Effect of Seed Size on Pervaporation Performances Through FAU Zeolite Membrane
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. FAU Zeolite Seed Preparation and Modification
2.3. FAU Zeolite Membranes Synthesis
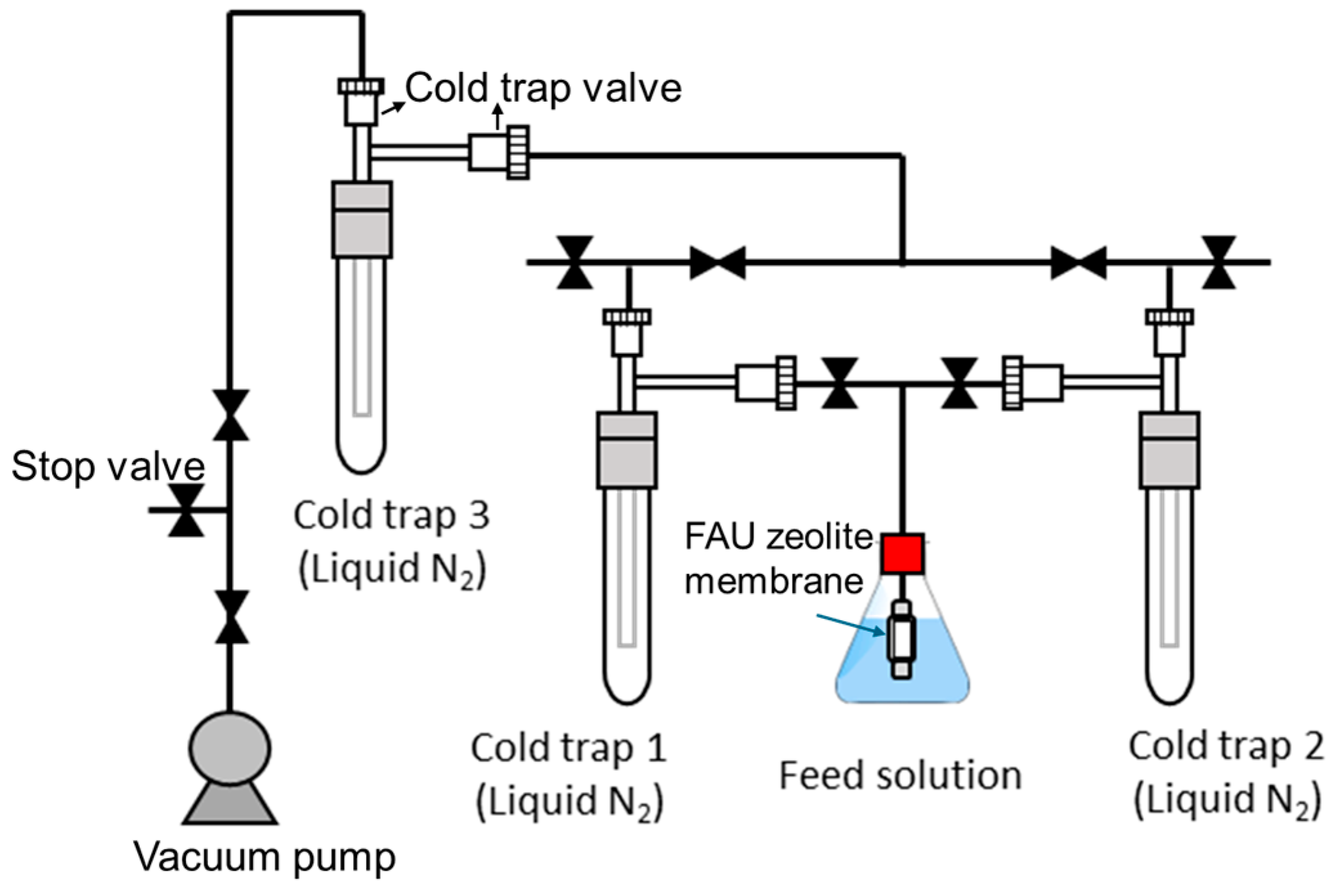
2.4. Characterization and Evaluation
3. Results and Discussion
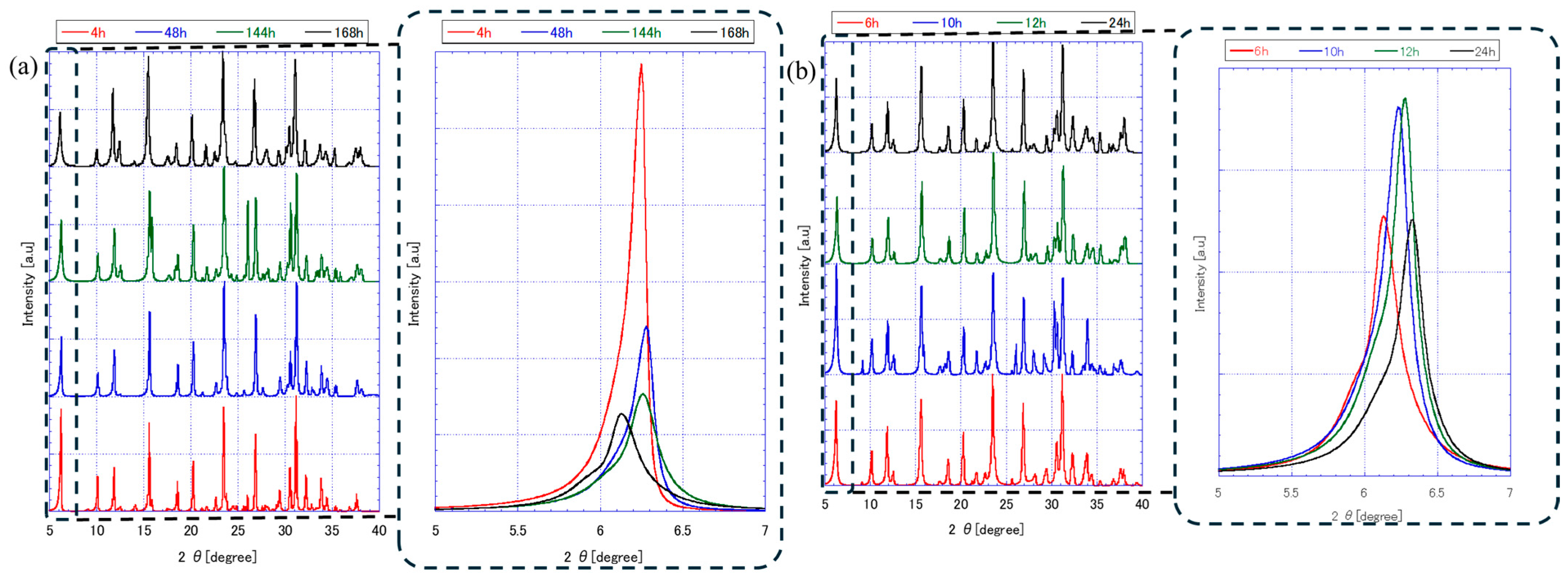
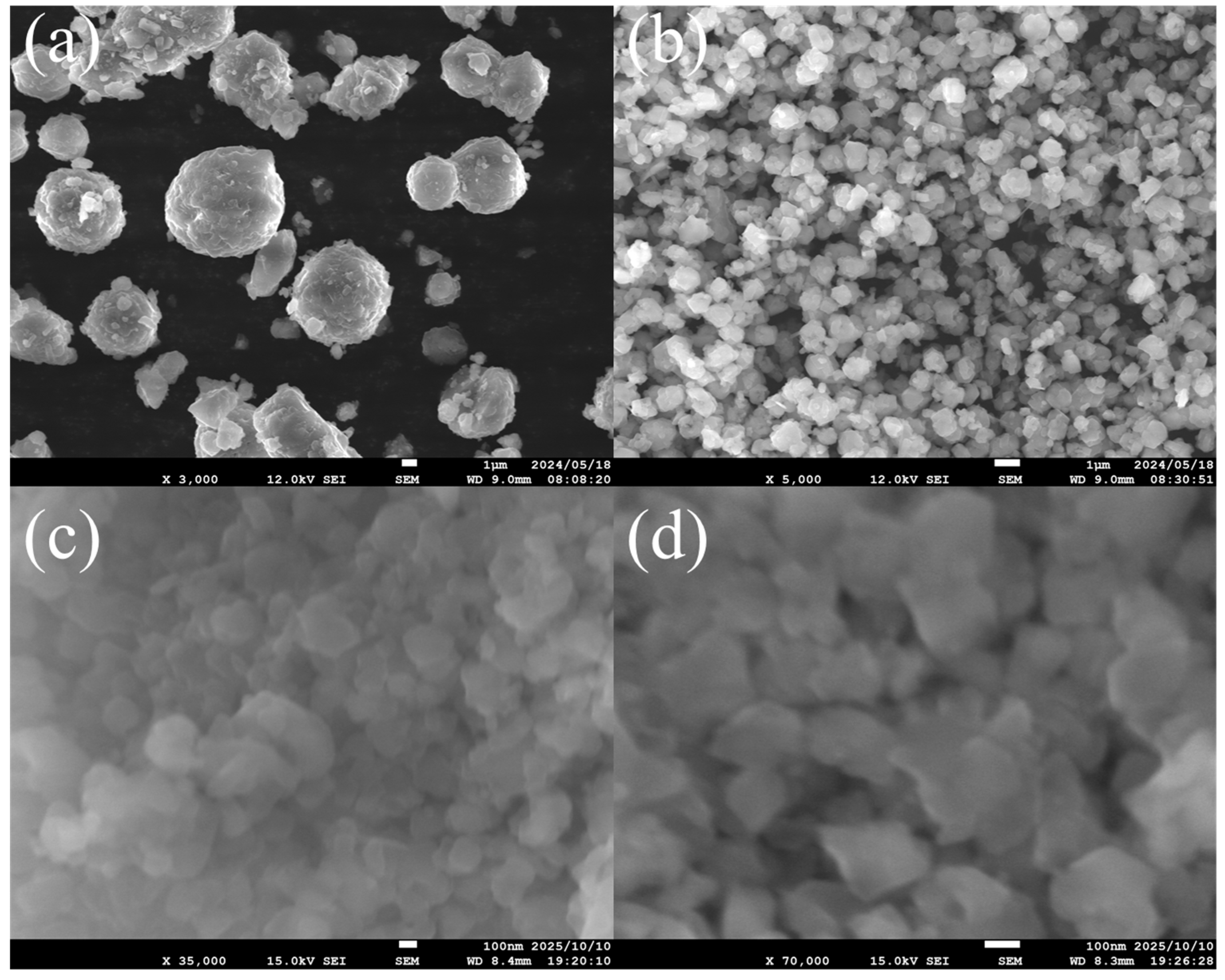
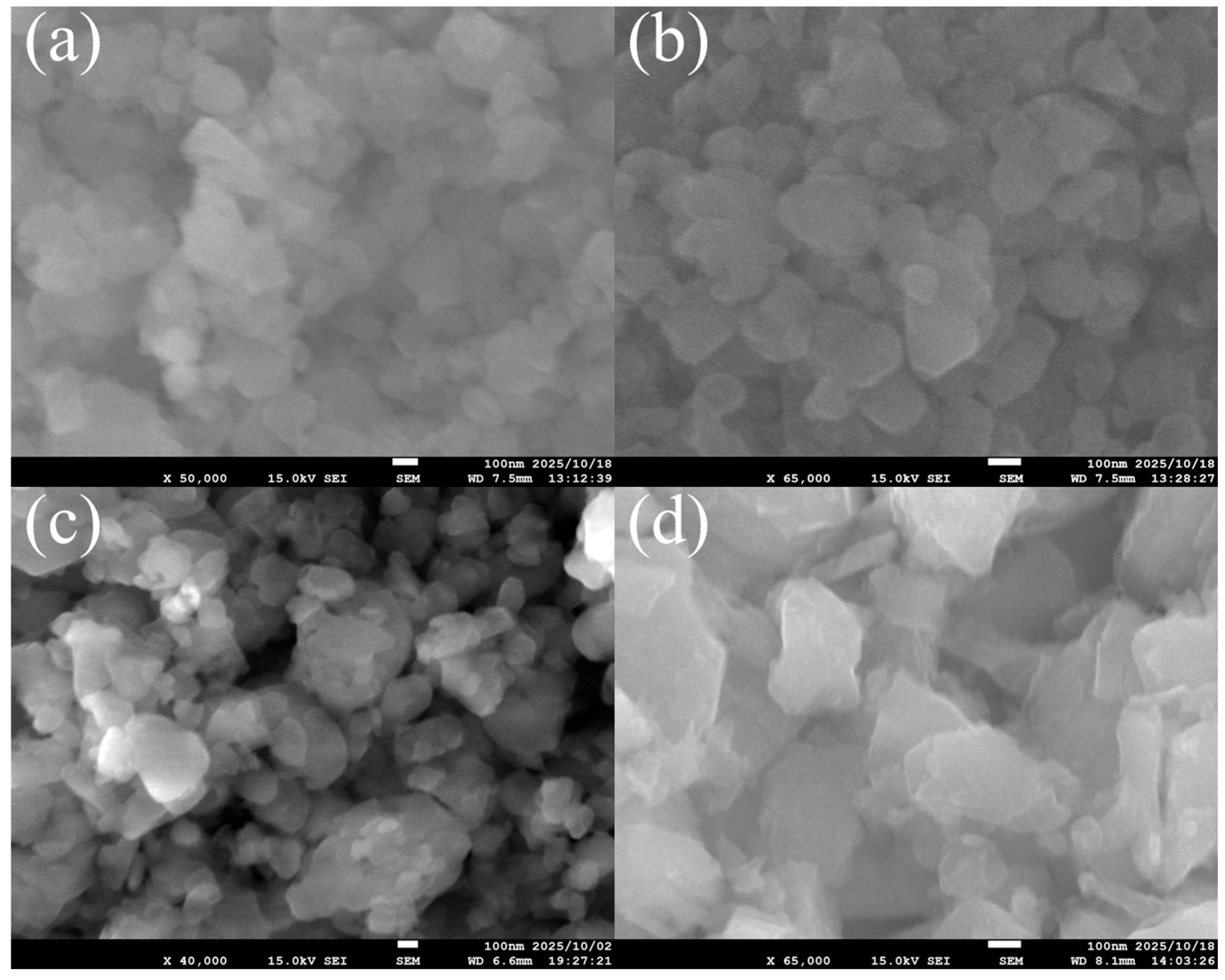
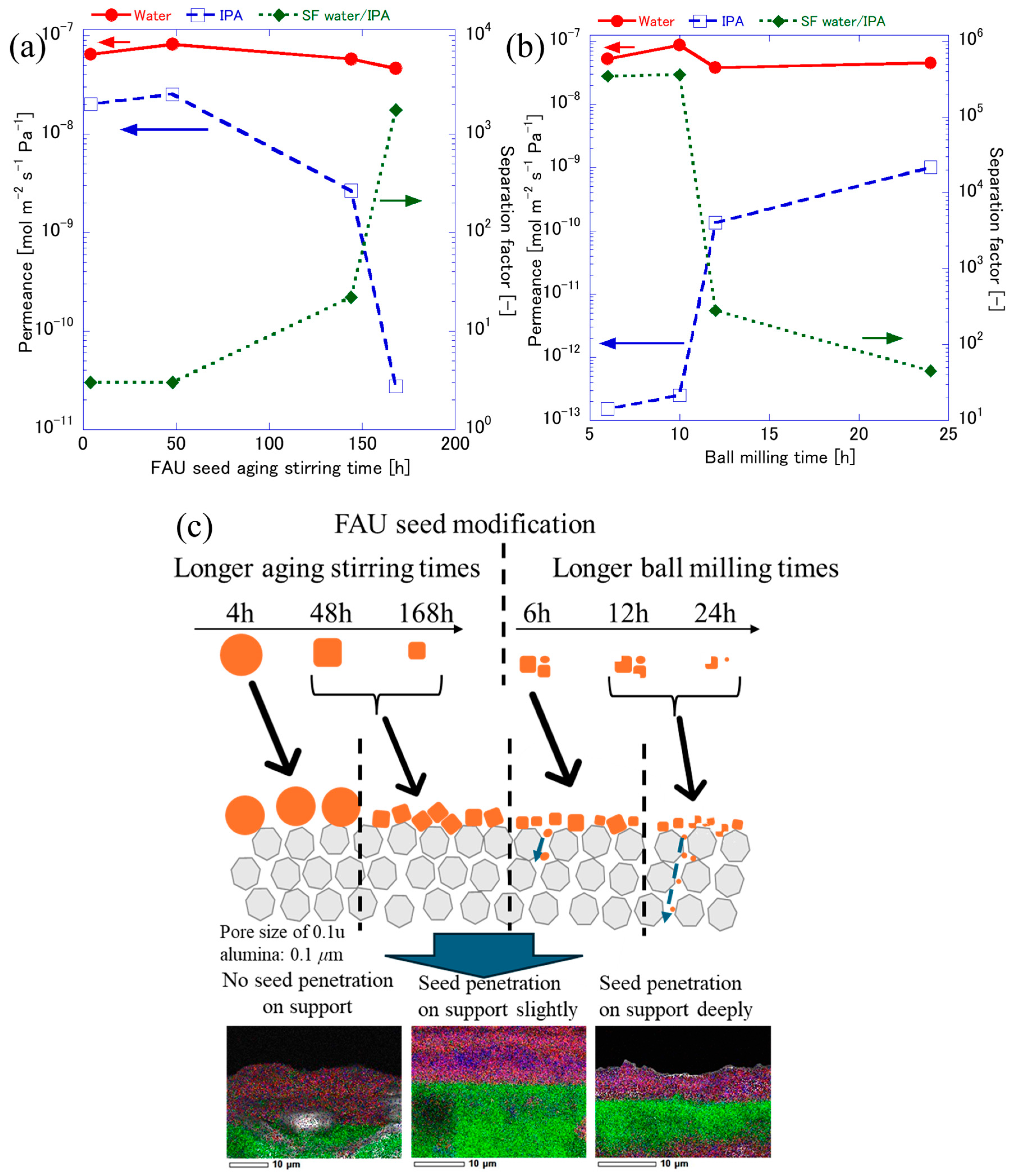
3.1. FAU Zeolite Seed Characterizations
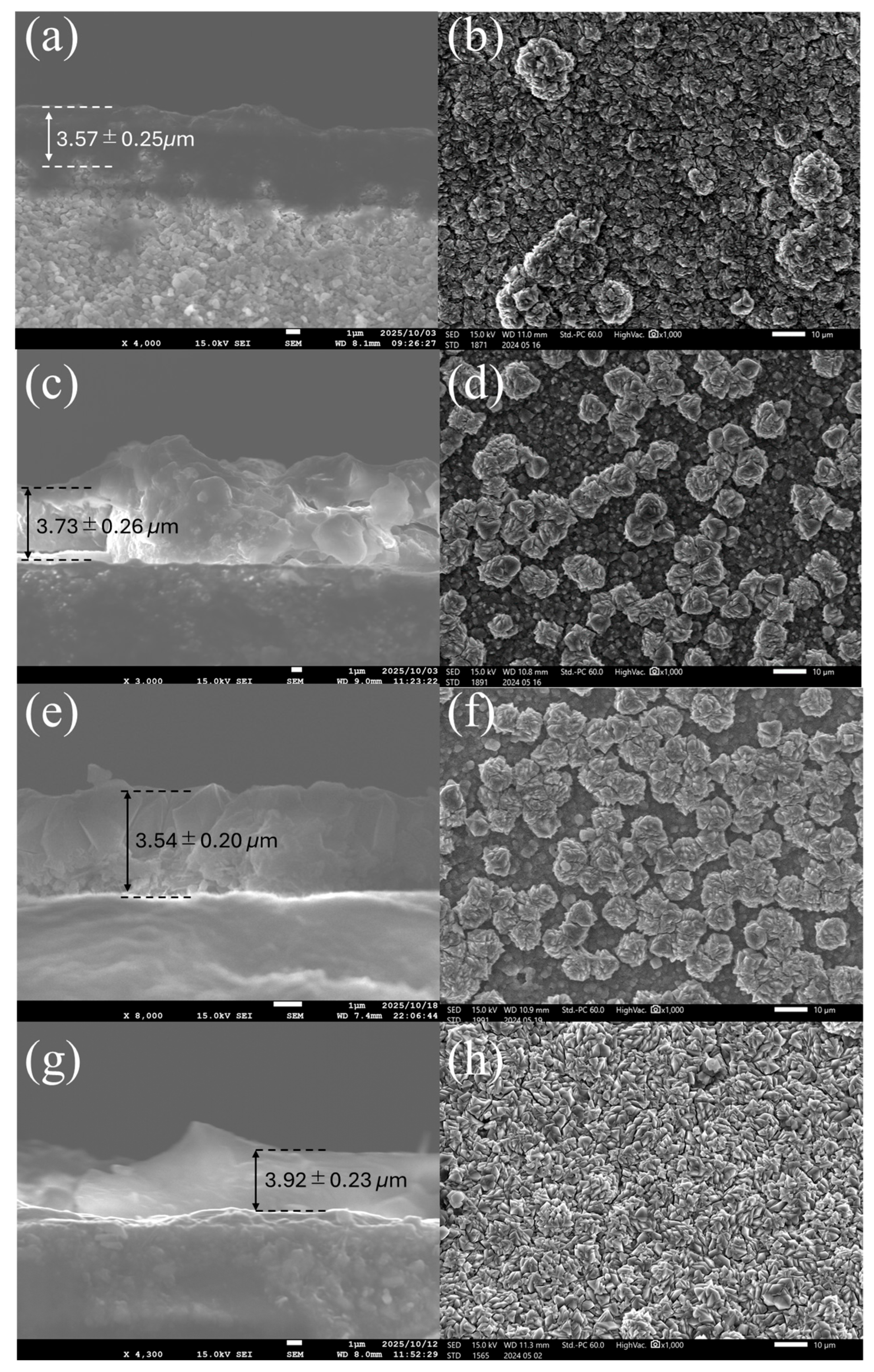
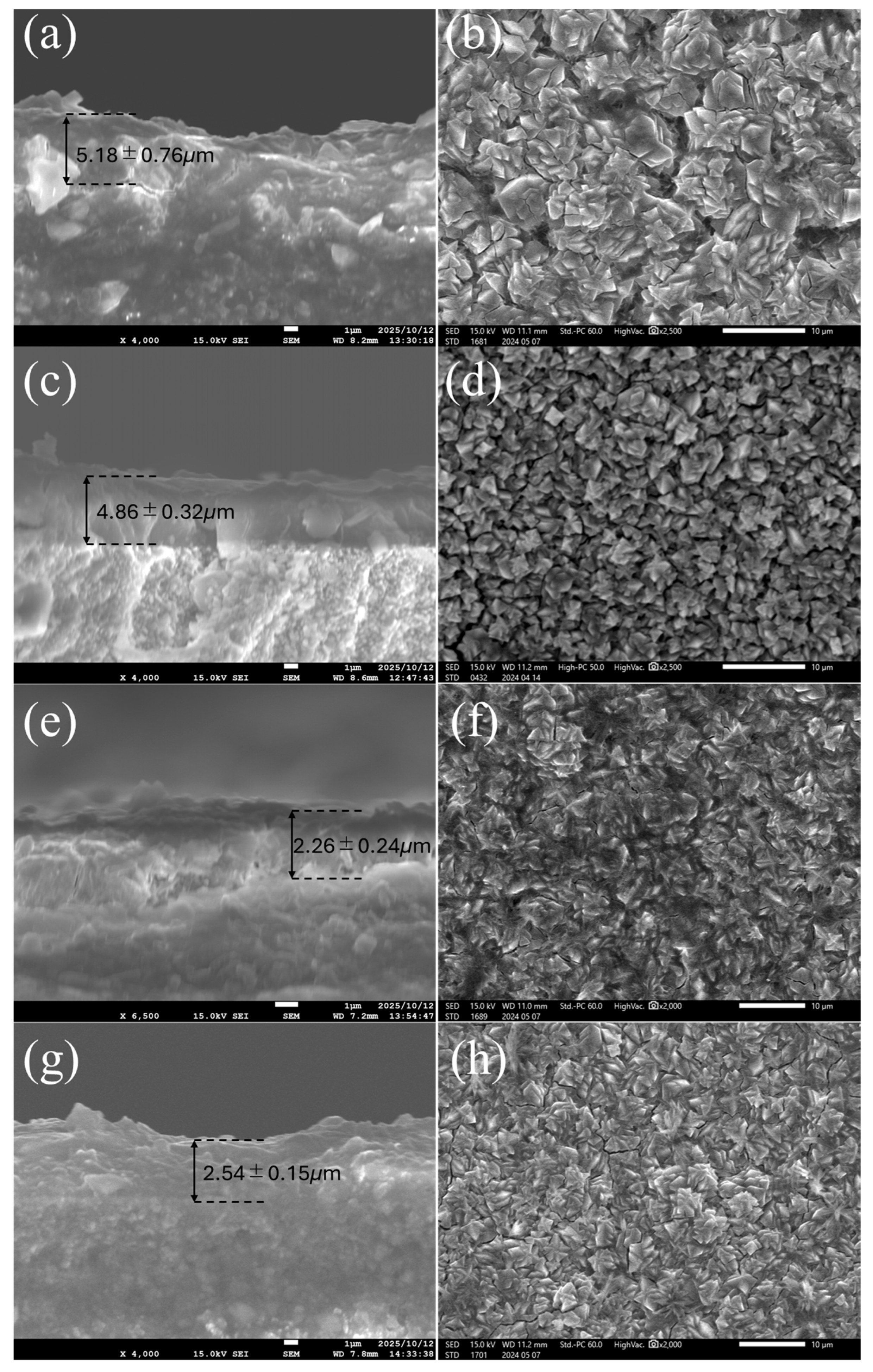
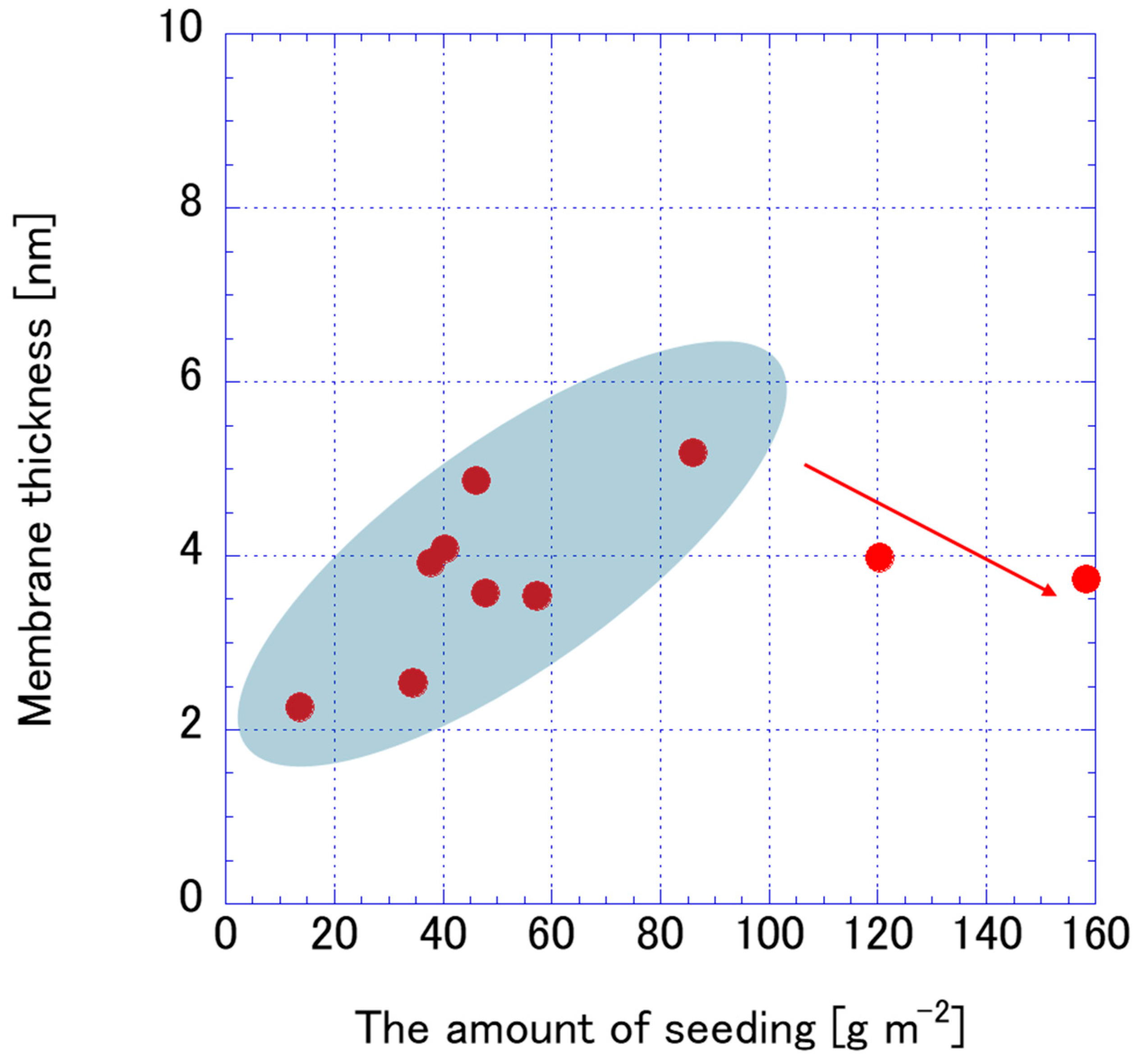
3.2. FAU Zeolite Membrane Characterizations
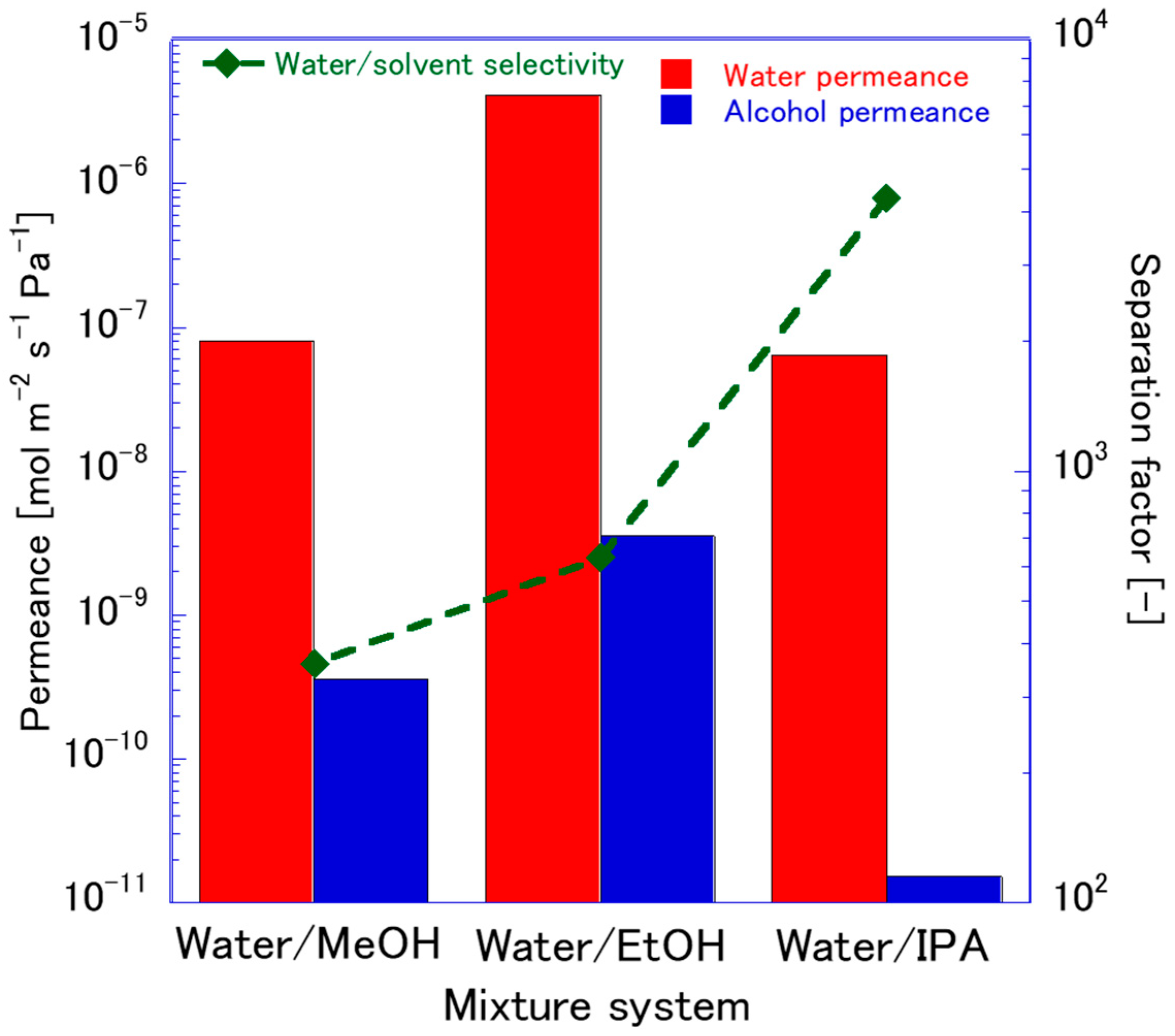
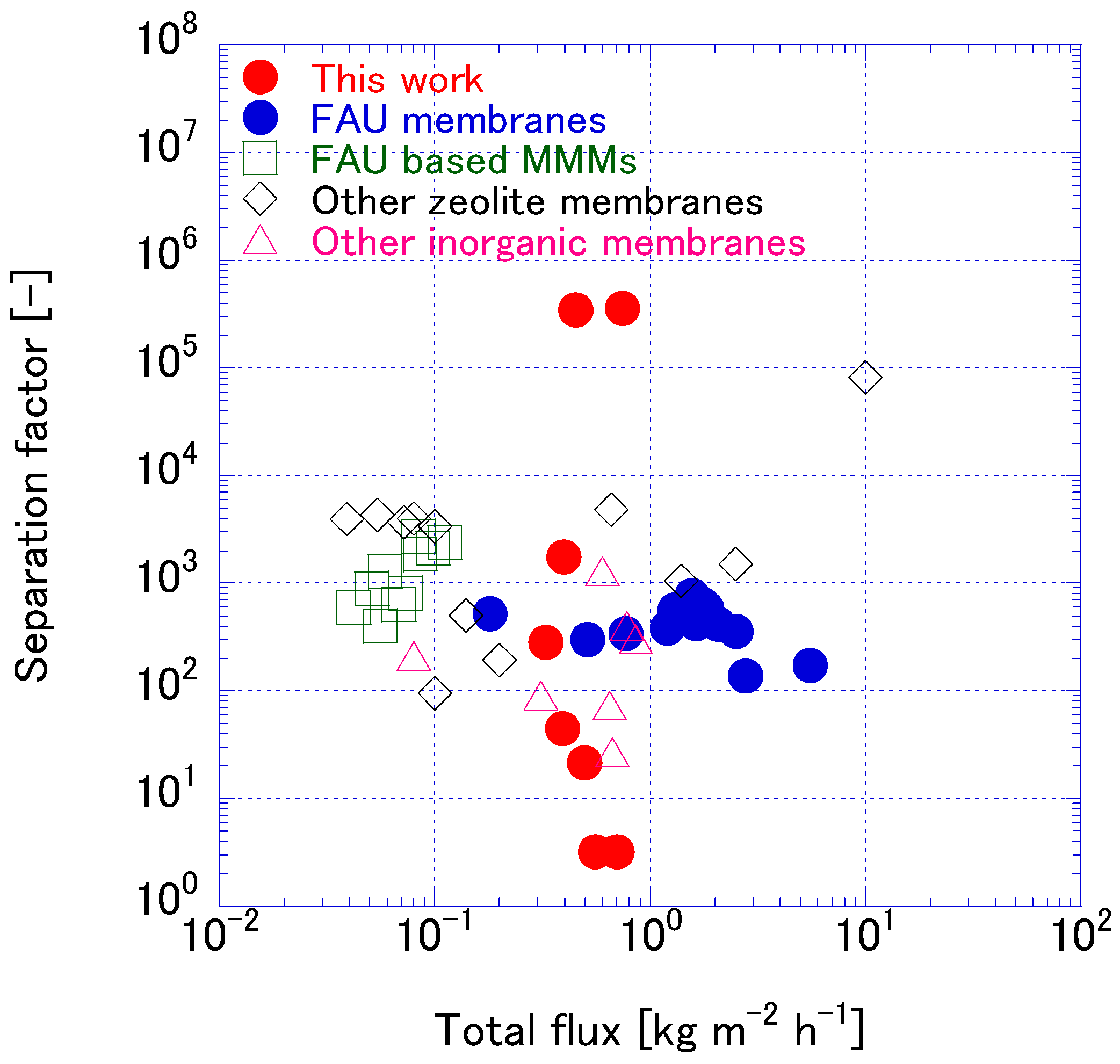
3.3. FAU Zeolite Membrane Pervaporation Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Q.; Xu, W.; Li, J.; Wang, Z.; Xu, H.; Zhou, M.; Wang, Y.; Li, X.; Zhong, L.; Cui, P. Molecular Mechanism of Efficient Separation of Isopropyl Alcohol and Isooctane by Extractive Distillation. Chem. Eng. Res. Des. 2024, 204, 269–281. [Google Scholar] [CrossRef]
- Vane, L.M. Review of Pervaporation and Vapor Permeation Process Factors Affecting the Removal of Water from Industrial Solvents. J. Chem. Technol. Biotechnol. 2020, 95, 495–512. [Google Scholar] [CrossRef]
- Janković, T.; Straathof, A.J.J.; Kiss, A.A. Advanced Purification of Isopropanol and Acetone from Syngas Fermentation. J. Chem. Technol. Biotechnol. 2024, 99, 714–726. [Google Scholar] [CrossRef]
- Khan, M.N.; Boorsma, E.; Vandezande, P.; Lammerink, I.; de Lange, R.; Buekenhoudt, A.; Van Dael, M. Unlocking the Benefits of Hybrid and Standalone Pervaporation for Sustainable Isopropanol Dehydration with HybSi® AR Membranes. Membranes 2025, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Matsuura, W.; Abe, C.; Lundin, S.-T.B.; Hasegawa, Y. Evaluation of FAU-Type Zeolite Membrane Stability in Transesterification Reaction Conditions. Membranes 2023, 13, 68. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, Y.; Xu, N.; Liu, Q.; Wang, B.; Fan, L.; Zhang, L.; Zhou, R. FAU Zeolite Membranes Synthesized Using Nanoseeds—Separation Mechanism and Optimization for the Pervaporation Dehydration of Various Organic Solvents. J. Memb. Sci. 2024, 696, 122522. [Google Scholar] [CrossRef]
- Widyanto, A.R.; Nomura, M. Advances in FAU Zeolite Membranes for Separation and Catalytic Reactor Applications: A Comprehensive Review. Microporous Mesoporous Mater. 2026, 401, 113956. [Google Scholar] [CrossRef]
- Sawamura, K.I.; Furuhata, T.; Sekine, Y.; Kikuchi, E.; Subramanian, B.; Matsukata, M. Zeolite Membrane for Dehydration of Isopropylalcohol-Water Mixture by Vapor Permeation. ACS Appl. Mater. Interfaces 2015, 7, 13728–13730. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Boczkaj, G. Pervaporation Zeolite-Based Composite Membranes for Solvent Separations. Molecules 2021, 26, 1242. [Google Scholar] [CrossRef]
- Nazir, L.S.M.; Yeong, Y.F.; Chew, T.L. Study on the Effect of Seed Particle Size toward the Formation of NaX Zeolite Membranes via Vacuum-Assisted Seeding Technique. J. Asian Ceram. Soc. 2021, 9, 586–597. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, H.; He, F.; Liu, Q.; Xu, N.; Fan, L.; Wang, C.; Zhang, L.; Zhou, R. High-Performance FAU Zeolite Membranes Derived from Nano-Seeds for Gas Separation. Membranes 2023, 13, 858. [Google Scholar] [CrossRef]
- Ding, W.; Xiang, S.; Ye, F.; Gui, T.; Li, Y.; Zhang, F.; Hu, N.; Zhu, M.; Chen, X. Effects of Seed Crystals on the Growth and Catalytic Performance of TS-1 Zeolite Membranes. Membranes 2020, 10, 41. [Google Scholar] [CrossRef]
- Qiu, L.; Kumakiri, I.; Tanaka, K.; Chen, X.; Kita, H. Effect of Seed Crystal Size on the Properties of Silicalite-1 Membranes Synthesized in a Fluoride Containing Medium. J. Chem. Eng. Jpn. 2017, 50, 345–350. [Google Scholar] [CrossRef]
- Widyanto, A.R.; Caralin, I.S.; Nakai, Y.; Nomura, M. Effects of Ion Exchange on Permeation Properties through FAU Zeolite Membranes. SEATUC J. Sci. Technol, 2025; in press. [Google Scholar]
- Widiastuti, N.; Rahman, R.A.; Cipta Dharma, H.N.; Widyanto, A.R.; Nareswari, C.; Kayadoe, V.; Raharjo, Y.; Saiful; Nomura, M. Carbon-Modified Zeolite Derivates Based Filler in Mixed Matrix Membrane Adsorbers (MMMAs) for Enhancing Urea and Creatinine Removal from Hemodialysis Spent Dialysate. J. Ind. Eng. Chem. 2025, 147, 584–597. [Google Scholar] [CrossRef]
- Widiastuti, N.; Widyanto, A.R.; Caralin, I.S.; Gunawan, T.; Wijiyanti, R.; Wan Salleh, W.N.; Ismail, A.F.; Nomura, M.; Suzuki, K. Development of a P84/ZCC Composite Carbon Membrane for Gas Separation of H2/CO2 and H2/CH4. ACS Omega 2021, 6, 15637–15650. [Google Scholar] [CrossRef]
- JCPDS-International Centre for Diffraction Data, Card Number; ICDD: Newton Square, PA, USA, 1995; pp. 39–1380.
- Endang, P.S.; Rahadian, A.R.; Ulva, T.I.M.; Alvin, R.W.; Rendy, M.I.; Widiastuti, N. The MnO2/Zeolite NaY Catalyzed Oxidation of CO Emission in Catalytic Converter System. Mater. Sci. Forum 2019, 964, 199–208. [Google Scholar] [CrossRef]
- Tsujiguchi, M.; Kobashi, T.; Utsumi, Y.; Kakimori, N.; Nakahira, A. Synthesis of FAU Zeolite from Aluminoborosilicate Glass and Elution Behavior of Glass Components. J. Ceram. Soc. Jpn. 2014, 122, 104–109. [Google Scholar] [CrossRef]
- Awala, H.; Kunjir, S.M.; Aurélie, V.; Gilson, J.P.; Valtchev, V.; Seblani, H.; Retoux, R.; Lakiss, L.; Fernandez, C.; Bedard, R.; et al. Crystallization Pathway from a Highly Viscous Colloidal Suspension to Ultra-Small FAU Zeolite Nanocrystals. J. Mater. Chem. A 2021, 9, 17492–17501. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Y.; Chen, H.; Liu, J.; Yang, W. An in Situ Approach to Synthesize Pure Phase FAU-Type Zeolite Membranes: Effect of Aging and Formation Mechanism. J. Mater. Sci. 2008, 43, 3279–3288. [Google Scholar] [CrossRef]
- Kuznetsov, P.; Malyavin, V.; Dement’ev, K. Insights into Ball Milling for the Production of Highly Active Zeolites for Catalytic Cracking of VGO. Catalysts 2025, 15, 596. [Google Scholar] [CrossRef]
- Mazzeo, P.P.; Lampronti, G.I.; Michalchuk, A.A.L.; Belenguer, A.M.; Bacchi, A.; Emmerling, F. Accurate Extrinsic and Intrinsic Peak Broadening Modelling for Time-Resolved in Situ Ball Milling Reactions via Synchrotron Powder X-ray Diffraction. Faraday Discuss. 2022, 241, 289–305. [Google Scholar] [CrossRef]
- Ogura, M.; Kawazu, Y.; Takahashi, H.; Okubo, T. Aluminosilicate Species in the Hydrogel Phase Formed during the Aging Process for the Crystallization of FAU Zeolite. Chem. Mater. 2003, 15, 2661–2667. [Google Scholar] [CrossRef]
- Poulesquen, A.; Gomes Rodrigues, D.; Keshavarz, B.; Courtois, N.; Ilavsky, J.; McKinley, G.H. Aluminosilicate Colloidal Gels: From the Early Age to the Precipitation of Zeolites. Soft Matter 2024, 20, 5538–5552. [Google Scholar] [CrossRef]
- Sahu, A.; Singh, L.K.; Maurya, R.S. Effect of Milling Parameters and Milling Energy on Amorphization: A Review. Trans. Indian Inst. Met. 2023, 76, 2033–2042. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.S.; Sabry, Y.A. An Approach to the Micro-Strain Distribution inside Nanoparticle Structure. Int. J. Non-Linear Mech. 2024, 161, 104670. [Google Scholar] [CrossRef]
- Samanta, P.K. Effect of Microstrain on the Crystallite Size of ZnO Nanoparticles: X-Ray Peak Profile and Rietveld Analysis. Next Mater. 2025, 8, 100841. [Google Scholar] [CrossRef]
- Severance, M.; Wang, B.; Ramasubramanian, K.; Zhao, L.; Ho, W.S.W.; Dutta, P.K. Rapid Crystallization of Faujasitic Zeolites: Mechanism and Application to Zeolite Membrane Growth on Polymer Supports. Langmuir 2014, 30, 6929–6937. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.S.D.; Zapelini, I.W.; Silva, L.L.; Mintova, S.; Martins, L. Impacts of Ball-Milling on ZSM-5 Zeolite Properties and Its Catalytic Activity in the Two-Phase Glycerol Ketalization with Acetone. Catal. Today 2024, 441, 114842. [Google Scholar] [CrossRef]
- Kosanović, C.; Čižmek, A.; Subotić, B.; Šmit, I.; Stubičar, M.; Tonejc, A. Mechanochemistry of Zeolites. Part 4: Influence of Cations on the Rate of Amorphization of Zeolite A by Ball Milling. Zeolites 1995, 15, 632–636. [Google Scholar] [CrossRef]
- Mukhtar, N.Z.F.; Borhan, M.Z.; Rusop, M.; Abdullah, S. Effect of Milling Time on Particle Size and Surface Morphology of Commercial Zeolite by Planetary Ball Mill. Adv. Mater. Res. 2013, 795, 711–715. [Google Scholar] [CrossRef]
- Xue, Q.; Zhu, J.; Meng, W.; Zhang, K. Effect of MXene Nanosheet Dispersed Phases on the Fabrication of Polyamide Nanofiltration Membranes. ACS Appl. Eng. Mater. 2023, 1, 679–689. [Google Scholar] [CrossRef]
- Jia, Z.; Li, J.; Gao, L.; Yang, D.; Kanaev, A. Dynamic Light Scattering: A Powerful Tool for In Situ Nanoparticle Sizing. Colloids Interfaces 2023, 7, 15. [Google Scholar] [CrossRef]
- Nazir, L.S.M.; Yeong, Y.F.; Chew, T.L. Methods and Synthesis Parameters Affecting the Formation of FAU Type Zeolite Membrane and Its Separation Performance: A Review. J. Asian Ceram. Soc. 2020, 8, 553–571. [Google Scholar] [CrossRef]
- Huo, Z.; Xu, X.; Lv, Z.; Song, J.; He, M.; Li, Z.; Wang, Q.; Yan, L.; Li, Y. Thermal Study of NaP Zeolite with Different Morphologies. J. Therm. Anal. Calorim. 2013, 111, 365–369. [Google Scholar] [CrossRef]
- Kumakiri, I.; Sasaki, Y.; Shimidzu, W.; Hashimoto, K.; Kita, H.; Yamaguchi, T.; Nakao, S. ichi Micro-Structure Change of Polycrystalline FAU Zeolite Membranes during a Hydrothermal Synthesis in a Dilute Solution. Microporous Mesoporous Mater. 2018, 272, 53–60. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Alassmy, Y.A.; Sebakhy, K.O. A Survey on Zeolite Synthesis and the Crystallization Process: Mechanism of Nucleation and Growth Steps. Crystals 2023, 13, 959. [Google Scholar] [CrossRef]
- Shao, J.; Ge, Q.; Shan, L.; Wang, Z.; Yan, Y. Influences of Seeds on the Properties of Zeolite Naa Membranes on Alumina Hollow Fibers. Ind. Eng. Chem. Res. 2011, 50, 9718–9726. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, Z.; Zhang, L.; Fu, Q.; Wang, M. Competitive Adsorptive Mechanism of H2/N2 in LTA/FAU Zeolites by Molecular Simulations and Experiments. Molecules 2024, 29, 3686. [Google Scholar] [CrossRef] [PubMed]
- Tahraoui, Z.; Nouali, H.; Marichal, C.; Forler, P.; Klein, J.; Daou, T.J. Influence of the Compensating Cation Nature on the Water Adsorption Properties of Zeolites. Molecules 2020, 25, 944. [Google Scholar] [CrossRef] [PubMed]
- Matsukata, M.; Sekine, Y.; Kikuchi, E.; Sakai, M.; Subramanian, B.; Toyoda, M.; Furuhata, T. Synthesis of FAU-Zeolite Membrane by a Secondary Growth Method: Influence of Seeding on Membrane Growth and Its Performance in the Dehydration of Isopropyl Alcohol-Water Mixture. ACS Omega 2021, 6, 9834–9842. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Humplik, T.; Bevilacqua, A.; Tsapatsis, M.; Chiavazzo, E.; Wang, E.N.; Asinari, P. Interplay between Hydrophilicity and Surface Barriers on Water Transport in Zeolite Membranes. Nat. Commun. 2016, 7, 12762. [Google Scholar] [CrossRef] [PubMed]
- Villaluenga, J.P.G.; Khayet, M.; Godino, P.; Seoane, B.; Mengual, J.I. Analysis of the Membrane Thickness Effect on the Pervaporation Separation of Methanol/Methyl Tertiary Butyl Ether Mixtures. Sep. Purif. Technol. 2005, 47, 80–87. [Google Scholar] [CrossRef]
- Wang, Q.; Qian, C.; Guo, C.; Xu, N.; Liu, Q.; Wang, B.; Fan, L.; Hu, K. Pervaporation Dehydration Mechanism and Performance of High-Aluminum ZSM-5 Zeolite Membranes for Organic Solvents. Int. J. Mol. Sci. 2024, 25, 7723. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Abe, C.; Matsuura, W.; Hasegawa, Y. Development of Methanol Permselective FAU-Type Zeolite Membranes and Their Permeation and Separation Performances. Membranes 2021, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Matsuura, W.; Abe, C.; Ikeda, A. Influence of Organic Solvent Species on Dehydration Behaviors of NaA-Type Zeolite Membrane. Membranes 2021, 11, 347. [Google Scholar] [CrossRef]
- Lin, X.; Kikuchi, E.; Matsukata, M. Preparation of Mordenite Membranes on α-Alumina Tubular Supports for Pervaporation of Water–Isopropyl Alcohol Mixtures. Chem. Commun. 2000, 11, 957–958. [Google Scholar] [CrossRef]
- Nakai, Y.; Widyanto, A.R.; Nomura, M. Water Permeation from IPA Solution Containing Sodium Chloride through MOR Zeolite Membranes. J. Phys. Conf. Ser. 2025, 3107, 12019. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, Y.; Peng, L.; Zhang, C.; Wu, Z.; Gu, X.; Wang, X.; Murad, S. Fabrication and Stability Exploration of Hollow Fiber Mordenite Zeolite Membranes for Isopropanol/Water Mixture Separation. Microporous Mesoporous Mater. 2019, 274, 347–355. [Google Scholar] [CrossRef]
- Du, J.; Jiang, J.; Xue, Z.; Hu, Y.; Liu, B.; Zhou, R.; Xing, W. Template-Free Synthesis of High Dehydration Performance CHA Zeolite Membranes with Increased Si/Al Ratio Using SSZ-13 Seeds. Membranes 2024, 14, 78. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Ikeda, A. Pervaporative Dehydration of Organic Solvents Using High-Silica CHA-Type Zeolite Membrane. Membranes 2021, 11, 229. [Google Scholar] [CrossRef]
- Qiu, H.; Jiang, J.; Peng, L.; Liu, H.; Gu, X. Choline Chloride Templated CHA Zeolite Membranes for Solvents Dehydration with Improved Acid Stability. Microporous Mesoporous Mater. 2019, 284, 170–176. [Google Scholar] [CrossRef]
- Sekulić, J.; Luiten, M.W.J.; ten Elshof, J.E.; Benes, N.E.; Keizer, K. Microporous Silica and Doped Silica Membrane for Alcohol Dehydration by Pervaporation. Desalination 2002, 148, 19–23. [Google Scholar] [CrossRef]
- Liao, M.; Guan, H.; Zuo, H.; Ren, G.; Gong, G. High-Performance Flexible Hybrid Silica Membranes with an Ultrasonic Atomization-Assisted Spray-Coated Active Layer on Polymer for Isopropanol Dehydration. Membranes 2024, 14, 154. [Google Scholar] [CrossRef]
- Sousa, P.B.F.; Bieseki, L.; Pergher, S.B.C. Seed-Assisted Crystallization in the Hydrothermal Synthesis of FAU Zeolite from Acid-Treated Residue Glass Powder. Materials 2025, 18, 1393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, L.; Hu, N.; Bu, N.; Zhou, R.; Chen, X. Preparation of NaY Zeolite Membranes in Fluoride Media and Their Application in Dehydration of Bio-Alcohols. Sep. Purif. Technol. 2014, 129, 9–17. [Google Scholar] [CrossRef]
- Wang, Z.; Kumakiri, I.; Tanaka, K.; Chen, X.; Kita, H. NaY Zeolite Membranes with High Performance Prepared by a Variable-Temperature Synthesis. Microporous Mesoporous Mater. 2013, 182, 250–258. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Y.; Zhou, H.; Liu, J.; Yang, W. FAU-Type Zeolite Membranes Synthesized by Microwave Assisted In Situ Crystallization. Mater. Lett. 2008, 62, 4357–4359. [Google Scholar] [CrossRef]
- Zhu, M.; An, X.; Gui, T.; Wu, T.; Li, Y.; Chen, X. Effects of Ion-Exchange on the Pervaporation Performance and Microstructure of NaY Zeolite Membrane. Chin. J. Chem. Eng. 2023, 59, 176–181. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Kittur, A.A.; Kariduraganavar, M.Y.; Davis, F.J. Pervaporation Dehydration of Isopropyl Alcohol with NaY Zeolite Incorporated Hybrid Membranes. J. Appl. Polym. Sci. 2008, 109, 2043–2053. [Google Scholar] [CrossRef]
- Kwon, Y.; Chaudhari, S.; Kim, C.; Son, D.; Park, J.; Moon, M.; Shon, M.; Park, Y.; Nam, S. Ag-Exchanged NaY Zeolite Introduced Polyvinyl Alcohol/Polyacrylic Acid Mixed Matrix Membrane for Pervaporation Separation of Water/Isopropanol Mixture. RSC Adv. 2018, 8, 20669–20678. [Google Scholar] [CrossRef] [PubMed]
- Premakshi, H.G.; Ramesh, K.; Kariduraganavar, M.Y. Modification of Crosslinked Chitosan Membrane Using NaY Zeolite for Pervaporation Separation of Water–Isopropanol Mixtures. Chem. Eng. Res. Des. 2015, 94, 32–43. [Google Scholar] [CrossRef]
- Li, G.; Kikuchi, E.; Matsukata, M. Separation of Water–Acetic Acid Mixtures by Pervaporation Using a Thin Mordenite Membrane. Sep. Purif. Technol. 2003, 32, 199–206. [Google Scholar] [CrossRef]
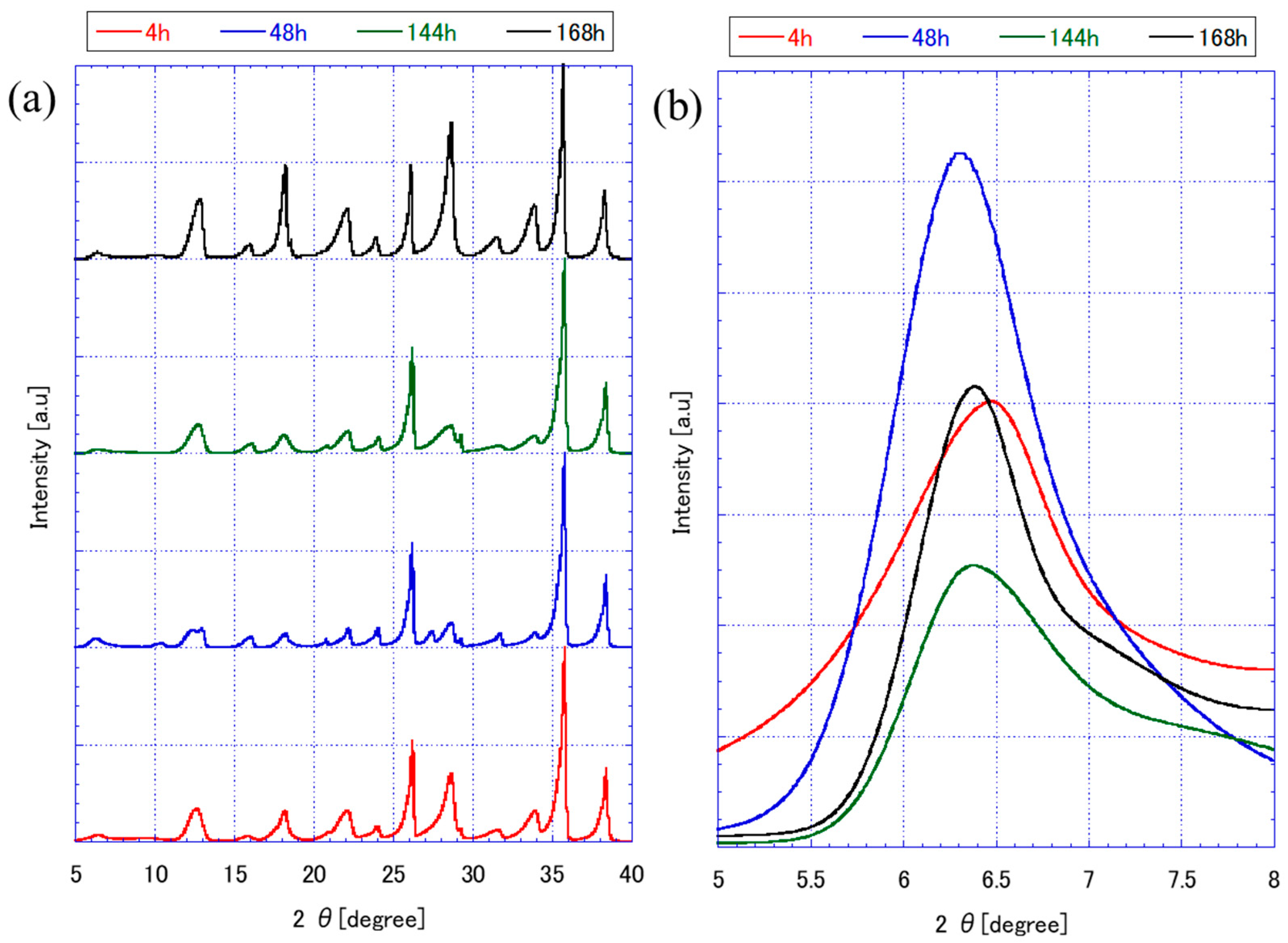
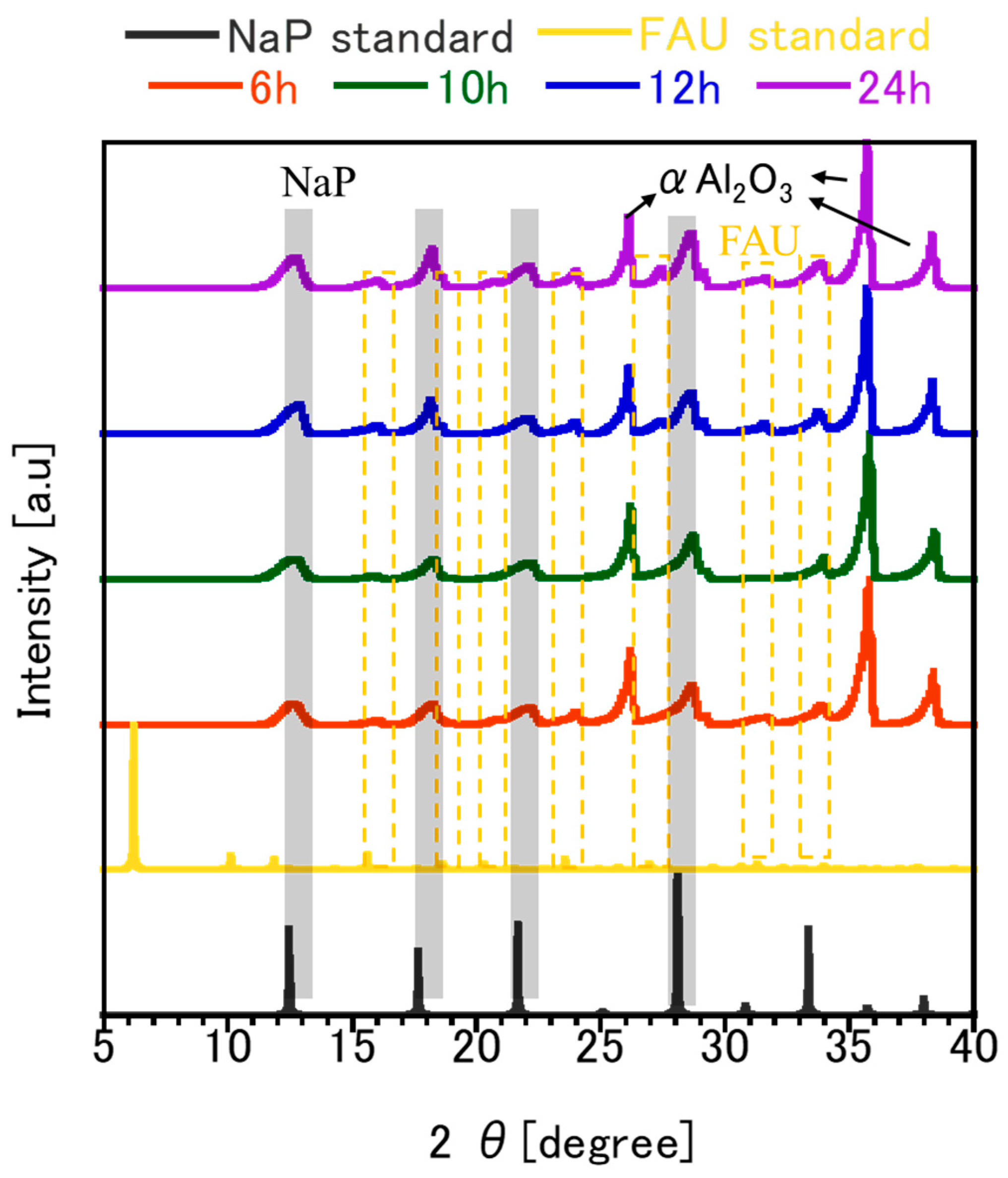


| FAU Seed with Different Preparation Conditions | Crystallite Size (nm) by XRD | Micro Strain (%) | Relative Crystallinity (%) | Particle Size (nm) by SEM Images | DLS Analysis | ||
|---|---|---|---|---|---|---|---|
| Stirring Time (h) | Ball Milling Time (h) | Particle Size (nm) | Polydispersity (nm) | ||||
| 4 | - | 36.7 ± 3.7 | 4.82 | 83.55 | 6106.99 ± 1075.92 | 431.95 ± 14.19 | 3.25 × 10−6 |
| 48 | - | 33.4 ± 4.7 | 5.40 | 95.53 | 985.35 ± 111.02 | 545.86 ± 35.30 | 1.90 × 10−6 |
| 144 | - | 24.6 ± 0.7 | 4.06 | 80.44 | 158.37 ± 33.42 | 450.30 ± 18.74 | 6.10 × 10−7 |
| 168 | - | 25.7 ± 1.2 | 3.94 | 83.9 | 125.04 ± 23.21 | 315.64 ± 10.02 | 5.85 × 10−8 |
| 168 | 6 | 27.6 ± 1.4 | 6.86 | 91.44 | 109.80 ± 4.04 | 372.77 ± 10.95 | 3.70 |
| 168 | 10 | 27.1 ± 0.5 | 8.28 | 79.72 | 95.51 ± 8.52 | 355.25 ± 1.96 | 5.72 |
| 168 | 12 | 26.1 ± 0.8 | 6.99 | 86.93 | 91.84 ± 14.63 | 297.22 ± 3.98 | 7.82 × 10−7 |
| 168 | 24 | 26.4 ± 2.5 | 6.03 | 87.77 | 72.08 ± 6.54 | 353.63 ± 2.00 | 13.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widyanto, A.R.; Nomura, M. Effect of Seed Size on Pervaporation Performances Through FAU Zeolite Membrane. Membranes 2025, 15, 355. https://doi.org/10.3390/membranes15120355
Widyanto AR, Nomura M. Effect of Seed Size on Pervaporation Performances Through FAU Zeolite Membrane. Membranes. 2025; 15(12):355. https://doi.org/10.3390/membranes15120355
Chicago/Turabian StyleWidyanto, Alvin Rahmad, and Mikihiro Nomura. 2025. "Effect of Seed Size on Pervaporation Performances Through FAU Zeolite Membrane" Membranes 15, no. 12: 355. https://doi.org/10.3390/membranes15120355
APA StyleWidyanto, A. R., & Nomura, M. (2025). Effect of Seed Size on Pervaporation Performances Through FAU Zeolite Membrane. Membranes, 15(12), 355. https://doi.org/10.3390/membranes15120355







