Gas and Steam Permeation Properties of Cation-Exchanged ZSM-5 Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the NaZSM-5 Powder and Membrane
2.2. Cation Exchange
2.3. Characterization of ZSM-5 Powder and Membrane
2.4. Permeation and Separation Tests
3. Results and Discussions
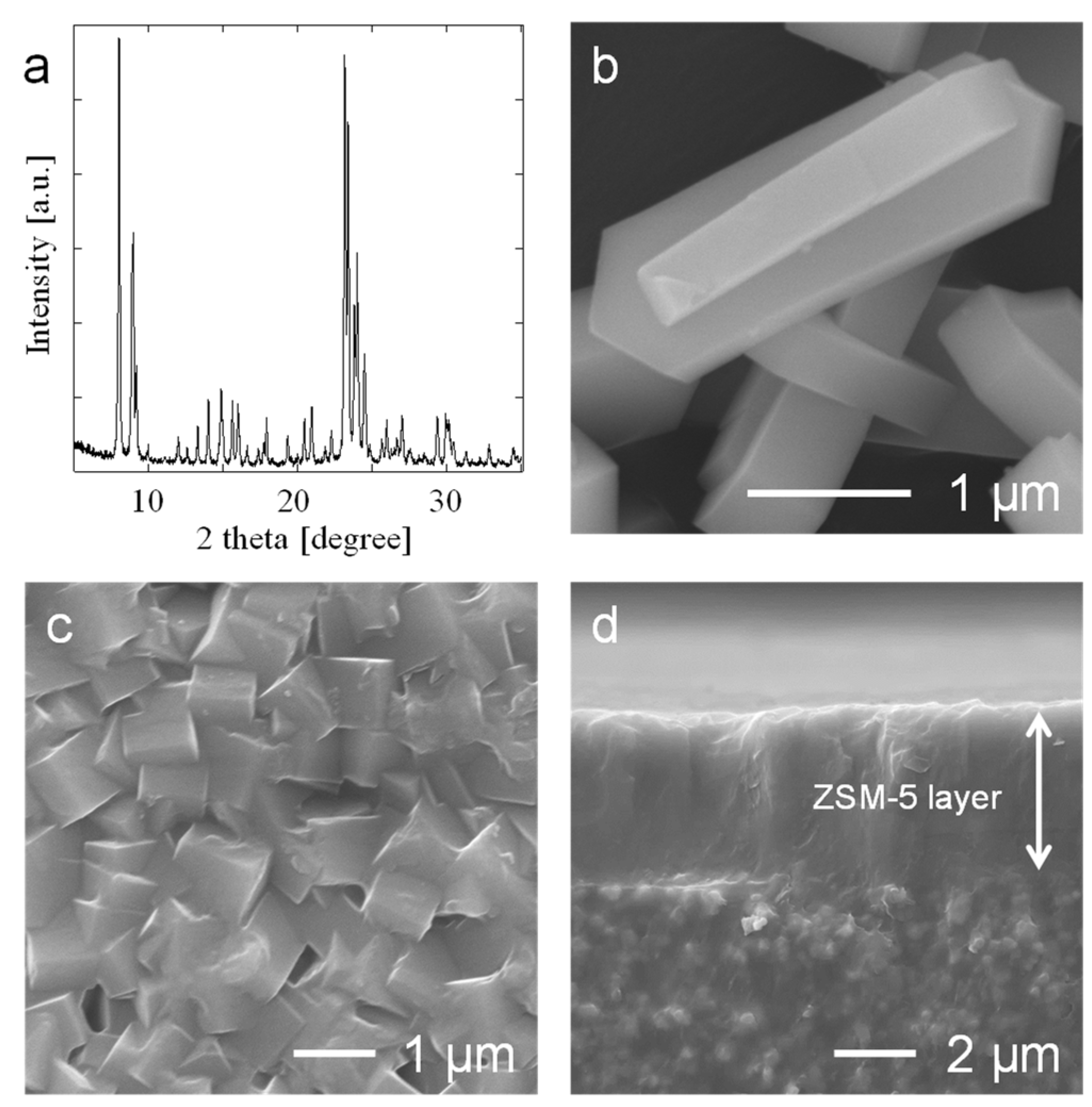
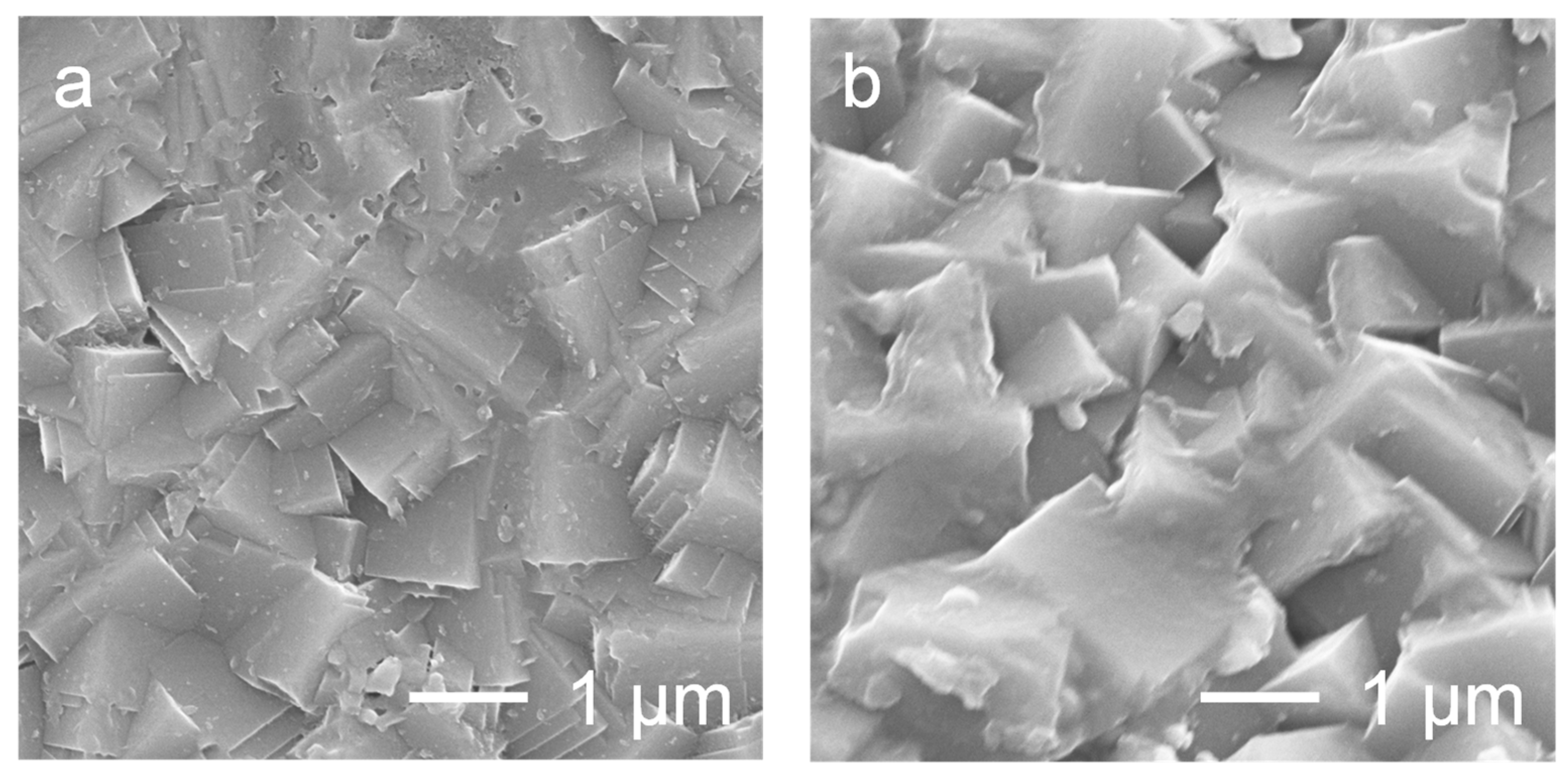
3.1. Preparation of the ZSM-5 Powder and Membrane
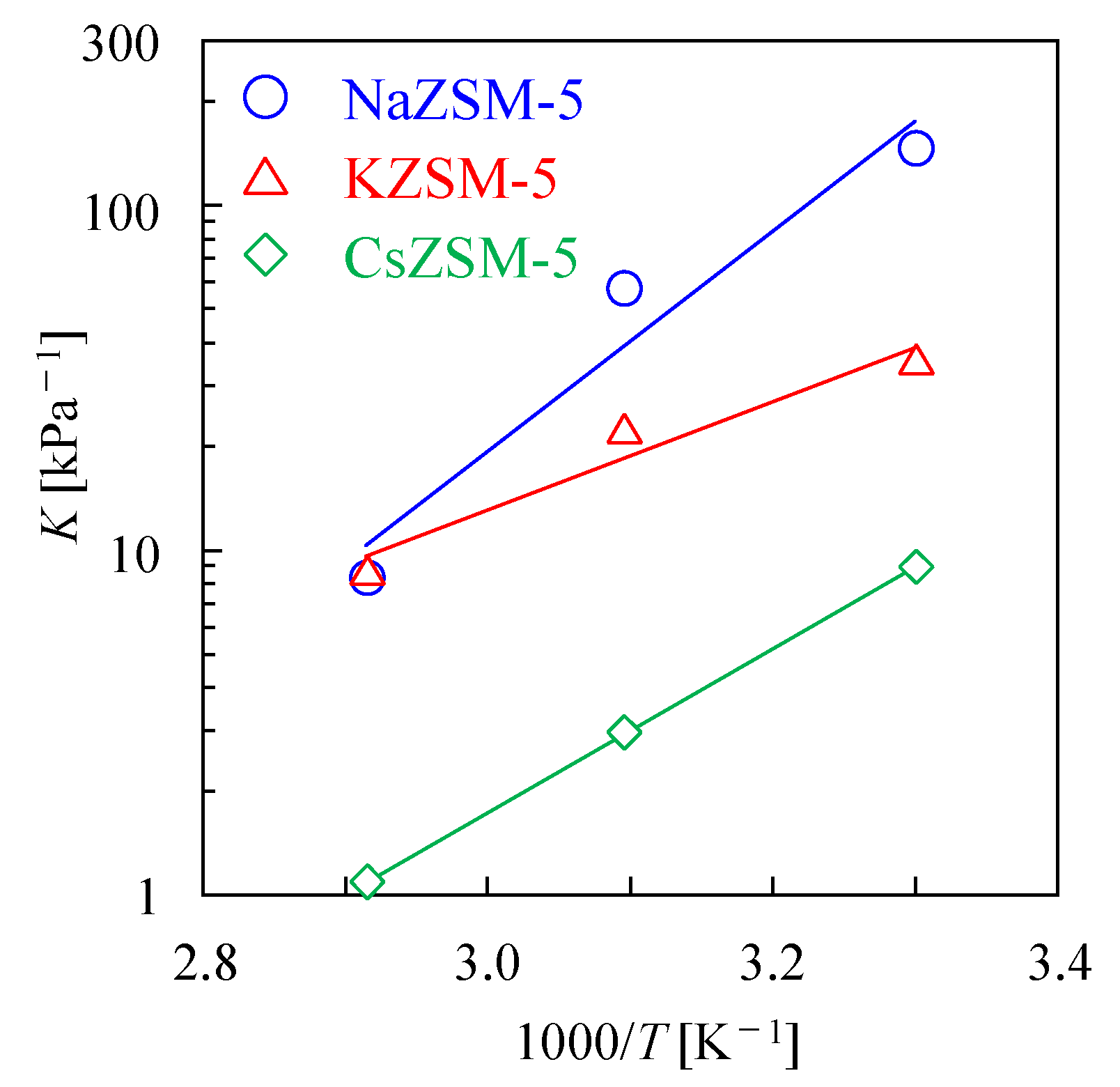
3.2. Effect of Cations on Microporosity and H2O Adsorption
3.3. Effect on Gas Permeability and Effective Zeolitic Pore Size
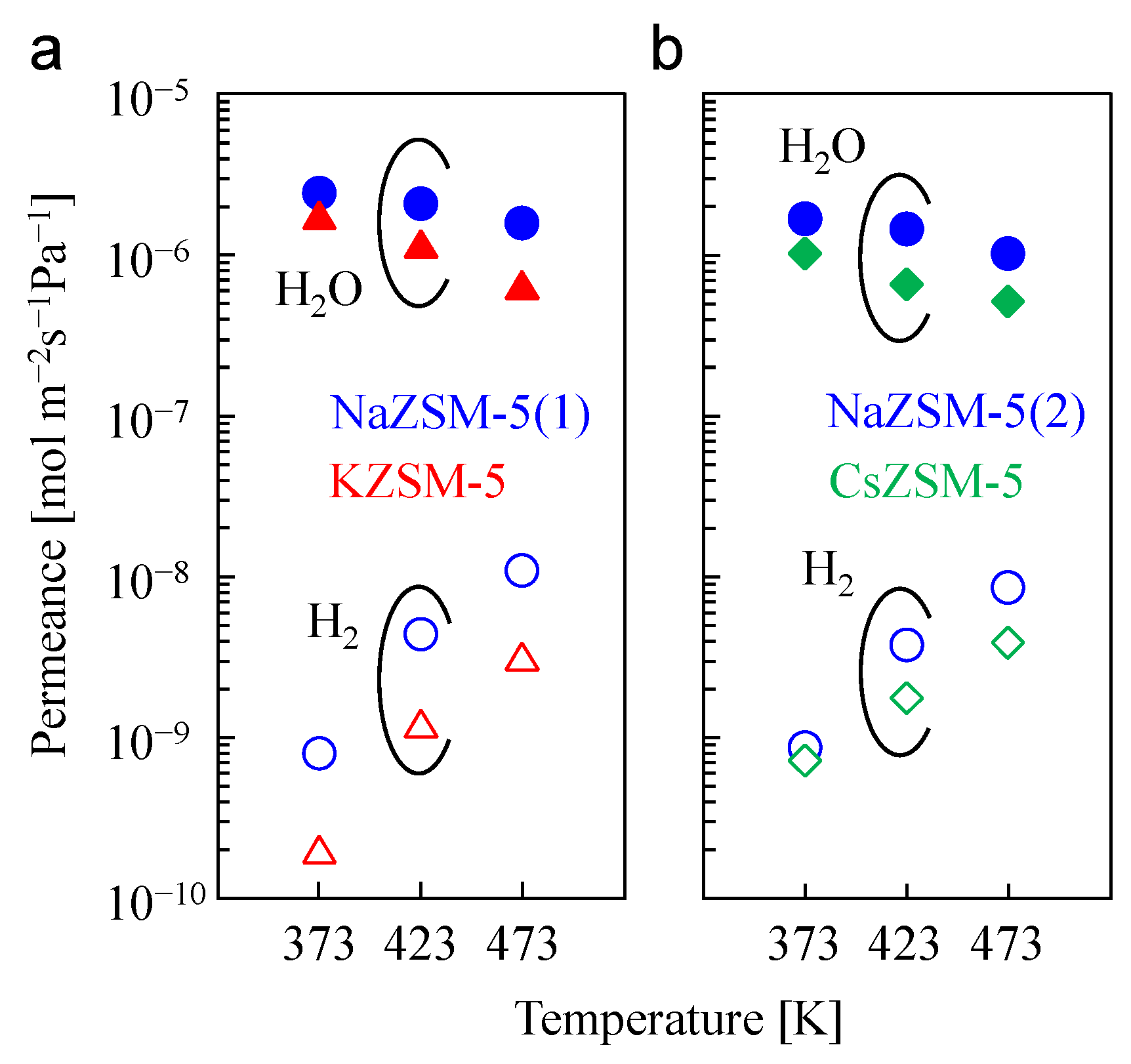
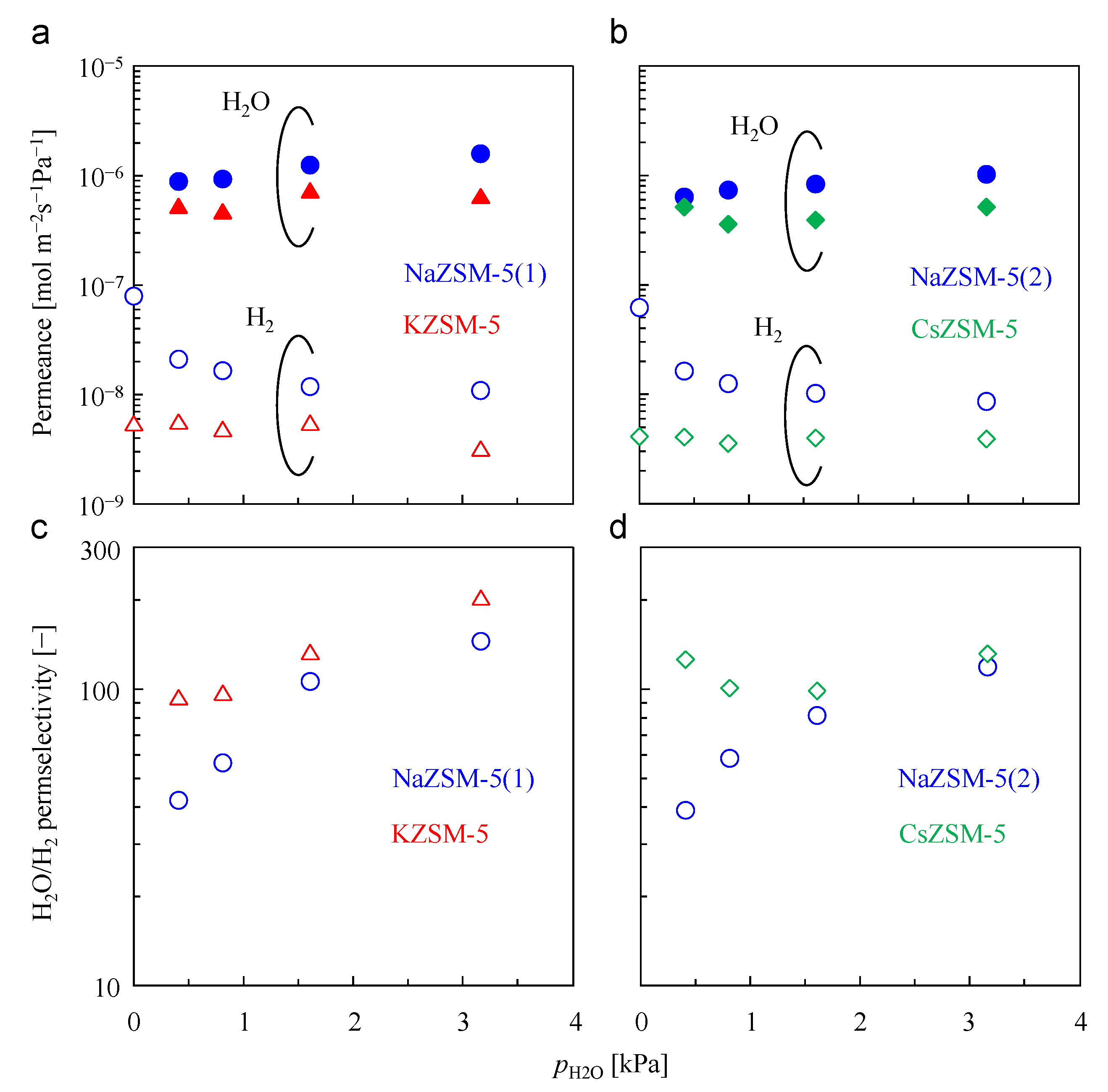
3.4. Effect on H2O/H2 Permeation and Separation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Struis, R.P.W.J.; Stucki, S.; Wiedorn, M. A membrane reactor for methanol synthesis. J. Membr. Sci. 1996, 113, 93–100. [Google Scholar] [CrossRef]
- Chen, G.; Yuan, Q. Methanol synthesis from CO2 using a silicone rubber/ceramic composite membrane reactor. Sep. Purif. Technol. 2004, 34, 227–237. [Google Scholar] [CrossRef]
- Piera, E.; Salomón, M.A.; Coronas, J.; Menéndez, M.; Santamaría, J. Synthesis, characterization and separation properties of a composite mordenite/ZSM-5/chabazite hydrophilic membrane. J. Membr. Sci. 1998, 149, 99–114. [Google Scholar] [CrossRef]
- Aoki, K.; Kusakabe, K.; Morooka, S. Separation of gases with an A-type zeolite membrane. Ind. Eng. Chem. Res. 2000, 39, 2245–2251. [Google Scholar] [CrossRef]
- Sato, K.; Sugimoto, K.; Sekine, Y.; Takada, M.; Matsukata, M.; Nakane, T. Application of FAU-type zeolite membranes to vapor/gasseparation under high pressure and high temperature up to 5 MPa and 180 °C. Micropor. Mesopor. Mater. 2007, 101, 312–318. [Google Scholar] [CrossRef]
- Sawamura, K.; Shirai, T.; Ohsuna, T.; Hagino, T.; Takada, M.; Sekine, Y.; Kikuchi, E.; Matsukata, M. Separation behavior of steam from hydrogen and methanol through mordenite membrane. J. Chem. Eng. Jpn. 2008, 41, 870–877. [Google Scholar] [CrossRef]
- Sawamura, K.; Izumi, T.; Kawasaki, K.; Daikohara, S.; Ohsuna, T.; Takada, M.; Sekine, Y.; Kikuchi, E.; Matsukata, M. Reverse-selective microporous membrane for gas separation. Chem. Asian J. 2009, 4, 1070–1077. [Google Scholar] [CrossRef]
- Sandström, L.; Palomino, M.; Hedlund, J. High flux zeolite X membranes. J. Membr. Sci. 2010, 354, 171–177. [Google Scholar] [CrossRef]
- Wang, H.; Lin, Y.S. Effects of water vapor on gas permeation and separation properties of MFI zeolite membranes at high temperatures. AIChE J. 2012, 58, 153–162. [Google Scholar] [CrossRef]
- Hirota, Y.; Yamamoto, Y.; Nakai, T.; Hayami, S.; Nishiyama, N. Application of silylated ionic liquid-derived organosilica membranes to simultaneous separation of methanol and H2O from H2 and CO2 at high temperature. J. Membr. Sci. 2018, 563, 345–350. [Google Scholar] [CrossRef]
- Moriyama, N.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Selective water vapor permeation from steam/non-condensable gas mixtures via organosilica membranes at moderate-to-high temperatures. J. Membr. Sci. 2019, 589, 117254. [Google Scholar] [CrossRef]
- Moriyama, N.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Improved performance of organosilica membranes for steam recovery at moderate-to-high temperatures via the use of a hydrothermally stable intermediate layer. J. Membr. Sci. 2021, 620, 118895. [Google Scholar] [CrossRef]
- Li, Z.; Deng, Y.; Wang, Z.; Hu, J.; Haw, K.G.; Wang, G.; Kawi, S. A superb water permeable membrane for potential applications in CO2 to liquid fuel process. J. Membr. Sci. 2021, 639, 119682. [Google Scholar] [CrossRef]
- Seshimo, M.; Liu, B.; Lee, H.R.; Yogo, K.; Yamaguchi, Y.; Shigaki, N.; Mogi, Y.; Kita, H.; Nakao, S. Membrane reactor for methanol synthesis using Si-rich LTA zeolite membrane. Membranes 2021, 11, 505. [Google Scholar] [CrossRef]
- Sakai, M.; Tanaka, K.; Matsukata, M. An experimental study of a zeolite membrane reactor for reverse water gas shift. Membranes 2022, 12, 1272. [Google Scholar] [CrossRef]
- van Leeuwen, M.E. Derivation of Stockmayer potential parameters for polar fluids. Fluid Phase Equilib. 1994, 99, 1–18. [Google Scholar] [CrossRef]
- Moriyama, N.; Takeyama, A.; Yamamoto, T.; Sawamura, K.; Gonoi, K.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Steam recovery from flue gas by organosilica membranes for simultaneous harvesting of water and energy. Nat. Commun. 2023, 14, 7641. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Aoki, K.; Tuan, V.A.; Falconer, J.L.; Noble, R.D. Gas permeation properties of ion-exchanged ZSM-5 zeolite membranes. Micropor. Mesopor. Mater. 2000, 39, 485–492. [Google Scholar] [CrossRef]
- Kusakabe, K.; Kuroda, T.; Uchino, K.; Hasegawa, Y.; Morooka, S. Gas permeation properties of ion-exchanged faujasite-type zeolite membranes. AIChE J. 1999, 45, 1220–1226. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Watanabe, K.; Kusakabe, K.; Morooka, S. The separation of CO2 using Y-type zeolite membranes ion-exchanged with alkali metal cations. Sep. Purif. Technol. 2001, 22–23, 319–325. [Google Scholar] [CrossRef]
- Sakai, M.; Sasaki, Y.; Tomono, T.; Seshimo, M.; Matsukata, M. Olefin selective Ag-exchanged X-type zeolite membrane for propylene/propane and ethylene/ethane separation. ACS Appl. Mater. Interfaces 2019, 11, 4145–4151. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Fujimaki, N.; Sasaki, Y.; Yasuda, N.; Seshimo, M.; Matsukata, M. Preferential adsorption of propylene over propane on a Ag-exchanged X-type zeolite membrane. ACS Appl. Mater. Interfaces 2020, 12, 24086–24092. [Google Scholar] [CrossRef]
- Zhu, M.; An, X.; Gui, T.; Wu, T.; Li, Y.; Chen, X. Effects of ion-exchange on the pervaporation performance and microstructure of NaY zeolite membrane. Chin. J. Chem. Eng. 2023, 59, 176–181. [Google Scholar] [CrossRef]
- Sakai, M.; Tsuzuki, Y.; Fujimaki, N.; Matsukata, M. Olefin recovery by *BEA-type zeolite membrane: Affinity-based separation with olefin–Ag+ interaction. Chem. Asian J. 2021, 16, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Li, S.; Funke, H.F.; Falconer, J.L.; Noble, R.D. Ion-exchanged SAPO-34 membranes for light gas separations. Micropor. Mesopor. Mater. 2007, 106, 140–146. [Google Scholar] [CrossRef]
- Biligetu, T.; Wang, Y.; Nishitoba, T.; Otomo, R.; Park, S.; Mochizuki, H.; Kondo, J.N.; Tatsumi, T.; Yokoi, T. Al distribution and catalytic performance of ZSM-5 zeolites synthesized with various alcohols. J. Catal. 2017, 353, 1–10. [Google Scholar] [CrossRef]
- Watanabe, R.; Yokoi, T.; Tatsumi, T. Synthesis and application of colloidal nanocrystals of the MFI-type zeolites. J. Colloid Interface Sci. 2011, 356, 434–441. [Google Scholar] [CrossRef]
- Hirota, Y.; Betsuno, R.; Hotta, Y.; Li, X.; Miyake, K.; Nishiyama, N. Catalytic performance of Zn-containing MFI zeolites in acetone-to-aromatics reactions. Micropor. Mesopor. Mater. 2022, 341, 112070. [Google Scholar] [CrossRef]
- Tang, Z.; Kim, S.-J.; Gu, X.; Dong, J. Microwave synthesis of MFI-type zeolite membranes by seeded secondary growth without the use of organic structure directing agents. Micropor. Mesopor. Mater. 2009, 118, 224–231. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Natsui, M.; Ikeda, A. Gas permeation properties of high-silica CHA-type zeolite membrane. Membranes 2021, 11, 249. [Google Scholar] [CrossRef]
- Olson, D.H.; Haag, W.O.; Borghard, W.S. Use of water as a probe of zeolitic properties: Interaction of water with HZSM-5. Micropor. Mesopor. Mater. 2000, 35–36, 435–446. [Google Scholar] [CrossRef]
- Bolis, V.; Busco, C.; Ugliengo, P. Thermodynamic study of water adsorption in high-silica zeolites. J. Phys. Chem. B 2006, 110, 14849–14859. [Google Scholar] [CrossRef] [PubMed]
- Ohlin, L.; Bazin, P.; Thibault-Starzyk, F.; Hedlund, J.; Grahn, M. Adsorption of CO2, CH4, and H2O in zeolite ZSM-5 studied using in situ ATR-FTIR spectroscopy. J. Phys. Chem. C 2013, 117, 16972–16982. [Google Scholar] [CrossRef]
- Yang, S.; Navrotsky, A. Energetics of formation and hydration of ion-exchanged zeolite Y. Micropor. Mesopor. Mater. 2000, 37, 175–186. [Google Scholar] [CrossRef]
- Sakai, M.; Sasaki, Y.; Kaneko, T.; Matsukata, M. Contribution of pore-connectivity to permeation performance of silicalite-1 membrane; Part I, Pore volume and effective pore size. Membranes 2021, 11, 382. [Google Scholar] [CrossRef]
- Hedlund, J.; Noack, M.; Kölsch, P.; Crease, D.; Caro, J.; Sterte, J. ZSM-5 membranes synthesized without organic templates using a seeding technique. J. Membr. Sci. 1999, 159, 263–273. [Google Scholar] [CrossRef]
- Noack, M.; Kölsch, P.; Caro, J.; Schneider, M.; Toussaint, P.; Sieber, I. MFI membranes of different Si/Al ratios for pervaporation and steam permeation. Micropor. Mesopor. Mater. 2000, 35–36, 253–265. [Google Scholar] [CrossRef]
- Au, L.T.Y.; Chau, J.L.H.; Ariso, C.T.; Yeung, K.L. Preparation of supported Sil-1, TS-1 and VS-1 membranes Effects of Ti and V metal ions on the membrane synthesis and permeation properties. J. Membr. Sci. 2001, 183, 269–291. [Google Scholar] [CrossRef]
- Au, L.T.Y.; Yeung, K.L. An investigation of the relationship between microstructure and permeation properties of ZSM-5 membranes. J. Membr. Sci. 2001, 194, 33–55. [Google Scholar] [CrossRef]
- Algieri, C.; Bernardo, P.; Golemme, G.; Barbieri, G.; Drioli, E. Permeation properties of a thin silicalite-1 (MFI) membrane. J. Membr. Sci. 2003, 222, 181–190. [Google Scholar] [CrossRef]
- Lai, Z.; Tsapatsis, M. Gas and organic vapor permeation through b-oriented MFI membranes. Ind. Eng. Chem. Res. 2004, 43, 3000–3007. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Chu, N.; Yin, X.; Yang, J.; Kong, C.; Wang, A.; Lu, J. Preparation of high-permeance MFI membrane with the modified secondary growth method on the macroporous α-alumina tubular support. J. Membr. Sci. 2008, 320, 303–309. [Google Scholar] [CrossRef]
- Xiao, W.; Yang, J.; Lu, J.; Wang, J. Preparation and characterization of silicalite-1 membrane by counter-diffusion secondary growth. J. Membr. Sci. 2009, 345, 183–190. [Google Scholar] [CrossRef]
- Xiao, W.; Yang, J.; Lu, J.; Wang, J. A novel method to synthesize high performance silicalite-1 membrane. Sep. Purif. Technol. 2009, 67, 58–63. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, H.; Lin, Y.S. Effect of the membrane quality on gas permeation and chemical vapor deposition modification of MFI-type zeolite membranes. Ind. Eng. Chem. Res. 2020, 49, 10026–10033. [Google Scholar] [CrossRef]
- Xiao, W.; Yang, J.; Shen, D.; Lu, J.; Wang, J. Synthesis and property of silicalite-1 membranes by restricting growth method with dilute solution. Micropor. Mesopor. Mater. 2010, 129, 22–29. [Google Scholar] [CrossRef]
- Qiu, L.; Kumakiri, I.; Tanaka, K.; Chen, X.; Kita, H. Effect of seed crystal size on the properties of silicalite-1 membranes synthesized in a fluoride containing medium. J. Chem. Eng. Jpn. 2017, 50, 345–350. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, A.; Zhong, S.; Wang, B.; Zhou, R. Highly (h0h)-oriented silicalite-1 membranes for butane isomer separation. J. Membr. Sci. 2017, 540, 50–59. [Google Scholar] [CrossRef]
- Wu, A.; Tang, C.; Zhong, S.; Wang, B.; Zhou, J.; Zhou, R. Synthesis optimization of (h0h)-oriented silicalite-1 membranes for butane isomer separation. Sep. Purif. Technol. 2019, 214, 51–60. [Google Scholar] [CrossRef]
- Tanizume, S.; Yoshimura, T.; Ishii, K.; Nomura, M. Control of sequential MTO reactions through an MFI-type zeolite membrane contactor. Membranes 2020, 10, 26. [Google Scholar] [CrossRef]
- Ma, B.; Zhu, Y.; Hong, H.; Cui, L.; Gao, H.; Zhao, D.; Wang, B.; Zhou, R.; Xing, W. Improved silicalite-1 membranes on 61-channel monolithic supports for n-butane/i-butane separation. Sep. Purif. Technol. 2022, 300, 121828. [Google Scholar] [CrossRef]




| Sample | Si/Al | |
|---|---|---|
| powder | NaZSM-5 | 23 |
| KZSM-5 | 22 | |
| CsZSM-5 | 21 | |
| membrane | NaZSM-5 | 18 |
| KZSM-5 | 21 | |
| CsZSM-5 | 17 | |
| Temperature (K) | Vs (cm3(STP) g−1) | ||
|---|---|---|---|
| NaZSM-5 | KZSM-5 | CsZSM-5 | |
| 303 | 39 | 39 | 40 |
| 323 | 42 | 43 | 42 |
| 343 | 48 | 42 | 42 |
| Zeolite | Si/Al | ΔH (kJ mol−1) | Ref |
|---|---|---|---|
| NaZSM-5 | 23 | −61 | this study |
| KZSM-5 | 22 | −30 | this study |
| CsZSM-5 | 21 | −45 | this study |
| HZSM-5 | 38 | −113 | [32] |
| HZSM-5 | 250 | −75 | [32] |
| HZSM-5 | 3.8 | −69 to −84 | [33] |
| silicalite-1 | ∞ | −61 to −68 | [33] |
| NaZSM-5 | 130 | −72 (site 1) −58 (site 2) | [34] |
| Membrane | Ideal Permselectivity * | ||
|---|---|---|---|
| H2/N2 | H2/SF6 | N2/SF6 | |
| NaZSM-5(1) | 21 | 1141 | 55 |
| KZSM-5 | 26 | 45 | 2 |
| NaZSM-5(2) | 17 | 329 | 20 |
| CsZSM-5 | 22 | 64 | 3 |
| Ep,H2O (kJ/mol) | ΔH (kJ mol−1) * | |
|---|---|---|
| NaZSM-5 | −6.1 (membrane(1)) −7.3 (membrane(2)) | −61 |
| KZSM-5 | −14 | −30 |
| CsZSM-5 | −10 | −45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirota, Y.; Nakai, M.; Tani, K.; Sakane, K.; Ikeda, A.; Hasegawa, Y.; Araki, S. Gas and Steam Permeation Properties of Cation-Exchanged ZSM-5 Membrane. Membranes 2025, 15, 70. https://doi.org/10.3390/membranes15030070
Hirota Y, Nakai M, Tani K, Sakane K, Ikeda A, Hasegawa Y, Araki S. Gas and Steam Permeation Properties of Cation-Exchanged ZSM-5 Membrane. Membranes. 2025; 15(3):70. https://doi.org/10.3390/membranes15030070
Chicago/Turabian StyleHirota, Yuichiro, Masaki Nakai, Kasumi Tani, Koya Sakane, Ayumi Ikeda, Yasuhisa Hasegawa, and Sadao Araki. 2025. "Gas and Steam Permeation Properties of Cation-Exchanged ZSM-5 Membrane" Membranes 15, no. 3: 70. https://doi.org/10.3390/membranes15030070
APA StyleHirota, Y., Nakai, M., Tani, K., Sakane, K., Ikeda, A., Hasegawa, Y., & Araki, S. (2025). Gas and Steam Permeation Properties of Cation-Exchanged ZSM-5 Membrane. Membranes, 15(3), 70. https://doi.org/10.3390/membranes15030070






