Photochemical Methods to Study the Radical-Induced Degradation of Anion-Exchange Membranes
Abstract
1. Introduction
E°(HO•, H+/H2O) = 2.73 V
E°(HOO•, H+/H2O2) = 1.46 V
radical path
non-radical path
2. Experimental Section
2.1. Chemicals and Reactants
2.2. Methods and Equipment
3. Results and Discussion
3.1. Photochemical Generation of Radicals
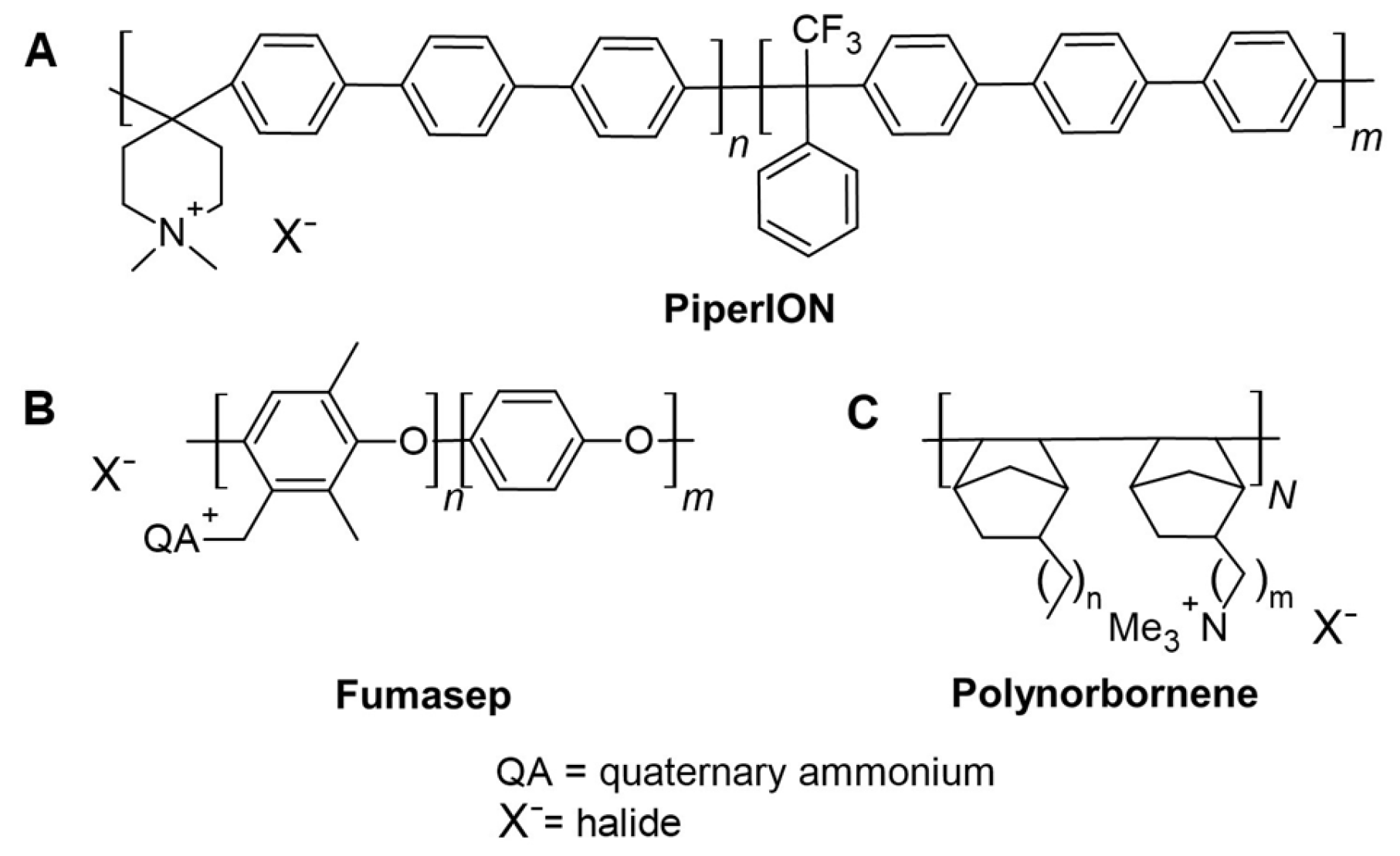
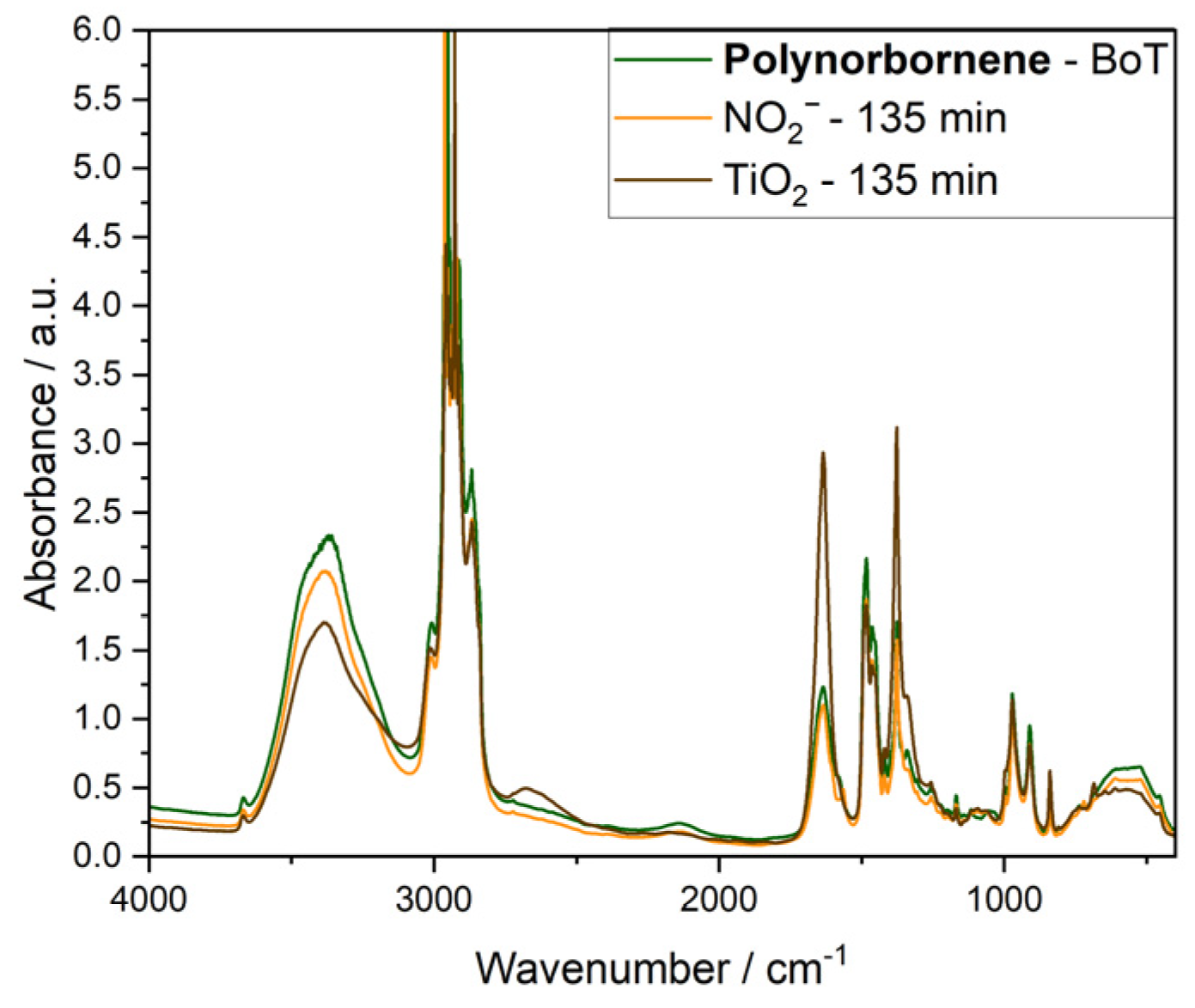
3.2. Radical-Induced Degradation of AEMs
3.3. Practical Considerations
k = 1.1 × 1010 M−1s−1
k = 1.2 × 1010 M−1s−1
k = 4 × 108 M−1s−1
k = 1.2 × 1010 M−1s−1
3.4. Applicability of Methods to Compare AEMs
k = 2.1 × 106 M−1s−1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Yuan, H.; Martinez, A.; Hong, P.; Xu, H.; Bockmiller, F.R. Polymer Electrolyte Membrane Fuel Cell and Hydrogen Station Networks for Automobiles: Status, Technology, and Perspectives. Adv. Appl. Energy 2021, 2, 100011. [Google Scholar] [CrossRef]
- Kim, M.; Lee, D.; Qi, M.; Kim, J. Techno-Economic Analysis of Anion Exchange Membrane Electrolysis Process for Green Hydrogen Production under Uncertainty. Energy Convers. Manag. 2024, 302, 118134. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Fateev, V.N.; Bessarabov, D.G.; Millet, P. Current Status, Research Trends, and Challenges in Water Electrolysis Science and Technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Willdorf-Cohen, S.; Mondal, A.N.; Dekel, D.R.; Diesendruck, C.E. Chemical Stability of Poly(Phenylene Oxide)-Based Ionomers in an Anion Exchange-Membrane Fuel Cell Environment. J. Mater. Chem. A 2018, 6, 22234–22239. [Google Scholar] [CrossRef]
- Gubler, L.; Dockheer, S.M.; Koppenol, W.H. Radical (HO•, H• and HOO•) Formation and Ionomer Degradation in Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2011, 158, B755–B769. [Google Scholar] [CrossRef]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability Challenges of Anion Exchange Membrane Fuel Cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Parrondo, J.; Wang, Z.; Jung, M.-S.J.; Ramani, V. Reactive Oxygen Species Accelerate Degradation of Anion Exchange Membranes Based on Polyphenylene Oxide in Alkaline Environments. Phys. Chem. Chem. Phys. 2016, 18, 19705–19712. [Google Scholar] [CrossRef]
- Zhang, Y.; Parrondo, J.; Sankarasubramanian, S.; Ramani, V. Detection of Reactive Oxygen Species in Anion Exchange Membrane Fuel Cells Using In Situ Fluorescence Spectroscopy. ChemSusChem 2017, 10, 3056–3062. [Google Scholar] [CrossRef]
- Espiritu, R.; Golding, B.T.; Scott, K.; Mamlouk, M. Degradation of Radiation Grafted Hydroxide Anion Exchange Membrane Immersed in Neutral pH: Removal of Vinylbenzyl Trimethylammonium Hydroxide Due to Oxidation. J. Mater. Chem. A 2017, 5, 1248–1267. [Google Scholar] [CrossRef]
- Wierzbicki, S.; Douglin, J.C.; Kostuch, A.; Dekel, D.R.; Kruczała, K. Are Radicals Formed During Anion-Exchange Membrane Fuel Cell Operation? J. Phys. Chem. Lett. 2020, 11, 7630–7636. [Google Scholar] [CrossRef]
- Wierzbicki, S.; Douglin, J.C.; Singh, R.K.; Dekel, D.R.; Kruczała, K. Operando EPR Study of Radical Formation in Anion-Exchange Membrane Fuel Cells. ACS Catal. 2023, 13, 2744–2750. [Google Scholar] [CrossRef]
- Marino, M.G.; Kreuer, K.D. Alkaline Stability of Quaternary Ammonium Cations for Alkaline Fuel Cell Membranes and Ionic Liquids. ChemSusChem 2015, 8, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Ayers, K.E.; Anderson, E.B.; Capuano, C.B.; Niedzwiecki, M.; Hickner, M.A.; Wang, C.-Y.; Leng, Y.; Zhao, W. Characterization of Anion Exchange Membrane Technology for Low Cost Electrolysis. ECS Trans. 2013, 45, 121–130. [Google Scholar] [CrossRef]
- Tsuneda, T. Fenton Reaction Mechanism Generating No OH Radicals in Nafion Membrane Decomposition. Sci. Rep. 2020, 10, 18144. [Google Scholar] [CrossRef]
- Koppenol, W.H. Ferryl for Real. The Fenton Reaction near Neutral pH. Dalton Trans. 2022, 51, 17496–17502. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, J.; Peng, J.; Xu, L.; Li, J.; Zhai, M. Study on the Chemical Stability of the Anion Exchange Membrane of Grafting Dimethylaminoethyl Methacrylate. J. Membr. Sci. 2011, 376, 70–77. [Google Scholar] [CrossRef]
- Mierzwa, J.C.; Rodrigues, R.; Teixeira, A.C.S.C. UV-Hydrogen Peroxide Processes. In Advanced Oxidation Processes for Waste Water Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 13–48. [Google Scholar] [CrossRef]
- Pędziwiatr, P.; Mikołajczyk, F.; Zawadzki, D.; Mikołajczyk, K.; Bedka, A. Decomposition of Hydrogen Peroxide—Kinetics and Review of Chosen Catalysts. Acta Innov. 2018, 26, 45–52. [Google Scholar] [CrossRef]
- Arges, C.G.; Ramani, V.; Wang, Z.; Ouimet, R.J. Assessing the Oxidative Stability of Anion Exchange Membranes in Oxygen Saturated Aqueous Alkaline Solutions. Front. Energy Res. 2022, 10, 871851. [Google Scholar] [CrossRef]
- Davis, R.E.; Horvath, G.L.; Tobias, C.W. The Solubility and Diffusion Coefficient of Oxygen in Potassium Hydroxide Solutions. Electrochim. Acta 1967, 12, 287–297. [Google Scholar] [CrossRef]
- Nemeth, T.; Nauser, T.; Gubler, L. On the Radical-Induced Degradation of Quaternary Ammonium Cations for Anion-Exchange Membrane Fuel Cells and Electrolyzers. ChemSusChem 2022, 15, e202201571. [Google Scholar] [CrossRef]
- Ghassemzadeh, L.; Peckham, T.J.; Weissbach, T.; Luo, X.; Holdcroft, S. Selective Formation of Hydrogen and Hydroxyl Radicals by Electron Beam Irradiation and Their Reactivity with Perfluorosulfonated Acid Ionomer. J. Am. Chem. Soc. 2013, 135, 15923–15932. [Google Scholar] [CrossRef]
- Takeda, K.; Fujisawa, K.; Nojima, H.; Kato, R.; Ueki, R.; Sakugawa, H. Hydroxyl Radical Generation with a High Power Ultraviolet Light Emitting Diode (UV-LED) and Application for Determination of Hydroxyl Radical Reaction Rate Constants. J. Photochem. Photobiol. A Chem. 2017, 340, 8–14. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (• OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O−2 Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Mack, J.; Bolton, J.R. Photochemistry of Nitrite and Nitrate in Aqueous Solution: A Review. J. Photochem. Photobiol. A Chem. 1999, 128, 1–13. [Google Scholar] [CrossRef]
- Buxton, G.V. Pulse Radiolysis of Aqueous Solutions. Some Rates of Reaction of OH and O– And pH Dependence of the Yield of O3–. Trans. Faraday Soc. 1969, 65, 2150. [Google Scholar] [CrossRef]
- Ransdell-Green, E.C.; Baranowska-Kortylewicz, J.; Wang, D. Advances in Fluorescence Techniques for the Detection of Hydroxyl Radicals near DNA and Within Organelles and Membranes. Antioxidants 2025, 14, 79. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Moon, G.; Kim, B.; Tachikawa, T.; Majima, T.; Hong, S.; Cho, K.; Kim, W.; Choi, W. Crystal Phase-Dependent Generation of Mobile OH Radicals on TiO2: Revisiting the Photocatalytic Oxidation Mechanism of Anatase and Rutile. Appl. Catal. B Environ. 2021, 286, 119905. [Google Scholar] [CrossRef]
- Al-Oubidy, E.A.; Kadhim, F.J. Photocatalytic Activity of Anatase Titanium Dioxide Nanostructures Prepared by Reactive Magnetron Sputtering Technique. Opt. Quant. Electron. 2019, 51, 23. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Setzler, B.P.; Rojas-Carbonell, S.; Ben Yehuda, C.; Amel, A.; Page, M.; Wang, L.; Hu, K.; Shi, L.; et al. Poly(Aryl Piperidinium) Membranes and Ionomers for Hydroxide Exchange Membrane Fuel Cells. Nat. Energy 2019, 4, 392–398. [Google Scholar] [CrossRef]
- Giovanelli, A.; Pozio, A.; Pucci, A.; Geppi, M.; Martini, F. Fumasep FAA-3-PK-130: Exploiting Multinuclear Solid-State NMR to Shed Light on Undisclosed Structural Properties. Polymer 2024, 311, 127536. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Kohl, P.A. Highly Conductive Anion-Exchange Membranes Based on Cross-Linked Poly(Norbornene): Vinyl Addition Polymerization. ACS Appl. Energy Mater. 2019, 2, 2447–2457. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Buxton, G.V.; Wood, N.D.; Dyster, S. Ionisation Constants of ˙OH and HO˙2 in Aqueous Solution up to 200 °C. A Pulse Radiolysis Study. J. Chem. Soc. Faraday Trans. 1 1988, 84, 1113. [Google Scholar] [CrossRef]
- Anbar, M.; Meyerstein, D.; Neta, P. The Reactivity of Aromatic Compounds toward Hydroxyl Radicals. J. Phys. Chem. 1966, 70, 2660–2662. [Google Scholar] [CrossRef]
- Bao, Z.-C.; Barker, J.R. Temperature and Ionic Strength Effects on Some Reactions Involving Sulfate Radical [SO4− (Aq)]. J. Phys. Chem. 1996, 100, 9780–9787. [Google Scholar] [CrossRef]
- Buxton, G.V. Pulse Radiolysis of Aqueous Solutions. Rate of Reaction of OH with OH? Trans. Faraday Soc. 1970, 66, 1656. [Google Scholar] [CrossRef]
- Guo, L.; Masuda, A.; Miyatake, K. Reinforcement Effect in Tandemly Sulfonated, Partially Fluorinated Polyphenylene PEMs for Fuel Cells. RSC Adv. 2023, 13, 11225–11233. [Google Scholar] [CrossRef]
- Clemens, A.L.; Jayathilake, B.S.; Karnes, J.J.; Schwartz, J.J.; Baker, S.E.; Duoss, E.B.; Oakdale, J.S. Tuning Alkaline Anion Exchange Membranes through Crosslinking: A Review of Synthetic Strategies and Property Relationships. Polymers 2023, 15, 1534. [Google Scholar] [CrossRef]
- Lewis, R.S.; Deen, W.M. Kinetics of the Reaction of Nitric Oxide with Oxygen in Aqueous Solutions. Chem. Res. Toxicol. 1994, 7, 568–574. [Google Scholar] [CrossRef]
- Uchino, T.; Tokunaga, H.; Ando, M.; Utsumi, H. Quantitative Determination of OH Radical Generation and Its Cytotoxicity Induced by TiO2–UVA Treatment. Toxicol. Vitr. 2002, 16, 629–635. [Google Scholar] [CrossRef]



| Reaction | Reactant | k /M−1s−1 | Conc/mM | k’ /s−1 | Yield /% |
|---|---|---|---|---|---|
| (7) | NO2− | 8 × 109 | 0.1 | 8 × 105 | 8 |
| (8) | BA | 2 × 109 | 4.5 | 9 × 106 | 92 |
| (7) | NO2− | 8 × 109 | 0.6 | 4.8 × 106 | 35 |
| (8) | BA | 2 × 109 | 4.5 | 9 × 106 | 65 |
| (7) | NO2− | 8 × 109 | 1 | 8 × 106 | 47 |
| (8) | BA | 2 × 109 | 4.5 | 9 × 106 | 53 |
| (7) | NO2− | 8 × 109 | 2 | 1.2 × 107 | 64 |
| (8) | BA | 2 × 109 | 4.5 | 9 × 106 | 36 |
| Remaining IEC 1/% | ||||||
|---|---|---|---|---|---|---|
| Time/min | Fumasep | Polynorbornene | PiperION | |||
| NO2− | TiO2 | NO2− | TiO2 | NO2− | TiO2 | |
| 0 | 100 ± 1 | 100 ± 3 | 100 ± 1 | 100 ± 1 | 100 ± 1 | 100 ± 1 |
| 30 | 96 ± 2 | 96 ± 1 | 92 ± 6 | 100 ± 3 | 93 ± 3 | 97 ± 1 |
| 60 | 90 ± 1 | 89 ± 3 | 83 ± 4 | 97 ± 8 | 94 ± 2 | 99 ± 1 |
| 90 | 92 ± 1 | 89 ± 3 | 74 ± 12 | 84 ± 15 | 86 ± 2 | 98 ± 2 |
| 135 | 86 ± 2 | 91 ± 7 | 83 ± 1 | 78 ± 15 | 82 ± 13 | 54 ± 2 |
| Method | Advantage | Limitation |
|---|---|---|
| Fenton’s | Established for PEMs, inexpensive | Ferryl radicals form instead of HO•/O•−, or O2•− at pH > 5 |
| EPR | Selective detection of radicals | Expensive, spin traps may react with radicals that are less relevant for AEMs |
| Radiolysis | Selective formation of radicals | Expensive, can only be used for dissolved compounds |
| Thermal H2O2 | Inexpensive and available | Low yield of radicals |
| Nitrite | Selective formation of HO•/O•−, inexpensive | Nitrite is a source and scavenger of radicals |
| TiO2 | Relevant radicals (HO•/O•−, or O2•−) form, inexpensive | Vigorous stirring is required to avoid sedimentation of particles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solyom, P.; Nauser, T.; Nemeth, T. Photochemical Methods to Study the Radical-Induced Degradation of Anion-Exchange Membranes. Membranes 2025, 15, 305. https://doi.org/10.3390/membranes15100305
Solyom P, Nauser T, Nemeth T. Photochemical Methods to Study the Radical-Induced Degradation of Anion-Exchange Membranes. Membranes. 2025; 15(10):305. https://doi.org/10.3390/membranes15100305
Chicago/Turabian StyleSolyom, Panna, Thomas Nauser, and Tamas Nemeth. 2025. "Photochemical Methods to Study the Radical-Induced Degradation of Anion-Exchange Membranes" Membranes 15, no. 10: 305. https://doi.org/10.3390/membranes15100305
APA StyleSolyom, P., Nauser, T., & Nemeth, T. (2025). Photochemical Methods to Study the Radical-Induced Degradation of Anion-Exchange Membranes. Membranes, 15(10), 305. https://doi.org/10.3390/membranes15100305







