Impacts of Cipargamin on Na+-ATPase and Osmoregulation of Trypanosoma cruzi
Abstract
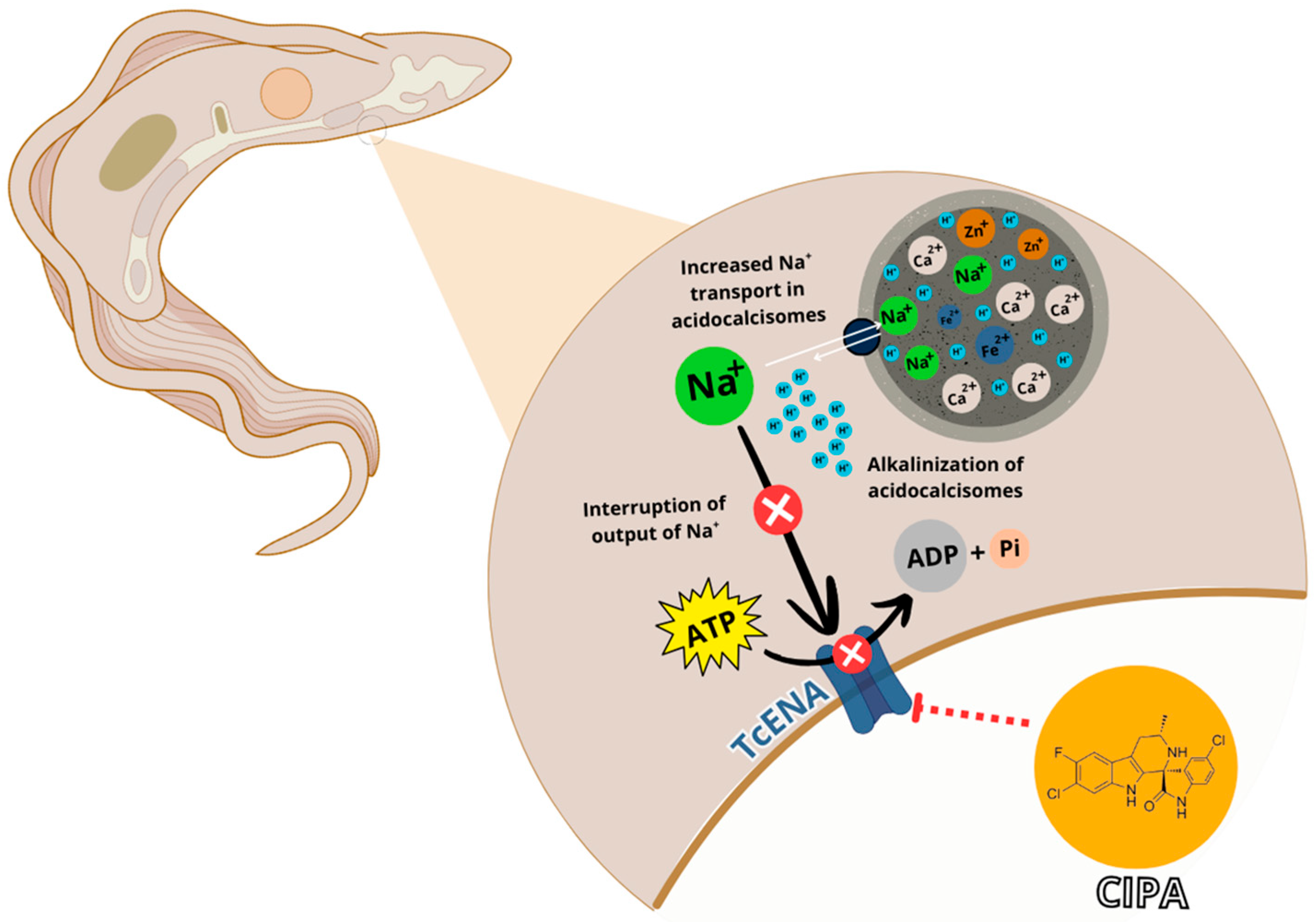
1. Introduction
2. Materials and Methods
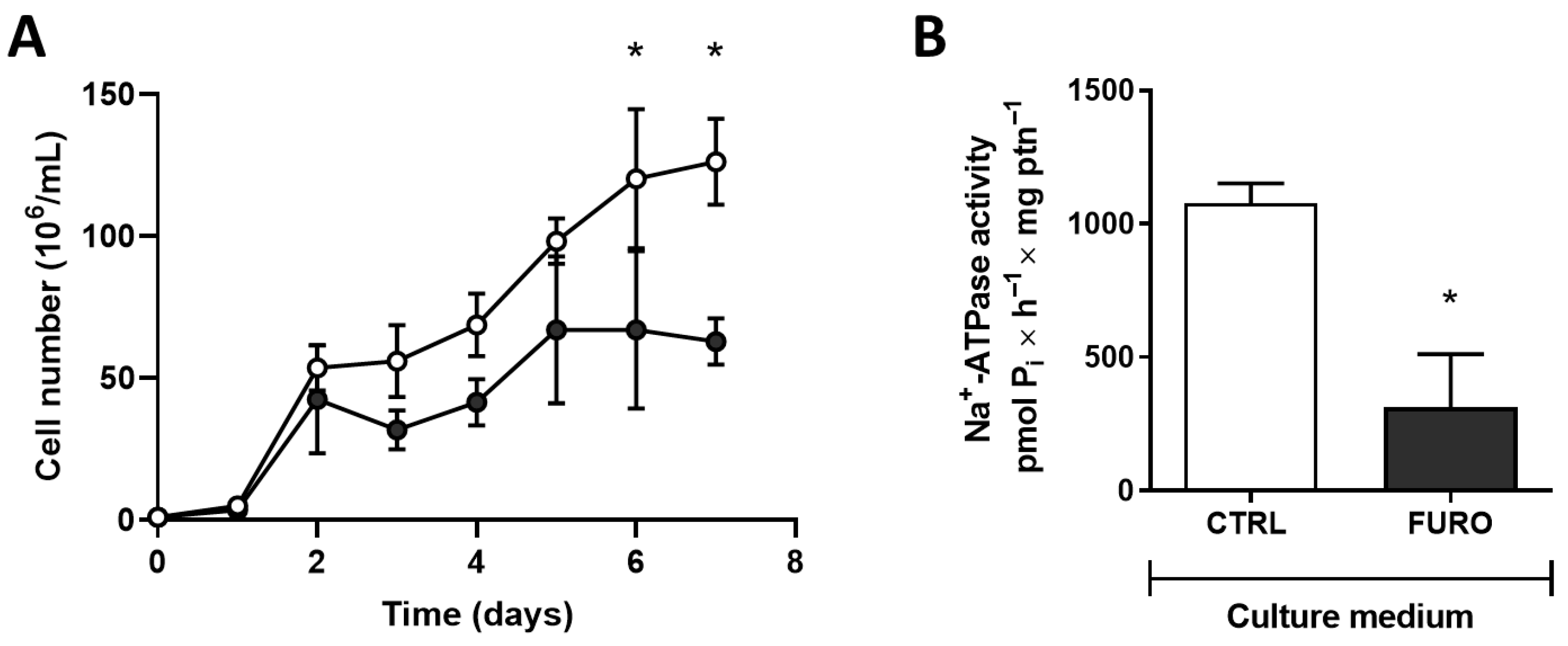
2.1. Cell Culture and Proliferation Profile
2.2. Cell Viability
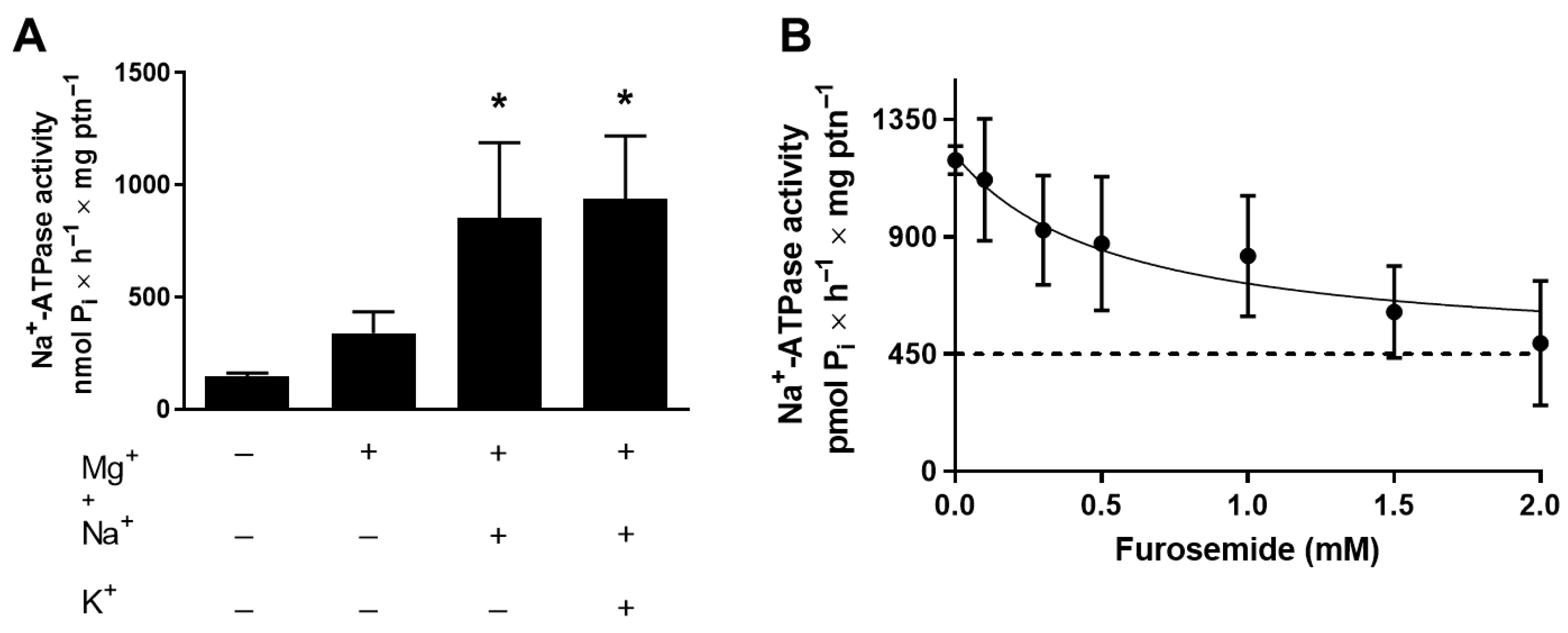
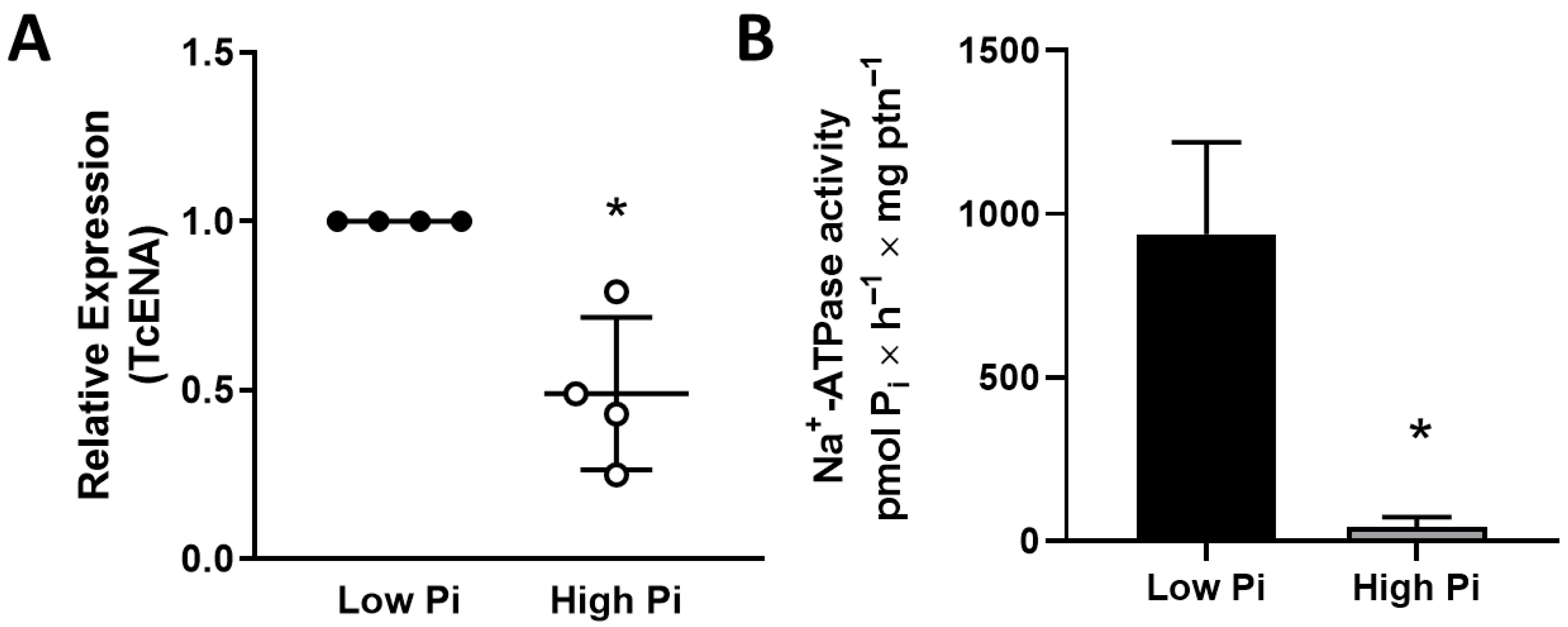
2.3. Na+-ATPase Activity in T. cruzi
2.4. Quantitative PCR
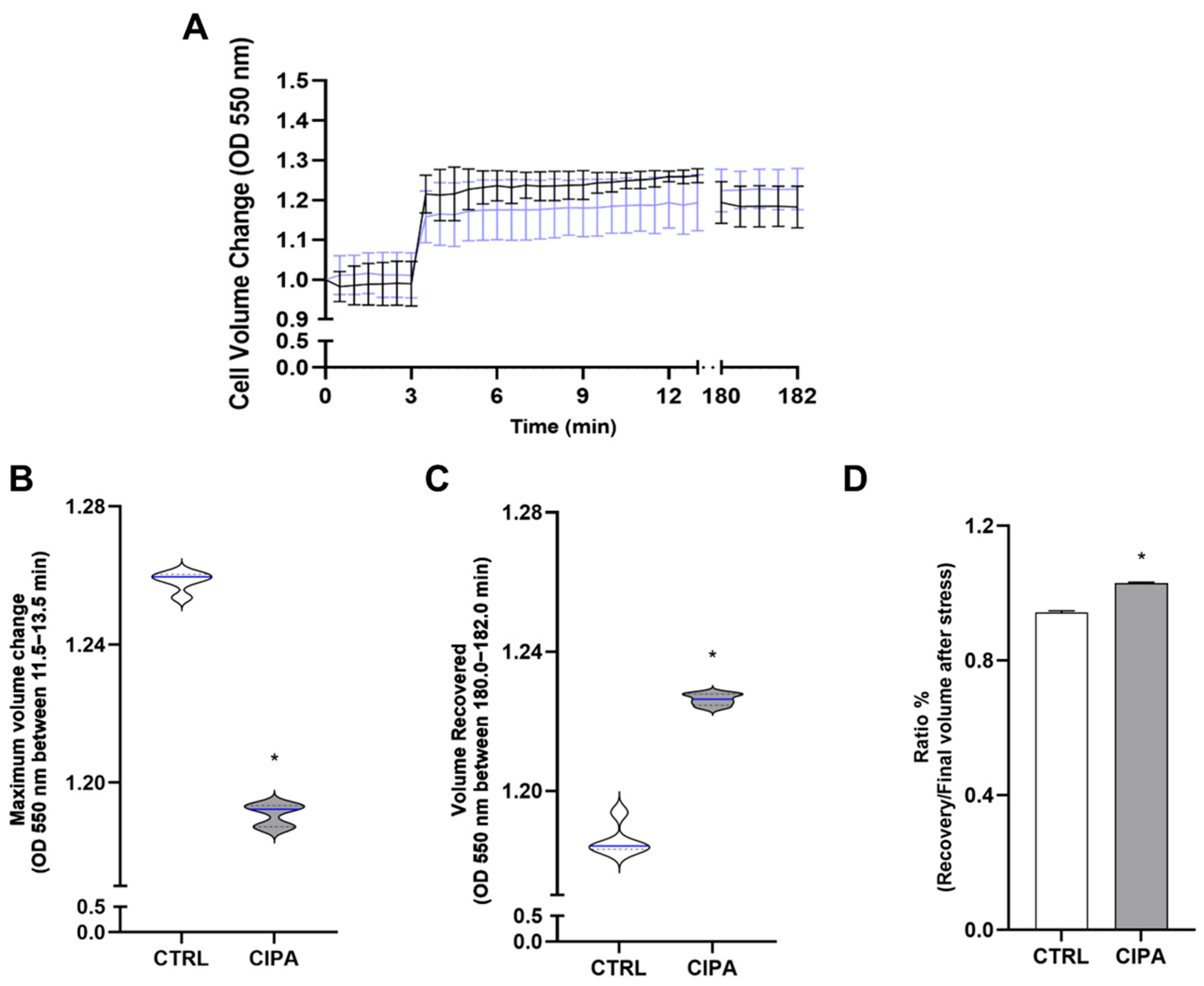
2.5. Regulatory Volume Changes Under Conditions of Osmotic Stress
2.6. Immunofluorescence
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chagas, C. Nova tripanozomiaze humana. Memórias Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef]
- Rassi, A.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Coura, J.R.; Junqueira, A.C.V.; Fernandes, O.; Valente, S.A.S.; Miles, M.A. Emerging Chagas disease in Amazonian Brazil. Trends Parasitol. 2002, 18, 171–176. [Google Scholar] [CrossRef]
- Pereira, K.S.; Schmidt, F.L.; Guaraldo, A.M.A.; Franco, R.M.B.; Dias, V.L.; Passos, L.A.C. Chagas’ disease as a foodborne illness. J. Food Prot. 2009, 72, 441–446. [Google Scholar] [CrossRef]
- Beyenbach, K.W. Kidneys sans glomeruli. Am. J. Physiol.-Ren. Physiol. 2004, 286, F811–F827. [Google Scholar] [CrossRef]
- Jakob, M.; Clausen, V.; Poulsen, H. Sodium/Potassium Homeostasis in the Cell. Met. Ions Life Sci. 2013, 12, 41–67. [Google Scholar]
- Landahl, H.D.; Baldwin, D.; Schwartz, T.B. A physicomathematical model of sodium balance. Bull. Math. Biol. 1964, 26, 193–198. [Google Scholar] [CrossRef]
- Skou, J.C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim. Biophys. Acta 1957, 23, 394–401. [Google Scholar] [CrossRef]
- Proverbio, F. Ouabain-insensitive Na+ stimulation of an Mg2+-dependent ATPase in kidney tissue. Biochim. Biophys. Acta 1975, 394, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Whittembury, G.; Proverbio, F. Two modes of Na extrusion in cells from guinea pig kidney cortex slices. Pflügers Arch. Eur. J. Physiol. 1970, 316, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Dick, C.F.; Meyer-Fernandes, J.R.; Vieyra, A. The Functioning of Na+-ATPases from Protozoan Parasites: Are These Pumps Targets for Antiparasitic Drugs? Cells 2020, 9, 2225. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, J.R.; Marín, R.; Proverbio, T.; Proverbio, T. Partial characterization of the ouabain-insensitive, Na+-stimulated atpase activity of kidney basal-lateral plasma membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 1982, 692, 61–68. [Google Scholar] [CrossRef]
- Iizumi, K.; Mikami, Y.; Hashimoto, M.; Nara, T.; Hara, Y.; Aoki, T. Molecular cloning and characterization of ouabain-insensitive Na+-ATPase in the parasitic protist, Trypanosoma cruzi. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 738–746. [Google Scholar] [CrossRef]
- Lehane, A.M.; Dennis, A.S.; Bray, K.O.; Li, D.; Rajendran, E.; McCoy, J.M.; McArthur, H.M.; Winterberg, M.; Rahimi, F.; Tonkin, C.J.; et al. Characterization of the ATP4 ion pump in Toxoplasma gondii. J. Biol. Chem. 2019, 294, 5720–5734. [Google Scholar] [CrossRef] [PubMed]
- Dick, C.F.; Dos-Santos, A.L.A.; Majerowicz, D.; Paes, L.S.; Giarola, N.L.; Gondim, K.C.; Vieyra, A.; Meyer-Fernandes, J.R. Inorganic phosphate uptake in Trypanosoma cruzi is coupled to K+ cycling and to active Na+ extrusion. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 4265–4273. [Google Scholar] [CrossRef]
- Spillman, N.J.; Allen, R.J.; McNamara, C.W.; Yeung, B.K.; Winzeler, E.A.; Diagana, T.T.; Kirk, K. Na+ regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe 2013, 13, 227–237. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A.; Benito, B. Sodium or potassium efflux ATPase. A fungal, bryophyte, and protozoal ATPase. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 1841–1853. [Google Scholar] [CrossRef]
- Rottmann, M.; McNamara, C.; Yeung, B.K.; Lee, M.C.; Zou, B.; Russell, B.; Seitz, P.; Plouffe, D.M.; Dharia, N.V.; Tan, J.; et al. Spiroindolones, a potent compound class for the treatment of malaria. Science 2010, 329, 1175–1180. [Google Scholar] [CrossRef]
- Bouwman, S.A.; Zoleko-Manego, R.; Renner, K.C.; Schmitt, E.K.; Mombo-Ngoma, G.; Grobusch, M.P. The early preclinical and clinical development of cipargamin (KAE609), a novel antimalarial compound. Travel Med. Infect. Dis. 2020, 36, 101765. [Google Scholar] [CrossRef]
- Spillman, N.J.; Kirk, K. The malaria parasite cation ATPase PfATP4 and its role in the mechanism of action of a new arsenal of antimalarial drugs. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 149–162. [Google Scholar] [CrossRef]
- White, N.J.; Pukrittayakamee, S.; Phyo, A.P.; Rueangweerayut, R.; Nosten, F.; Jittamala, P.; Jeeyapant, A.; Jain, J.P.; Lefèvre, G.; Li, R.; et al. Spiroindolone KAE609 for Falciparum and Vivax Malaria. N. Engl. J. Med. 2014, 371, 403–410. [Google Scholar] [CrossRef]
- Jiménez-Díaz, M.B.; Ebert, D.; Salinas, Y.; Pradhan, A.; Lehane, A.M.; Myrand-Lapierre, M.E.; O’Loughlin, K.G.; Shackleford, D.M.; Justino de Almeida, M.; Carrillo, A.K.; et al. (+)-SJ733, a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc. Natl. Acad. Sci. USA 2014, 111, E5455–E5462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Suwanarusk, R.; Malleret, B.; Cooke, B.M.; Nosten, F.; Lau, Y.L.; Dao, M.; Lim, C.T.; Renia, L.; Tan, K.S.; et al. A basis for rapid clearance of circulating ring-stage malaria parasites by the spiroindolone KAE609. J. Infect. Dis. 2016, 213, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lander, N.; Ulrich, P.N.; Docampo, R. Trypanosoma brucei vacuolar transporter chaperone 4 (TbVtc4) is an acidocalcisome polyphosphate kinase required for in vivo infection. J. Biol. Chem. 2013, 288, 34205–34216. [Google Scholar] [CrossRef]
- Chiurillo, M.A.; Carlson, J.; Bertolini, M.S.; Raja, A.; Lander, N. Dual localization of receptor-type adenylate cyclases and cAMP response protein 3 unveils the presence of two putative signaling microdomains in Trypanosoma cruzi. mBio 2023, 14, e0106423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vercesi, A.E.; Moreno, S.N.; Docampo, R. Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei. Biochem. J. 1994, 304, 227–233. [Google Scholar] [CrossRef]
- Huang, G.; Docampo, R. Acidocalcisome localization of membrane transporters and enzymes in Trypanosoma brucei. Microbiol. Spectr. 2024, 12, e0112824. [Google Scholar] [CrossRef] [PubMed]
- Lemercier, G.; Dutoya, S.; Luo, S.; Ruiz, F.A.; Rodrigues, C.O.; Baltz, T.; Docampo, R.; Bakalara, N. A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei. J. Biol. Chem. 2002, 277, 37369–37376. [Google Scholar] [CrossRef]
- Docampo, R. Advances in the cellular biology, biochemistry, and molecular biology of acidocalcisomes. Microbiol. Mol. Biol. Rev. 2024, 88, e0004223. [Google Scholar] [CrossRef]
- Docampo, R.; Jimenez, V.; Lander, N.; Li, Z.H.; Niyogi, S. New insights into roles of acidocalcisomes and contractile vacuole complex in osmoregulation in protists. Int. Rev. Cell Mol. Biol. 2013, 305, 69–113. [Google Scholar]
- Asady, B.; Dick, C.F.; Ehrenman, K.; Sahu, T.; Romano, J.D.; Coppens, I. A single Na+-Pi cotransporter in Toxoplasma plays key roles in phosphate import and control of parasite osmoregulation. PLoS Pathog. 2020, 16, e1009067. [Google Scholar] [CrossRef]
- Rosling, J.E.O.; Ridgway, M.C.; Summers, R.L.; Kirk, K.; Lehane, A.M. Biochemical characterization and chemical inhibition of PfATP4-associated Na-ATPase activity in Plasmodium falciparum membranes. J. Biol. Chem. 2018, 293, 13327–13337. [Google Scholar] [CrossRef] [PubMed]
- Lander, N.; Chiurillo, M.A.; Docampo, R. Signaling pathways involved in environmental sensing in Trypanosoma cruzi. Mol. Microbiol. 2021, 115, 819–828. [Google Scholar] [CrossRef]
- Dumoulin, P.C.; Burleigh, B.A. Metabolic flexibility in Trypanosoma cruzi amastigotes: Implications for persistence and drug sensitivity. Curr. Opin. Microbiol. 2021, 63, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Kollien, A.H.; Grospietsch, T.; Kleffmann, T.; Zerbst-Boroffka, I.; Schaub, G.A. Ionic composition of the rectal contents and excreta of the reduviid bug Triatoma infestans. J. Insect Physiol. 2001, 47, 739–747. [Google Scholar] [CrossRef]
- Lang, F. Mechanisms and Significance of Cell Volume Regulation. J. Am. Coll. Nutr. 2007, 26, 613S–623S. [Google Scholar] [CrossRef]
- McManus, M.L.; Churchwell, K.B.; Sthange, K. Regulation of cell volume in health and disease. N. Engl. J. Med. 2013, 26, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Alvarez, V.E.; De Gaudenzi, J.G.; Sant’Anna, C.; Frasch, A.C.; Cazzulo, J.J.; Docampo, R. Hyperosmotic stress induces aquaporin-dependent cell shrinkage, polyphosphate synthesis, amino acid accumulation, and global gene expression changes in Trypanosoma cruzi. J. Biol. Chem. 2011, 286, 43959–43971. [Google Scholar] [CrossRef]
- Fang, J.; Rohloff, P.; Miranda, K.; Docampo, R. Ablation of a small transmembrane protein of Trypanosoma brucei (TbVTC1) involved in the synthesis of polyphosphate alters acidocalcisome biogenesis and function, and leads to a cytokinesis defect. Biochem. J. 2007, 407, 161–170. [Google Scholar] [CrossRef]
- Ruiz, F.A.; Rodrigues, C.O.; Docampo, R. Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi. J. Biol. Chem. 2001, 276, 26114–26121. [Google Scholar] [CrossRef] [PubMed]
- Zuma, A.A.; dos Santos Barrias, E.; de Souza, W. Basic Biology of Trypanosoma cruzi. Curr. Pharm. Des. 2020, 27, 1671–1732. [Google Scholar] [CrossRef] [PubMed]
- Bonansea, S.; Usorach, M.; Gesumaría, M.C.; Santander, V.; Gimenez, A.M.; Bollo, M.; Machado, E.E. Stress response to high osmolarity in Trypanosoma cruzi epimastigotes. Arch. Biochem. Biophys. 2012, 527, 6–15. [Google Scholar] [CrossRef] [PubMed]


| Primers Name | Forward Primer | Reverse Primer |
|---|---|---|
| TcENA | GCTCCTTTG CCGTCGGGG TC | GGCGAGAACACCCCAGTGCC |
| TcGAPDH | GCAGCTCCATCTACGACTCC | AGTATCCCCACTCGTTGTCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dick, C.F.; Silva, A.A.C.d.L.B.d.; de-Souza-Silva, A.; Frechiani, G.; Barbosa-de-Barros, J.; Vieyra, A.; Meyer-Fernandes, J.R. Impacts of Cipargamin on Na+-ATPase and Osmoregulation of Trypanosoma cruzi. Membranes 2025, 15, 349. https://doi.org/10.3390/membranes15120349
Dick CF, Silva AACdLBd, de-Souza-Silva A, Frechiani G, Barbosa-de-Barros J, Vieyra A, Meyer-Fernandes JR. Impacts of Cipargamin on Na+-ATPase and Osmoregulation of Trypanosoma cruzi. Membranes. 2025; 15(12):349. https://doi.org/10.3390/membranes15120349
Chicago/Turabian StyleDick, Claudia Fernanda, Ana Angelica Celso de Lima Barreto da Silva, Adriano de-Souza-Silva, Giovanna Frechiani, Juliana Barbosa-de-Barros, Adalberto Vieyra, and José Roberto Meyer-Fernandes. 2025. "Impacts of Cipargamin on Na+-ATPase and Osmoregulation of Trypanosoma cruzi" Membranes 15, no. 12: 349. https://doi.org/10.3390/membranes15120349
APA StyleDick, C. F., Silva, A. A. C. d. L. B. d., de-Souza-Silva, A., Frechiani, G., Barbosa-de-Barros, J., Vieyra, A., & Meyer-Fernandes, J. R. (2025). Impacts of Cipargamin on Na+-ATPase and Osmoregulation of Trypanosoma cruzi. Membranes, 15(12), 349. https://doi.org/10.3390/membranes15120349








