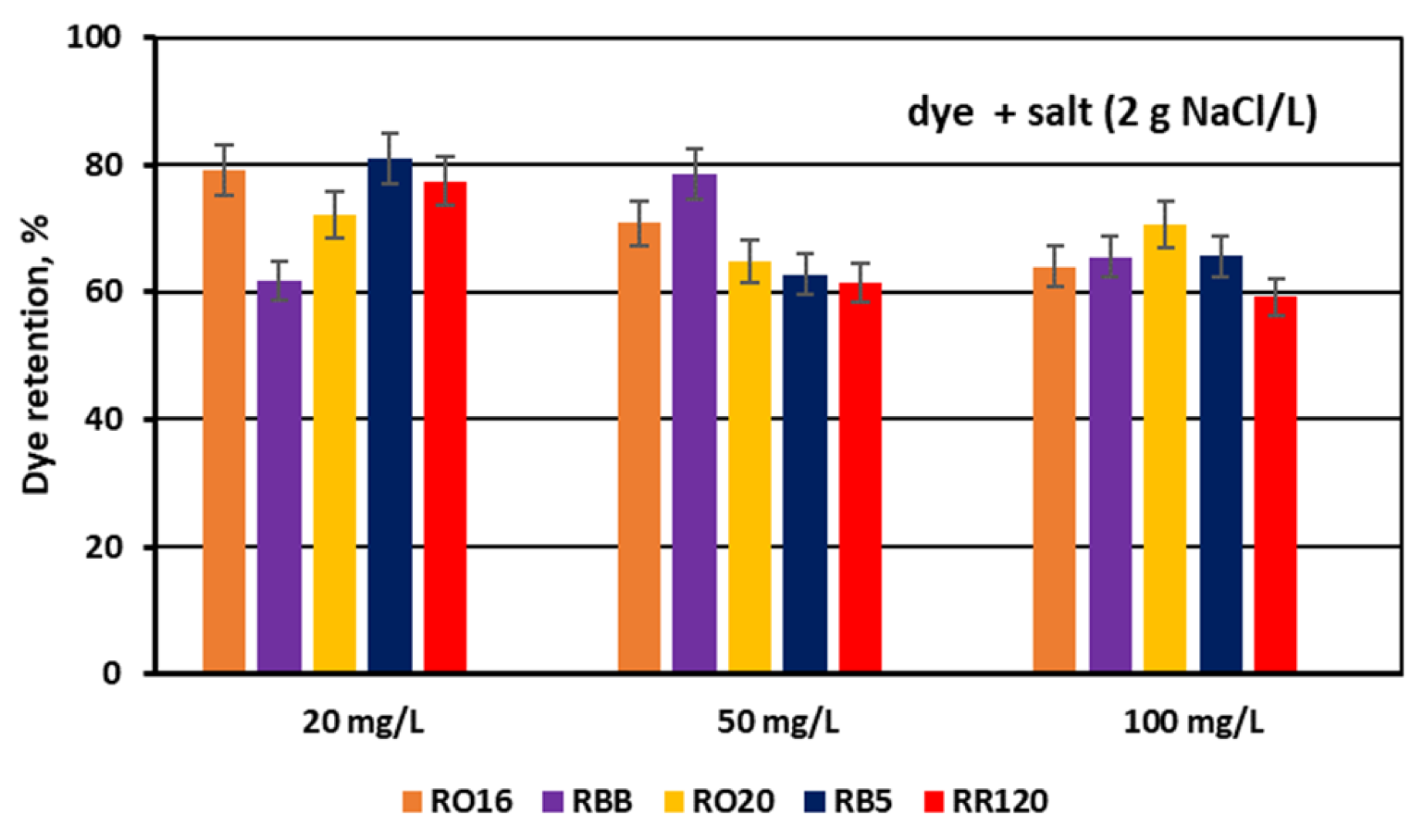
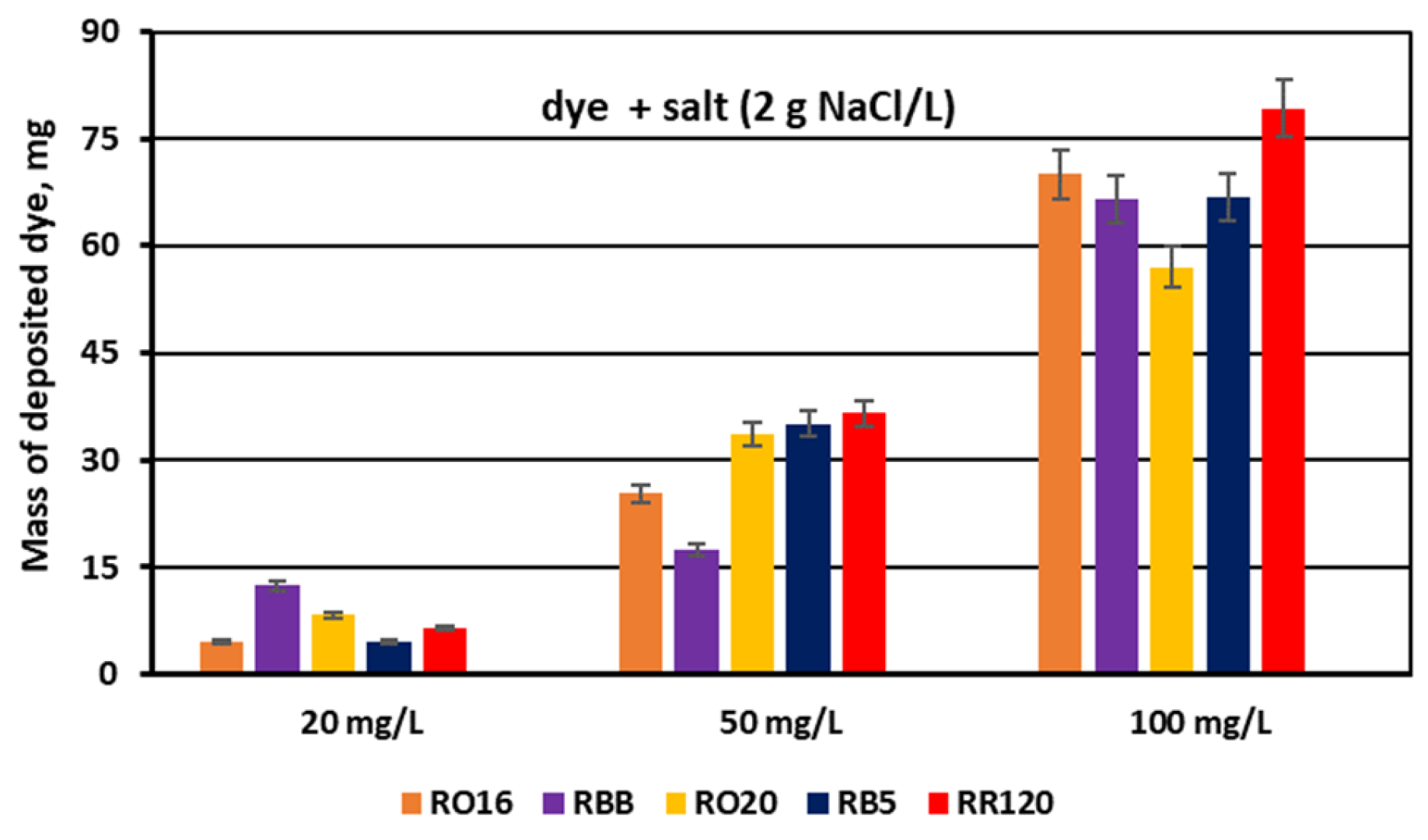
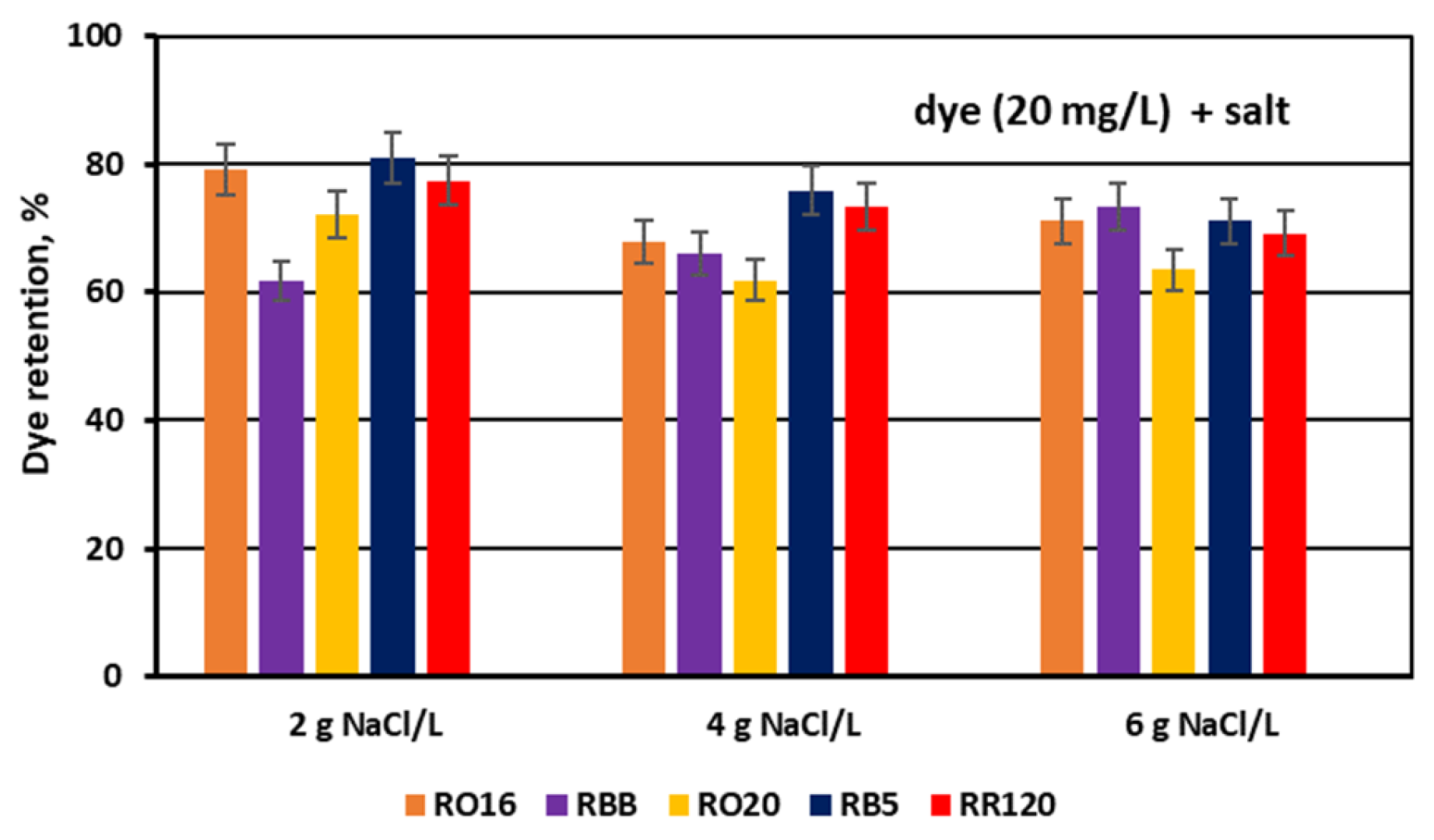
3.2. Reactive Dye Retention in the Course of the MASED Process
3.2.1. Dye Reactivity and Susceptibility to Adsorption by Ion-Exchange Membranes
3.2.2. Effect of Feed Dye Concentration on Dye Adsorption by PC-MVA Membranes
3.2.3. Effect of Feed Salt Concentration on Dye Adsorption by PC-MVA Membranes
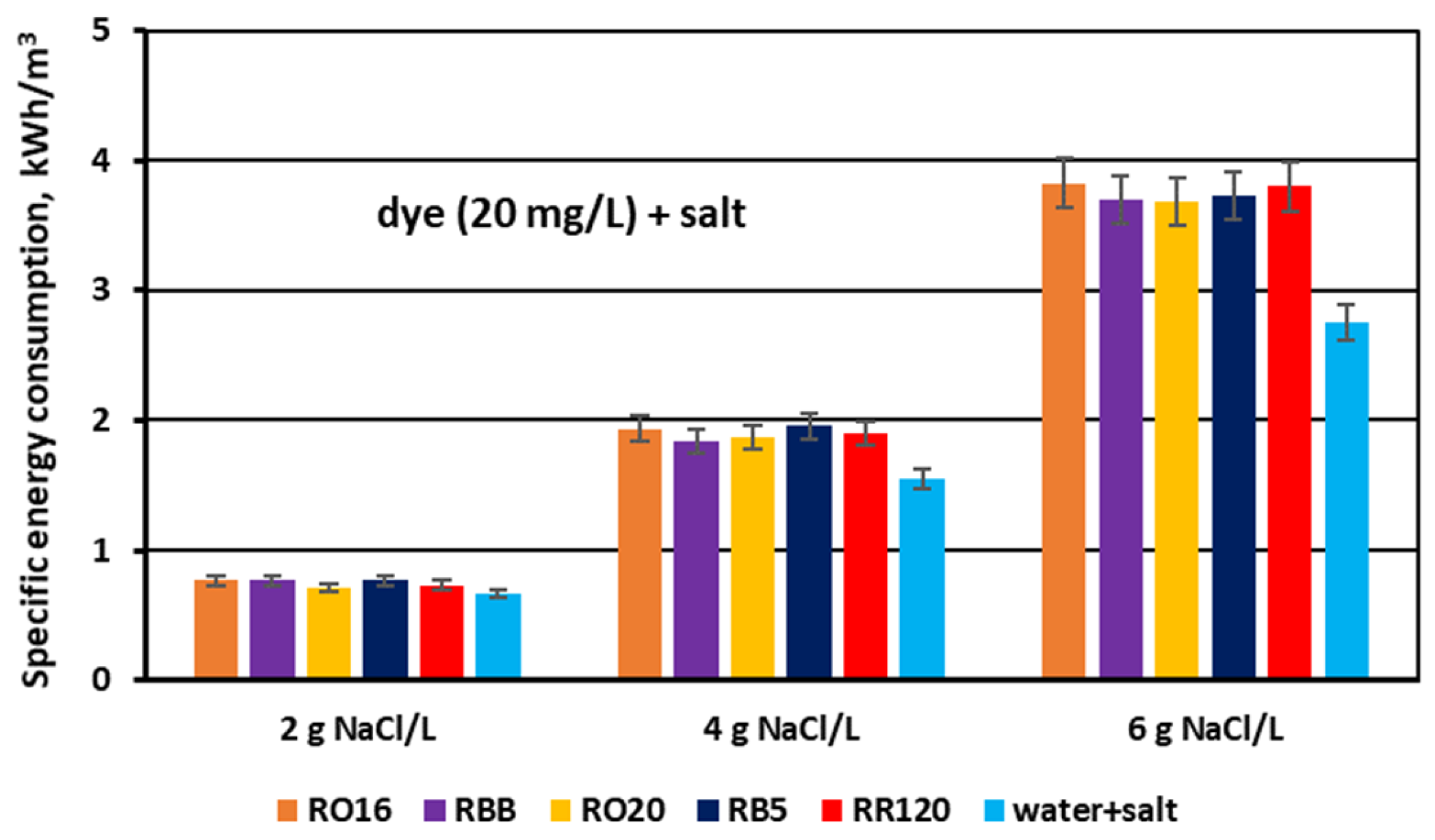
3.3. Power Demand for Desalination of Dye–Salt Mixtures by MASED
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Xu, Z.; Xu, J.; Wang, H.; Guo, X.; Gong, Z.; Zhang, C. Efficient Decolorization of Reactive Dyed Cotton Fabric with a Two-Step NaOH/Na2S2O4 Process. Cellulose 2024, 31, 1253–1265. [Google Scholar] [CrossRef]
- Shang, S.M. Process Control in Dyeing of Textiles. In Woodhead Publishing Series in Textiles, Process Control in Textile Manufacturing; Majumdar, A., Das, A., Alagirusamy, R., Kothari, V.K., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 300–338. ISBN 9780857090270. [Google Scholar] [CrossRef]
- Wolela, A.D. Effect and Role of Salt in Cellulosic Fabric Dyeing. Adv. Res. Text. Eng. 2021, 6, 1061. [Google Scholar]
- Singh, V.K.; Sett, A.; Karmakar, S. Waste to Wealth: Facile Activation of Red Mud Waste and Insights into Industrial Reactive Dye Removal from Wastewater. Chem. Eng. J. 2024, 481, 148373. [Google Scholar] [CrossRef]
- Allègre, C.; Moulin, P.; Maisseu, M.; Charbit, F. Treatment and Reuse of Reactive Dyeing Effluents. J. Membr. Sci. 2006, 269, 15–34. [Google Scholar] [CrossRef]
- Fadzli, J.; Hamid, K.H.K.; Him, N.R.N.; Puasa, S.W. A Critical Review on the Treatment of Reactive Dye Wastewater. Desal. Water Treat. 2022, 257, 185–203. [Google Scholar] [CrossRef]
- Rashidi, H.R.; Sulaiman, N.M.N.; Hashim, N.A.; Hassan, C.R.C.; Ramli, M.R. Synthetic Reactive Dye Wastewater Treatment by Using Nano-Membrane Filtration. Desal. Water Treat. 2015, 55, 86–95. [Google Scholar] [CrossRef]
- Moradihamedani, P. Recent Advances in Dye Removal from Wastewater by Membrane Technology: A Review. Polym. Bull. 2022, 79, 2603–2631. [Google Scholar] [CrossRef]
- Kamati, S.N.; Yan, J.; Fan, J. A Review on Progress in Reactive Dye-Containing Wastewater Treatment. Water Pract. Technol. 2024, 19, 2712–2733. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, X.; Sun, J.; Liu, J.; Liu, Z.; Wang, Y.; Pan, J. Zero Liquid Discharge and Resource Treatment of Low-Salinity Mineralized Wastewater Based on Combing Selectrodialysis with Bipolar Membrane Electrodialysis. Separations 2023, 10, 269. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, M.; Yan, B.; Sun, X.; Liu, Y.; Wang, Y.; Xu, T.; Zhang, Y.Z. Waste Conversion and Resource Recovery from Wastewater by Ion Exchange Membranes: State-of-the-Art and Perspective. Ind. Eng. Chem. Res. 2018, 57, 6025–6039. [Google Scholar] [CrossRef]
- Sedighi, M.; Usefi, M.M.B.; Ismail, A.F.; Ghasemi, M. Environmental Sustainability and Ions Removal Through Electrodialysis Desalination: Operating Conditions and Process Parameters. Desalination 2023, 549, 116319. [Google Scholar] [CrossRef]
- Majewska-Nowak, K. Treatment of Organic Dye Solutions by Electrodialysis. Membr. Water Treat. 2013, 4, 203–214. [Google Scholar] [CrossRef]
- Xue, C.; Chen, Q.; Liu Yang, Y.-Y.; Xu, D.; Xue, L.; Zhang, W.-M. Acid Blue 9 Desalting Using Electrodialysis. J. Membr. Sci. 2015, 493, 28–36. [Google Scholar] [CrossRef]
- Lafi, R.; Mabrouk, W.; Hafiane, A. Removal of Methylene Blue from Saline Solutions by Adsorption and Electrodialysis. Membr. Water Treat. 2019, 10, 139–148. [Google Scholar] [CrossRef]
- Lafi, R.; Gzara, L.; Lajimi, R.H.; Hafiane, A. Treatment of Textile Wastewater by a Hybrid Ultrafiltration/Electrodialysis Process. Chem. Eng. Process. 2018, 132, 105–113. [Google Scholar] [CrossRef]
- Ye, W.; Liu, R.; Chen, X.; Chen, Q.; Lin, J.; Lin, X.; Van der Bruggen, B.; Zhao, S. Loose Nanofiltration-Based Electrodialysis for Highly Efficient Textile Wastewater Treatment. J. Membr. Sci. 2020, 608, 118182. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Q.; Huang, X.; Yan, Z.; Lin, X.; Ye, W.; Arcadio, S.; Luis, P.; Bi, J.; Van der Bruggen, B.; et al. Integrated Loose Nanofiltration-Electrodialysis Process for Sustainable Resource Extraction from High-Salinity Textile Wastewater. J. Hazard. Mat. 2021, 419, 126505. [Google Scholar] [CrossRef]
- Rápó, E.; Tonk, S. Factors Affecting Synthetic Dye Adsorption; Desorption Studies: A Review of Results from the Last Five Years (2017–2021). Molecules 2021, 26, 5419. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Majewska-Nowak, K.; Grzegorzek, M. Removal of Reactive Dyes from Aqueous Solutions Using Ultrafiltration Membranes. Environ. Prot. Eng. 2021, 47, 109–121. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Majewska-Nowak, K.; Ahmed, M.; Grzegorzek, M.K. The Separation of Mineral Salt from a Dye-Salt Aqueous Mixture by Electrodialysis. Desal. Water Treat. 2023, 316, 532–541. [Google Scholar] [CrossRef]
- Rautenbach, R.; Albrecht, R. Membrane Processes; John Wiley&Sons Ltd.: West Sussex, UK, 1989. [Google Scholar]
- Hyder, A.G.; Morales, B.A.; Cappelle, M.A.; Percival, S.J.; Small, L.J.; Spoerke, E.D.; Rempe, S.B.; Walker, W.S. Evaluation of Electrodialysis Desalination Performance of Novel Bioinspired and Conventional Ion Exchange Membranes with Sodium Chloride Feed Solutions. Membranes 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, Y.; Yan, H.; Wu, K.; Xu, T. Purification of Methylsulfonylmethane from Mixtures Containing Salt by Conventional Electrodialysis. Membranes 2020, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Benkhaya, S.; M’rabet, S.; Harfi, A.E. Classifications, Properties, Recent Synthesis and Applications of Azo Dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef]
- Won, S.W.; Han, M.H.; Yun, Y.-S. Different Binding Mechanisms in Biosorption of Reactive Dyes According to Their Reactivity. Water Res. 2008, 42, 4847–4855. [Google Scholar] [CrossRef]
- Egabaierdi, G.; Yu, H.-B.; Li, Y.; Zhang, X.-Q.; Song, H.-O.; Du, M.-X. Effect of dye aggregation and salting-out on membrane fouling and control measures during nanofiltration of saline dye wastewater. J. Water Proc. Eng. 2022, 50, 103285. [Google Scholar] [CrossRef]
- Jiang, M.; Ye, K.; Lin, J.; Zhang, X.; Ye, W.; Zhao, S.; Van der Bruggen, B. Effective Dye Purification Using Tight Ceramic Ultrafiltration Membran. J. Membr. Sci. 2018, 566, 151–160. [Google Scholar] [CrossRef]
- Tanaka, N.; Nagase, M.; Higa, H. Organic fouling behavior of commercially available hydrocarbon-based anion-exchange membranes by various organic-fouling substances. Desalination 2012, 296, 81–86. [Google Scholar] [CrossRef]
- Cao, R.; Duan, F.; Ji, W.; Ruan, D.; Xu, Y.; Shi, S.; Li, Y.; Cao, H. Toward improved antifouling properties and monovalent anion selectivity of anion exchange membrane via interfacial polymerization modification. Desalination 2025, 601, 118562. [Google Scholar] [CrossRef]
- Berkessa, Y.W.; Lang, Q.; Yan, B.; Kuang, S.; Mao, D.; Shu, L.; Zhang, Y. Anion Exchange Membrane Organic Fouling and Mitigation in Salt Valorisation Process from High Salinity Textile Wastewater by Bipolar Membrane Electrodialysis. Desalination 2019, 465, 94–103. [Google Scholar] [CrossRef]
- Lee, H.-J.; Hong, M.-K.; Han, S.-D.; Cho, S.-H.; Moon, S.-H. Fouling of an Anion Exchange Membrane in the Electrodialysis Desalination Process in the Presence of Organic Foulants. Desalination 2009, 238, 60–69. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, S.; Cao, H.; Li, Y.; Van der Bruggen, B. Comparative Studies on Fouling of Homogeneous Anion Exchange Membranes by Different Structured Organics in Electrodialysis. J. Environ. Sci. 2019, 77, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Phukan, R.; Guttierez, L.; De Schepper, W.; Vanoppen, M.; Verbeken, K.; Raes, K.; Verliefde, A.; Cornelissen, E. Short Term Fouling Tests on Homogeneous and Heterogeneous Anion-Exchange Membranes from Food and Bio-Based Industrial Streams: Foulant Identification and Characterization. Sep. Purif. Technol. 2023, 322, 124247. [Google Scholar] [CrossRef]






| Dye Name | MW, Da | Reactive Group/Number of Reactive Groups | Dye Symbol | λmax 1, nm | Structural Formula |
|---|---|---|---|---|---|
| Reactive Orange 16 | 617.53 | Vinyl sulfone/1 | RO16 | 486 | C20H17N3Na2O11S3 |
| Remazol Brilliant Blue R | 626.53 | Vinyl sulfone/1 | RBB | 594 | C22H16N2Na2O11S3 |
| Reactive Orange 20 | 682.18 | Monochloro-triazine/1 | RO20 | 491 | C23H16ClN7O10S3 |
| Reactive Black 5 | 991.8 | Vinyl sulfone/2 | RB5 | 533 | C26H21N5Na4O19S6 |
| Reactive Red 120 | 1469.98 | Dichloro-triazine/2 | RR120 | 624 | C44H24Cl2N14Na6O20S6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska-Nowak, K.; Ahmed, A.E.; Grzegorzek, M.; Baraniec, K. Suitability of Electrodialysis with Monovalent Selective Anion-Exchange Membranes for Fractionation of Aqueous Mixture Containing Reactive Dye and Mineral Salt. Membranes 2025, 15, 85. https://doi.org/10.3390/membranes15030085
Majewska-Nowak K, Ahmed AE, Grzegorzek M, Baraniec K. Suitability of Electrodialysis with Monovalent Selective Anion-Exchange Membranes for Fractionation of Aqueous Mixture Containing Reactive Dye and Mineral Salt. Membranes. 2025; 15(3):85. https://doi.org/10.3390/membranes15030085
Chicago/Turabian StyleMajewska-Nowak, Katarzyna, Arif Eftekhar Ahmed, Martyna Grzegorzek, and Karolina Baraniec. 2025. "Suitability of Electrodialysis with Monovalent Selective Anion-Exchange Membranes for Fractionation of Aqueous Mixture Containing Reactive Dye and Mineral Salt" Membranes 15, no. 3: 85. https://doi.org/10.3390/membranes15030085
APA StyleMajewska-Nowak, K., Ahmed, A. E., Grzegorzek, M., & Baraniec, K. (2025). Suitability of Electrodialysis with Monovalent Selective Anion-Exchange Membranes for Fractionation of Aqueous Mixture Containing Reactive Dye and Mineral Salt. Membranes, 15(3), 85. https://doi.org/10.3390/membranes15030085






