Bacterial Contamination of Ultrafiltration Installation Applied to Carwash Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. UF Installation
2.2. Cleaning Solutions
2.3. Bacterial Analysis
Taxonomic Characteristics of the Isolated Bacteria
3. Results
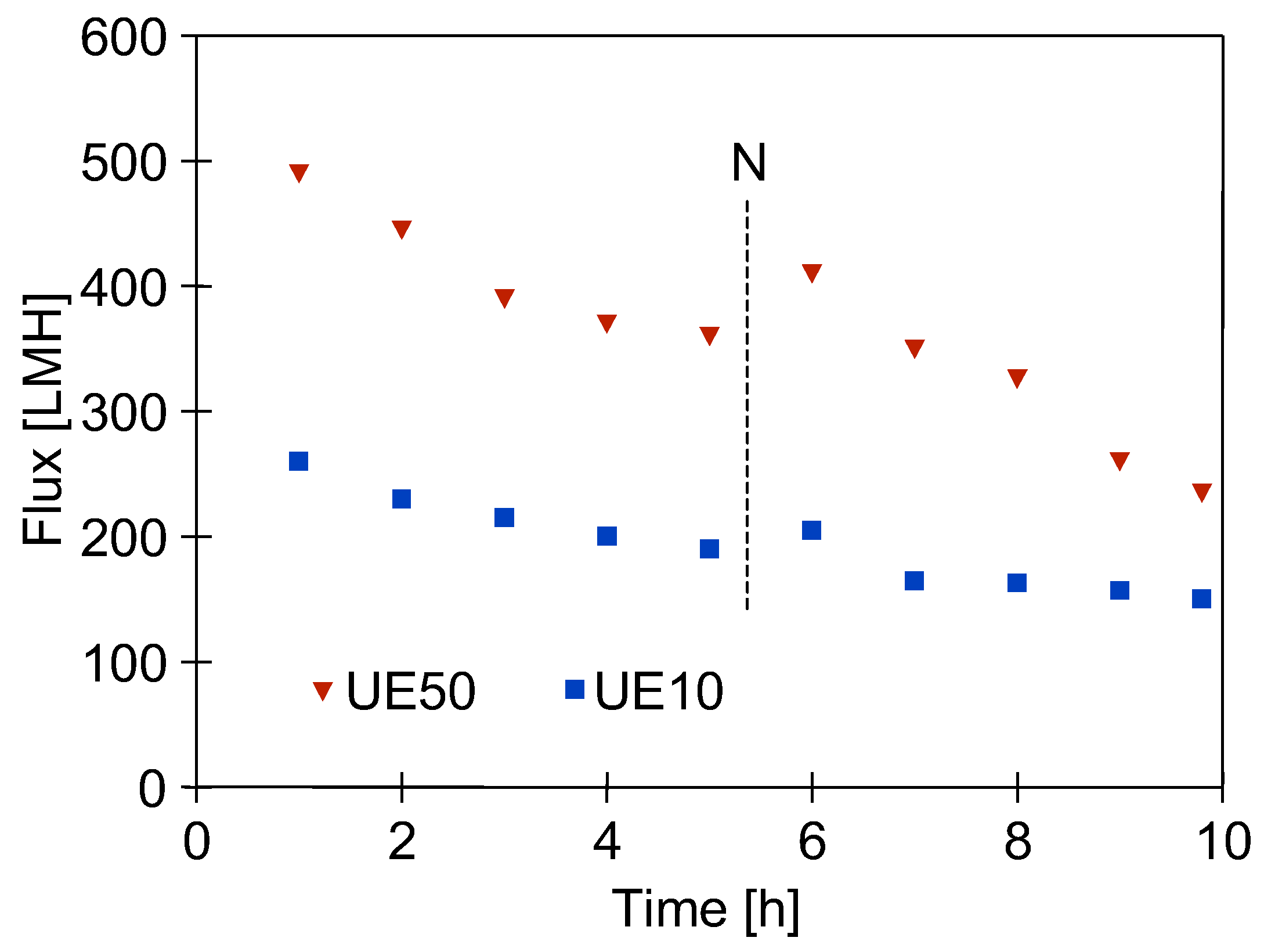
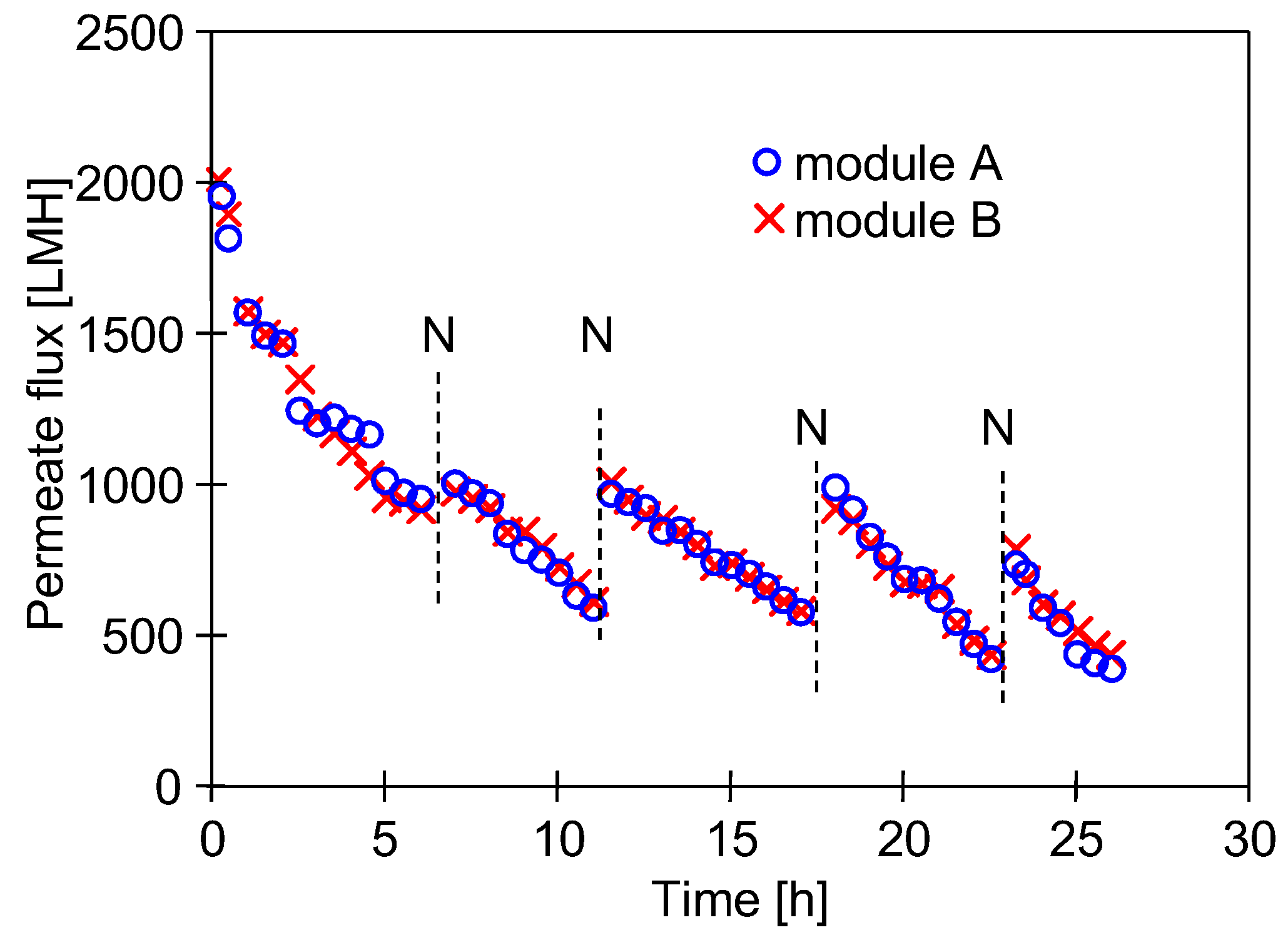
3.1. Restart of UF Installation
3.2. Influence of Membrane Permeability
3.3. Influence of Extended Cleaning Time
3.4. Nutrients and Bacterial Growth
3.5. Influence of High-Temperature Washing
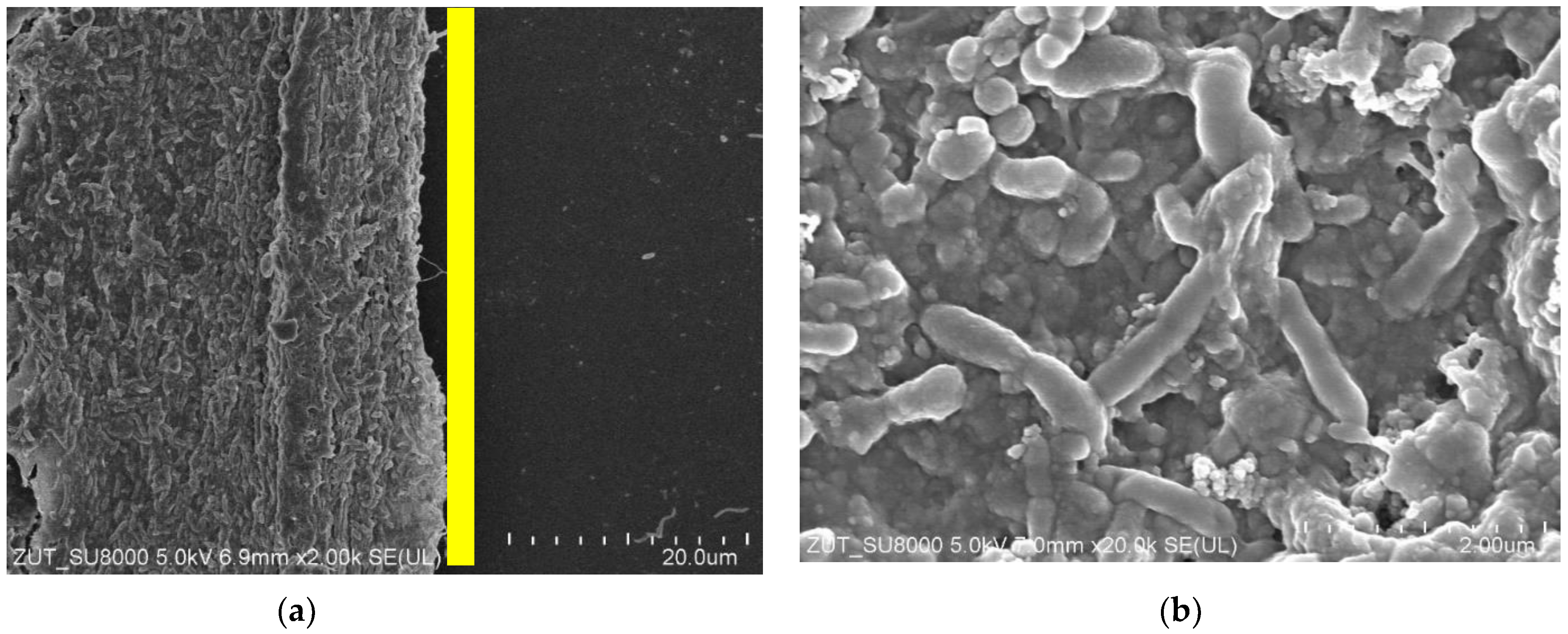
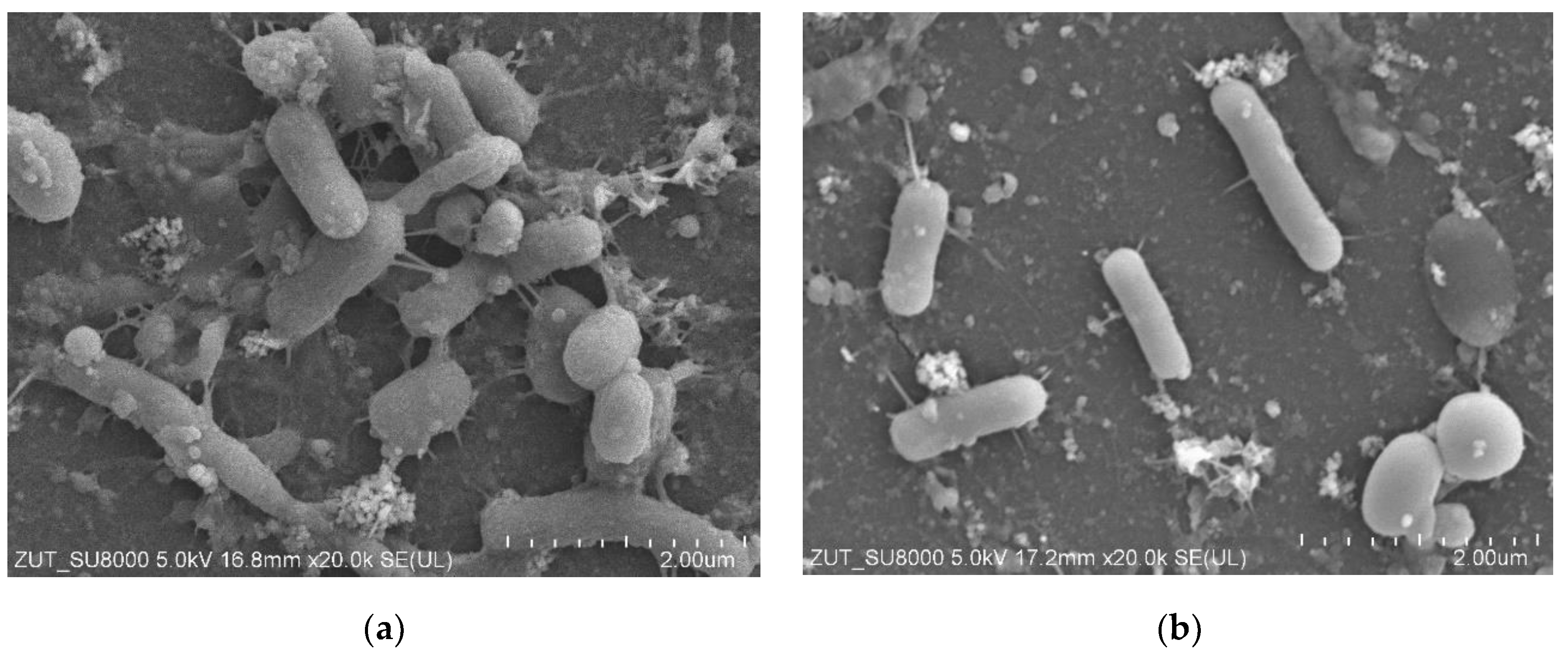
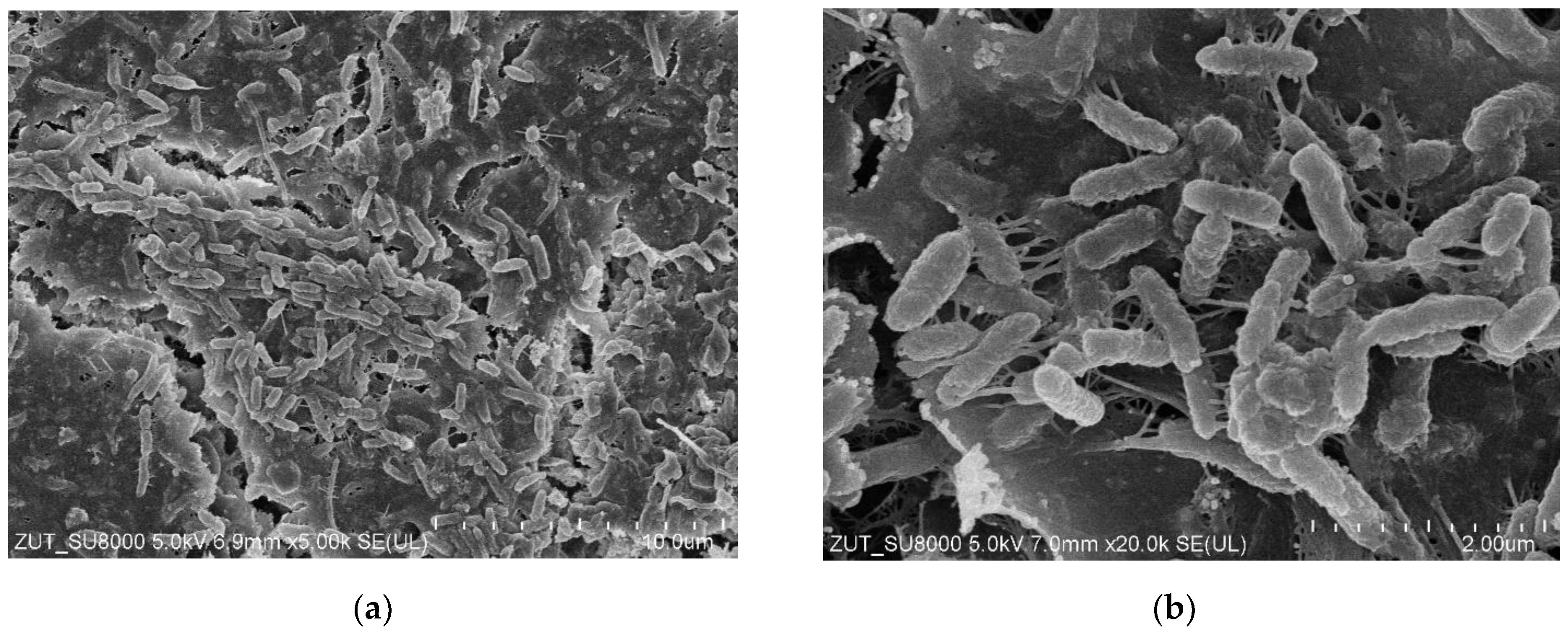
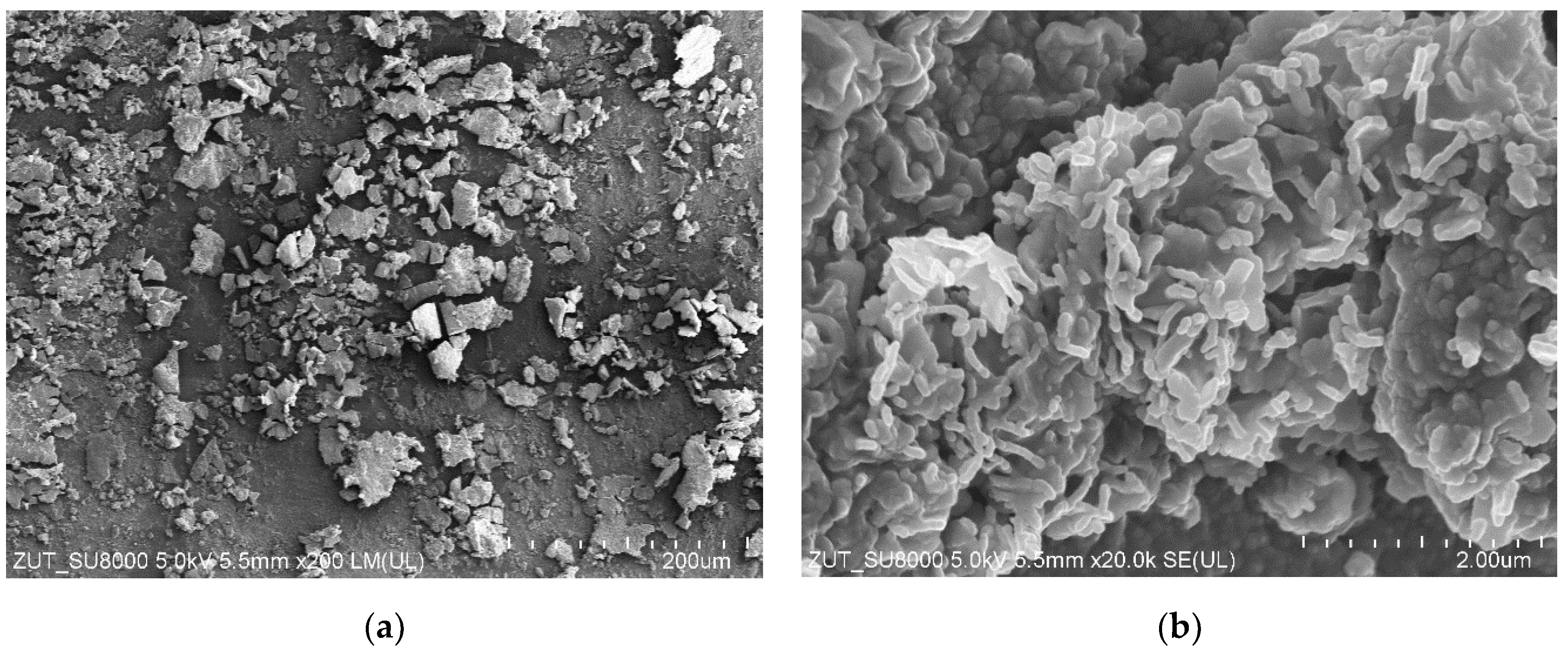
3.6. Bacterial Analysis
3.7. Influence of Chemical Washing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kuan, W.-H.; Hu, C.-Y.; Ke, L.-W.; Wu, J.-M. A Review of On-Site Carwash Wastewater Treatment. Sustainability 2022, 14, 5764. [Google Scholar] [CrossRef]
- Monney, I.; Donkor, E.A.; Buamah, R. Clean vehicles, polluted waters: Empirical estimates of water consumption and pollution loads of the carwash industry. Heliyon 2020, 6, e03952. [Google Scholar] [CrossRef]
- Boluarte, I.A.R.; Andersen, M.; Pramanik, B.K.; Chang, C.-Y.; Bagshaw, S.; Farago, L.; Jegatheesan, V.; Shu, L. Reuse of car wash wastewater by chemical coagulation and membrane bioreactor treatment processes. Int. Biodeterior. Biodegrad. 2016, 113, 44–48. [Google Scholar] [CrossRef]
- Zaneti, R.; Etchepare, R.; Rubio, J. Car wash wastewater reclamation. Full-scale application and upcoming features. Resour. Conserv. Recycl. 2011, 55, 953–959. [Google Scholar] [CrossRef]
- Mujumdar, M.M.; Rajagolkar, S.P.; Jadhav, P. Treatment of vehicle washing waste water for maximum reuse of treated water and reduce fresh water consumption. Int. J. Recent Res. Asp. 2020, 7, 1–5. Available online: https://www.academia.edu/43768187 (accessed on 27 October 2024).
- Elgaali, E.; Akram, M. Recycling and Reuse of Wastewater Generated in Car-Washing Facilities. Adv. Sci. Technol. Eng. Syst. J. 2021, 6, 521–525. [Google Scholar] [CrossRef]
- Uçar, D. Membrane processes for the reuse of car washing wastewater. J. Water Reuse Desalin. 2018, 8, 169–175. [Google Scholar] [CrossRef]
- Li, T.; Tan, X.-j.; Cui, F.-y.; Zhou, Q.; Yin, J. Reuse of carwash wastewater with hollow fiber membrane aided by enhanced coagulation and activated carbon treatments. Water Sci. Technol. 2007, 56, 111–118. [Google Scholar] [CrossRef]
- Moazzem, S.; Wills, J.; Fan, L.; Roddick, F.; Jegatheesan, V. Performance of ceramic ultrafiltration and reverse osmosis membranes in treating car wash wastewater for reuse. Environ. Sci. Pollut. Res. 2018, 25, 8654–8668. [Google Scholar] [CrossRef]
- Pinto, A.C.S.; de Barros Grossi, L.; de Melo, R.A.C.; de Assis, T.M.; Ribeiro, V.M.; Amaral, M.C.S.; de Souza Figueiredo, K.C. Carwash Wastewater Treatment by Micro and Ultrafiltration Membranes: Effects of Geometry, Pore Size, Pressure Difference and Feed Flow Rate in Transport Properties. J. Water Process Eng. 2017, 17, 143–148. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. The Application of Polyethersulfone Ultrafiltration Membranes for Separation of Car Wash Wastewaters: Experiments and Modelling. Membranes 2023, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Leon-Zerpa, F.A.; Vaswani-Reboso, J.; Tavares, T.; Ramos-Martín, A.; Mendieta-Pino, C.A. Advances in Drinking Water Treatment through Piloting with UF Membranes. Water 2023, 15, 1031. [Google Scholar] [CrossRef]
- Wan, K.; Fang, T.; Zhang, W.; Ren, G.; Tang, X.; Ding, Z.; Wang, Y.; Qi, P.; Liu, X. Enhanced antimony removal within lamellar nanoconfined interspaces through a self-cleaning MXene@CNF@FeOOH water purification membrane. Chem. Eng. J. 2023, 465, 143018. [Google Scholar] [CrossRef]
- Monney, I.; Buamah, R.; Donkor, E.A.; Etuaful, R.; Nota, H.K.; Ijzer, H. Treating waste with waste: The potential of synthesized alum from bauxite waste for treating car wash wastewater for reuse. Environ. Sci. Pollut. Res. 2019, 26, 12755–12764. [Google Scholar] [CrossRef]
- Hamada, T.; Miyazaki, Y. Reuse of carwash water with a cellulose acetate ultrafiltration membrane aided by flocculation and activated carbon treatments. Desalination 2004, 169, 257–267. [Google Scholar] [CrossRef]
- Vishwakarma, V.; Kandasamy, J.; Vigneswaran, S. Surface Treatment of Polymer Membranes for Effective Biofouling Control. Membranes 2023, 13, 736. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, W.; Woźniak, P.; Gryta, M.; Grzechulska-Damszel, J.; Daniluk, M. Cleaning of Ultrafiltration Membranes: Long-Term Treatment of Car Wash Wastewater as a Case Study. Membranes 2024, 14, 159. [Google Scholar] [CrossRef]
- Available online: https://www.grandviewresearch.com/industry-analysis/car-wash-service-market (accessed on 21 October 2024).
- Woźniak, P.; Dubicki, M.; Gryta, M. Microbiological Hazard Analysis of Car Wash Wastewater. Pol. J. Environ. Stud. 2023, 32, 3871–3882. [Google Scholar] [CrossRef]
- Semiào, A.J.C.; Habimana, O.; Casey, E. Bacterial adhesion onto nanofiltration and reverse osmosis membranes: Effect of permeate flux. Water Res. 2014, 63, 296–305. [Google Scholar] [CrossRef]
- de Vries, H.J.; Kleibusch, E.; Hermes, G.D.A.; van den Brink, P.; Plugge, C.M. Biofouling control: The impact of biofilm dispersal and membrane flushing. Water Res. 2021, 198, 117163. [Google Scholar] [CrossRef]
- Elkhatat, A.; Qiblawey, H. Biofouling in membrane systems for zero liquid discharge: A review on microbial dynamics, analytical approaches, and environmental influences. Environ. Technol. Innov. 2024, 35, 103635. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, W.N. Biofouling formation and structure on original and modified PVDF membranes: Role of microbial species and membrane properties. RSC Adv. 2017, 7, 37990–38000. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Q.; Song, X.; Wang, J.; Cai, W. Microbial Responses to Various Types of Chemical Regents during On-Line Cleaning of UF Membranes. Membranes 2022, 12, 920. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Zhu, X.; Herzberg, M.; Walker, S.; Jassby, D. Impact of Physical and Chemical Cleaning Agents on Specific Biofilm Components and the Implications for Membrane Biofouling Management. Ind. Eng. Chem. Res. 2018, 57, 3359–3370. [Google Scholar] [CrossRef]
- Bremer, P.J.; Fillery, S.; McQuillan, A.J. Laboratory scale Clean-In-Place (CIP) studies on the effectiveness of different caustic and acid wash steps on the removal of dairy biofilms. Int. J. Food Microbiol. 2006, 106, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhu, X. Biofilm formation and food safety in food industries. Trends Food Sci. Technol. 2009, 20, 407–413. [Google Scholar] [CrossRef]
- Silva, H.O.; Lima, J.A.S.; Aguilar, C.E.G.; Rossi, G.A.M.; Mathias, L.A.; Vidal, A.M.C. Efficiency of Different Disinfectants on Bacillus cereus Sensu Stricto Biofilms on Stainless-Steel Surfaces in Contact With Milk. Front. Microbiol. 2018, 9, 2934. [Google Scholar] [CrossRef]
- Lee, E.-S.; Kim, J.-H.; Oh, M.-H. Inhibitory Effects of Combinations of Chemicals on Escherichia coli, Bacillus cereus, and Staphylococcus aureus Biofilms during the Clean-in-Place Process at an Experimental Dairy Plant. J. Food Prot. 2020, 83, 1302–1306. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Svensson, B.; Guinebretiere, M.H.; Lindback, T.; Andersson, M.; Schulz, A.; Fricker, M.; Christiansson, A.; Granum, P.E.; Martlbauer, E.; et al. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 2005, 151, 183–197. [Google Scholar] [CrossRef]
- Burgess, S.A.; Flint, S.H.; Lindsay, D. Characterization of thermophilic bacilli from a milk powder processing plant. J. Appl. Microbiol. 2014, 116, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, P.; Gryta, M. Application of Polymeric Tubular Ultrafiltration Membranes for Separation of Car Wash Wastewater. Membranes 2024, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Nilusha, R.T.; Wei, Y. New Insights into the Microbial Diversity of Cake Layer in Yttria Composite Ceramic Tubular Membrane in an Anaerobic Membrane Bioreactor (AnMBR). Membranes 2021, 11, 108. [Google Scholar] [CrossRef]
- Gryta, M.; Woźniak, P. Polyethersulfone membrane fouling mitigation during ultrafiltration of wastewaters from car washes. Desalination 2024, 574, 117254. [Google Scholar] [CrossRef]
- Weis, A.; Bird, M.R.; Nyström, M. The chemical cleaning of polymeric UF membranes fouled with spent sulphite liquor over multiple operational cycles. J. Membr. Sci. 2003, 216, 67–79. [Google Scholar] [CrossRef]
- Weis, A.; Bird, M.R. The influence of multiple fouling and cleaning cycles upon the membrane processing of lignosulphonates. Food Bioprod. Process. 2001, 79, 184–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Z.; Bai, H.; Zhao, L.; He, L.; Shi, C. A Pilot-Scale Treatment of Steel Plant Wastewater by PVDF Hollow Fiber Ultrafiltration Membrane with Low Packing Density. Separations 2022, 9, 37. [Google Scholar] [CrossRef]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- El Batouti, M.; Alharby, N.F.; Elewa, M.M. Review of New Approaches for Fouling Mitigation in Membrane Separation Processes in Water Treatment Applications. Separations 2022, 9, 1. [Google Scholar] [CrossRef]
- Gryta, M. Long-term performance of membrane distillation process. J. Membr. Sci. 2005, 265, 153–159. [Google Scholar] [CrossRef]
- Khasheii, B.; Mahmoodi, P.; Mohammadzadeh, A. Siderophores: Importance in bacterial pathogenesis and applications in medicine and industry. Microbiol. Res. 2021, 250, 126790. [Google Scholar] [CrossRef]
- Javier, L.; Pulido-Beltran, L.; Vrouwenvelder, J.S.; Farhat, N.M. Permeation Increases Biofilm Development in Nanofiltration Membranes Operated with Varying FeedWater Phosphorous Concentrations. Membranes 2022, 12, 335. [Google Scholar] [CrossRef] [PubMed]
- Vidic, J.; Chaix, C.; Manzano, M.; Heyndrickx, M. Food Sensing: Detection of Bacillus cereus Spores in Dairy Products. Biosensors 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Flint, S.H.; Palmer, J.S. Bacillus cereus spores and toxins–The potential role of biofilms. Food Microbiol. 2020, 90, 103493. [Google Scholar] [CrossRef]
- Zeinab Breijyeh, Z.; Buthaina Jubeh, B.; Rafik Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Sibanda, T.; Selvarajan, R.; Tekere, M. Targeted 165 rRNA amplicon analysis reveals the diversity of bacterial communities in carwash effluents. Int. Microbiol. 2019, 22, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Schwering, M.; Song, J.; Louie, M.; Turner, R.J.; Ceri, H. Multi-species biofilms defined from drinking water microorganisms provide increased protection against chlorine disinfection. Biofouling J. Bioadhesion Biofilm Res. 2013, 29, 917–928. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Erschen, S.; Krause, R.; Müller, H.; Cernava, T.; Berg, G. Enhanced survival of multi-species biofilms under stress is promoted by low-abundant but antimicrobial-resistant keystone species. J. Hazard. Mater. 2022, 422, 126836. [Google Scholar] [CrossRef]
- Zhu, Z.; Shan, L.; Li, X.; Hu, F.; Yuan, Y.; Zhong, D.; Zhang, J. Effects of interspecific interactions on biofilm formation potential and chlorine resistance: Evaluation of dual-species biofilm observed in drinking water distribution systems. J. Water Process Eng. 2020, 38, 101564. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Long-Term Performance of Ultrafiltration Membranes: Corrosion Fouling Aspect. Materials 2023, 16, 1673. [Google Scholar] [CrossRef]







| Cleaning Agent | Component | Concentration [wt.%] |
|---|---|---|
| NaOH | >40 | |
| P3 Ultrasil 11 | ethylenediaminetetraacetic acid tetrasodium salt | >30 |
| (powder) | anionic surfactants | <5 |
| non-ionic surfactants | <5 | |
| NaOH | 3–5 | |
| Insect | ethylenediaminetetraacetic acid tetrasodium salt | 3–5 |
| (solution) | diethylene glycol butyl ether | 3–5 |
| sulfonic acids, C14-16-alkane hydroxy and C14-16-alkene, sodium salts | 5–7 |
| Element | After UF | After Washing |
|---|---|---|
| C | 47.67 | 43.59 |
| O | 42.34 | 42.66 |
| S | 6.92 | 12.55 |
| Si | 2.07 | 0.99 |
| P | 0.29 | 0.14 |
| Mg | 0.12 | − |
| Ca | 0.17 | 0.07 |
| Fe | 0.42 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, P.; Gryta, M. Bacterial Contamination of Ultrafiltration Installation Applied to Carwash Wastewater Treatment. Membranes 2025, 15, 71. https://doi.org/10.3390/membranes15030071
Woźniak P, Gryta M. Bacterial Contamination of Ultrafiltration Installation Applied to Carwash Wastewater Treatment. Membranes. 2025; 15(3):71. https://doi.org/10.3390/membranes15030071
Chicago/Turabian StyleWoźniak, Piotr, and Marek Gryta. 2025. "Bacterial Contamination of Ultrafiltration Installation Applied to Carwash Wastewater Treatment" Membranes 15, no. 3: 71. https://doi.org/10.3390/membranes15030071
APA StyleWoźniak, P., & Gryta, M. (2025). Bacterial Contamination of Ultrafiltration Installation Applied to Carwash Wastewater Treatment. Membranes, 15(3), 71. https://doi.org/10.3390/membranes15030071






