A New Method for Preparing Cross-Sections of Polymer Composite Membranes for TEM Characterization by Substrate Stripping and Double-Orientation Embedding
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Direct Resin Embedding Method
- (1)
- Sample preparation. Firstly, membrane samples were cut into strips measuring 5 mm × 0.5 mm. Then, each strip was gently folded into an “L” shape and heated in an oven at 40 °C for 2 h.
- (2)
- Resin Impregnation and Embedding. The folded samples were placed into embedding molds and immersed in resin. Samples were soaked in the resin for 12 h to ensure complete penetration.
- (3)
- Orientation and Polymerization. The long arm of the “L” shape was adjusted to be parallel to the long axis of the embedding slot, and the short arm parallel to the short axis. The resin was polymerized in an oven at 70 °C for 24 h.
- (4)
- Sectioning. The embedded samples were trimmed to expose the target region, followed by preparation of 100 nm-thick sections using an ultramicrotome (UC7, Leica, Wetzlar, Germany). Finally, sections were collected on copper grids and allowed to air-dry.
2.2.2. Substrate Stripping Technique and Double-Orientation Embedding Method
- (1)
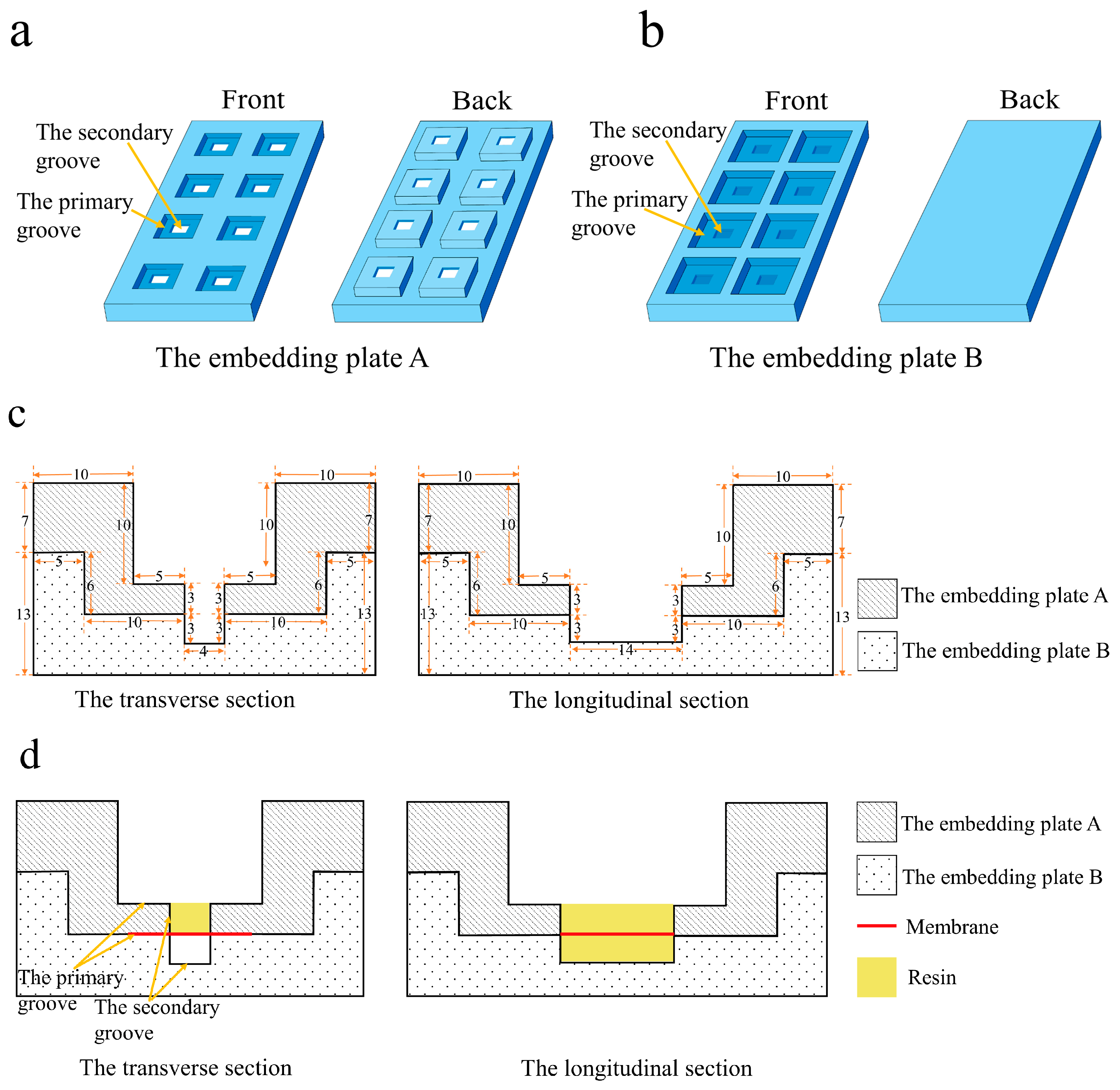
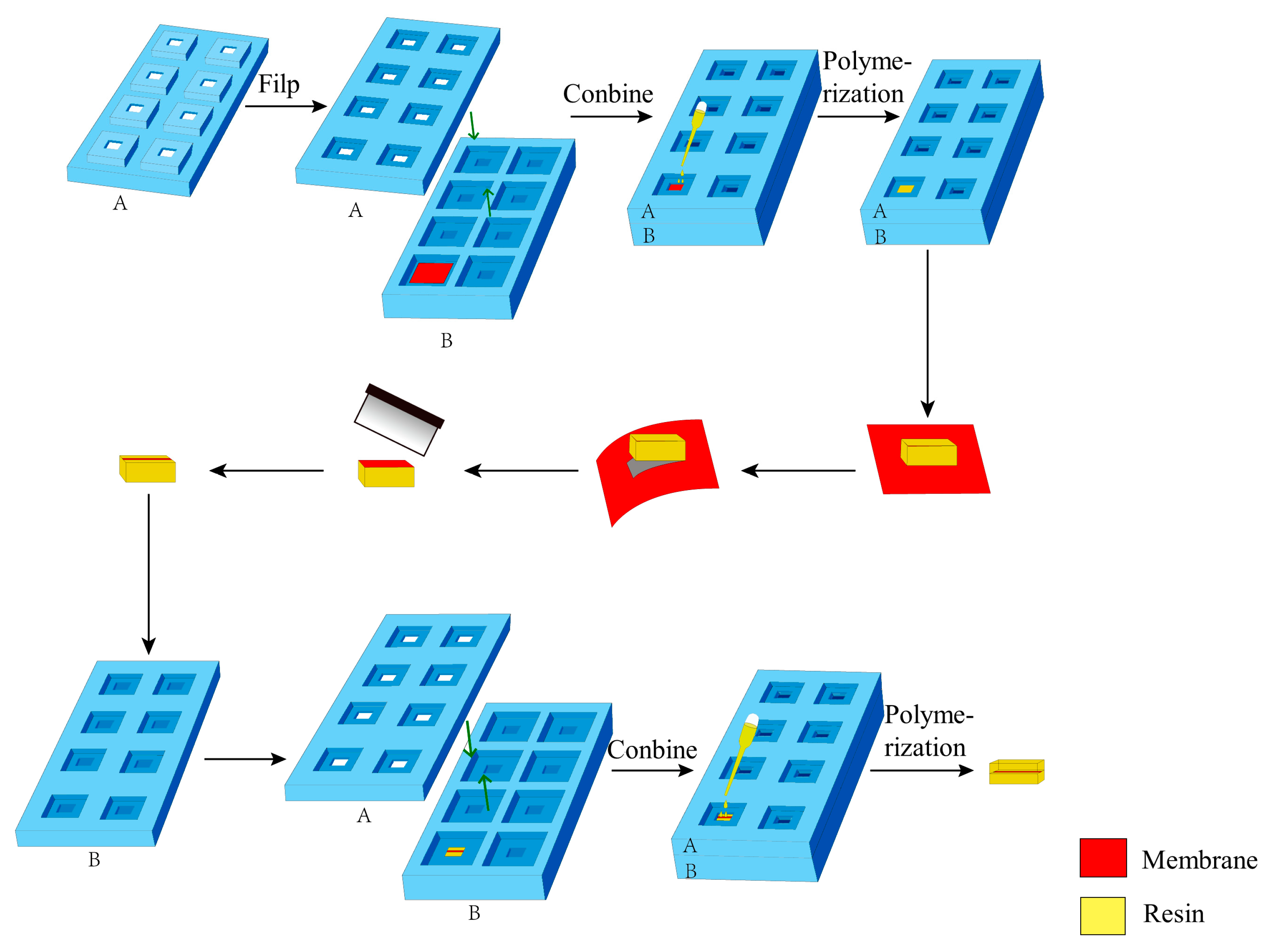
- Sample preparation. The membrane was cut into a rectangle approximately 2.5 cm on each side and heated in an oven at 40 °C for 2 h. Subsequently, the membrane was positioned in the primary groove of the embedding plate B with the functional layer facing upward. Embedding plate A was assembled with plate B, and the assembled plates were tightly clamped around the edges using a fixing device.
- (2)
- Resin Impregnation. 120 µL of epoxy resin was added to the sample in the secondary groove of embedding plate A, followed by impregnation for 1 h. Then, an additional 120 µL of epoxy resin was added to the sample in the same groove, extending impregnation to 12 h.
- (3)
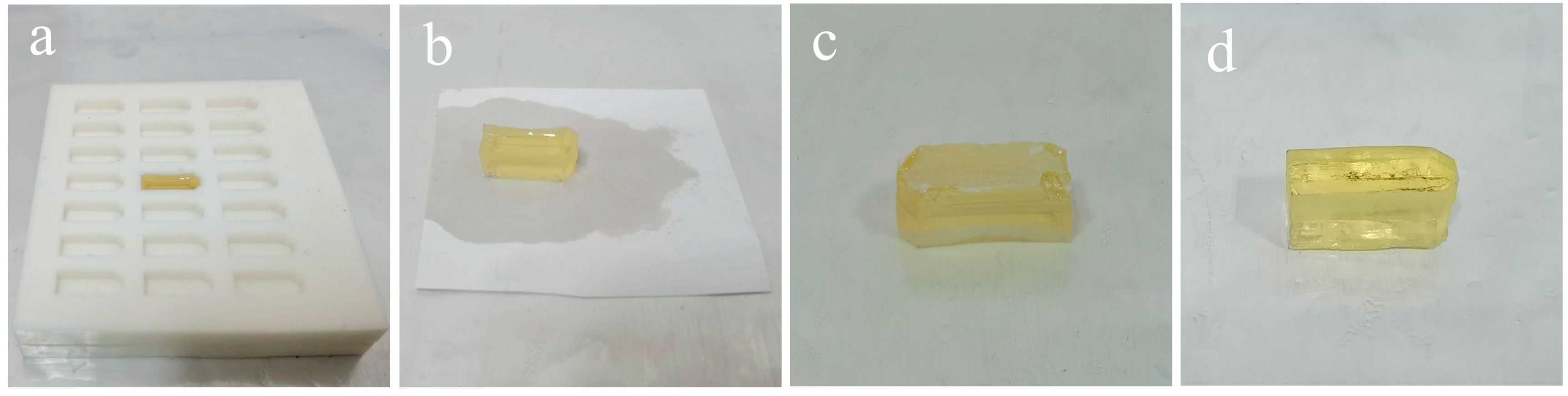
- First Resin Embedding and Polymerization. The secondary groove of the embedding plate A (approximately 3 mm in depth) was filled with epoxy resin, as shown in Figure 4a. Polymerization was conducted in an oven at 70 °C for 24 h.
- (4)
- Fiber Substrate Removal. The embedding plates A and B were disassembled to remove the embedding block (Figure 4b). Subsequently, the embedding block was clamped with tweezers, and the membrane adjacent to the block was manually peeled off. The functional layer of the membrane remained adhered to the bottom of the embedding block, as shown in Figure 4c.
- (5)
- Membrane Trimming and Marking. The lateral regions of the membrane functional layer were trimmed, preserving only a 1–2 mm wide central portion. Charcoal pencil marks were lightly applied to both sides of the membrane to facilitate sample position identification during trimming and sectioning.
- (6)
- Secondary Embedding. The embedding block was positioned in the secondary groove of the embedding plate B (membrane side upward), and plate A was assembled. After securing the assembly, the secondary groove of plate A was filled with resin and impregnated for 4 h.
- (7)
- Second Polymerization. The oven temperature was set to 70 °C for another 24 h polymerization.
- (8)
- Sectioning. The embedded block was trimmed. Then, ultra-thin sections were cut using an ultramicrotome, collected on copper grids, and air-dried.
2.2.3. TEM Characterization and EDS Analysis
3. Results and Discussion
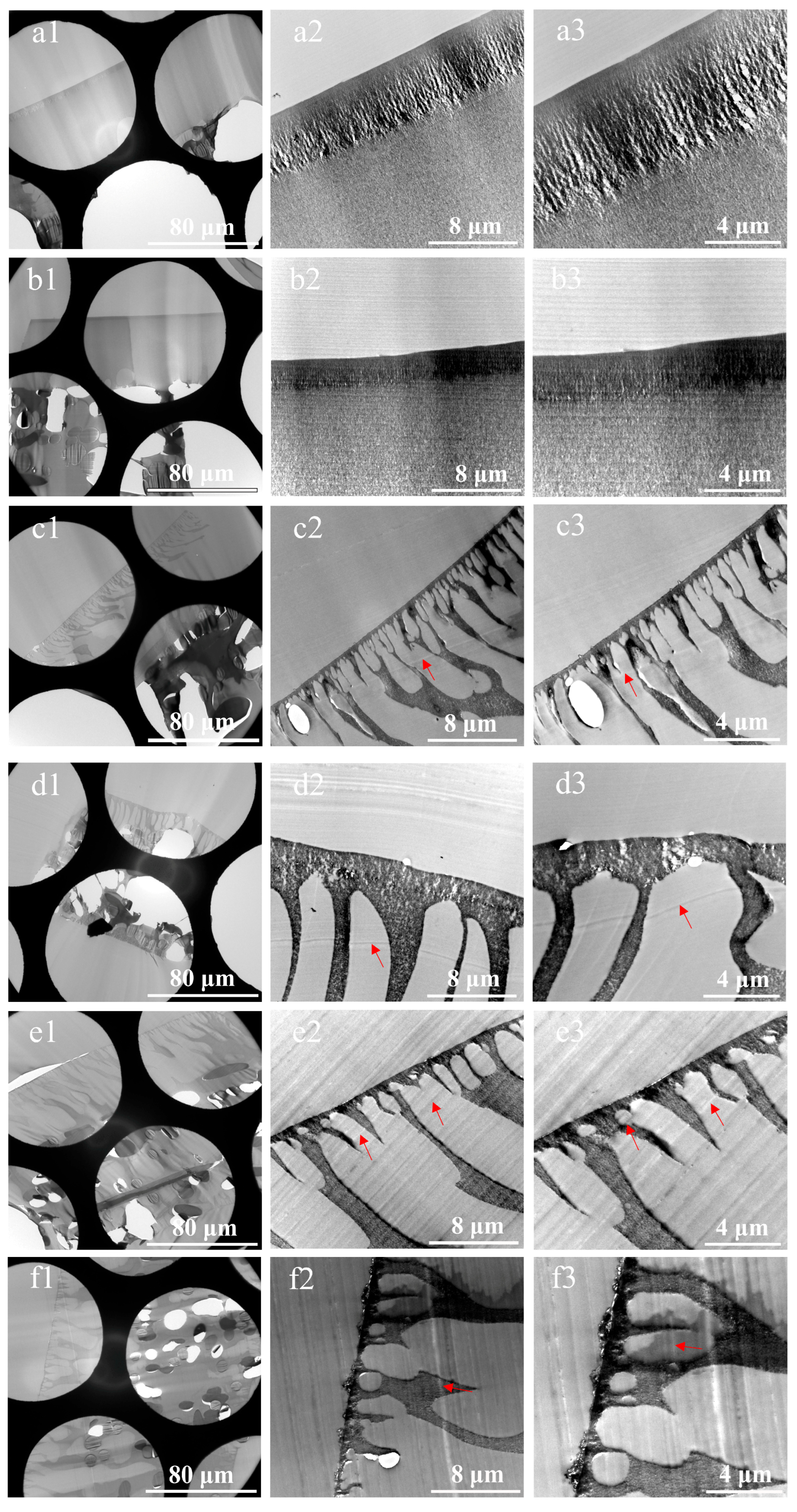
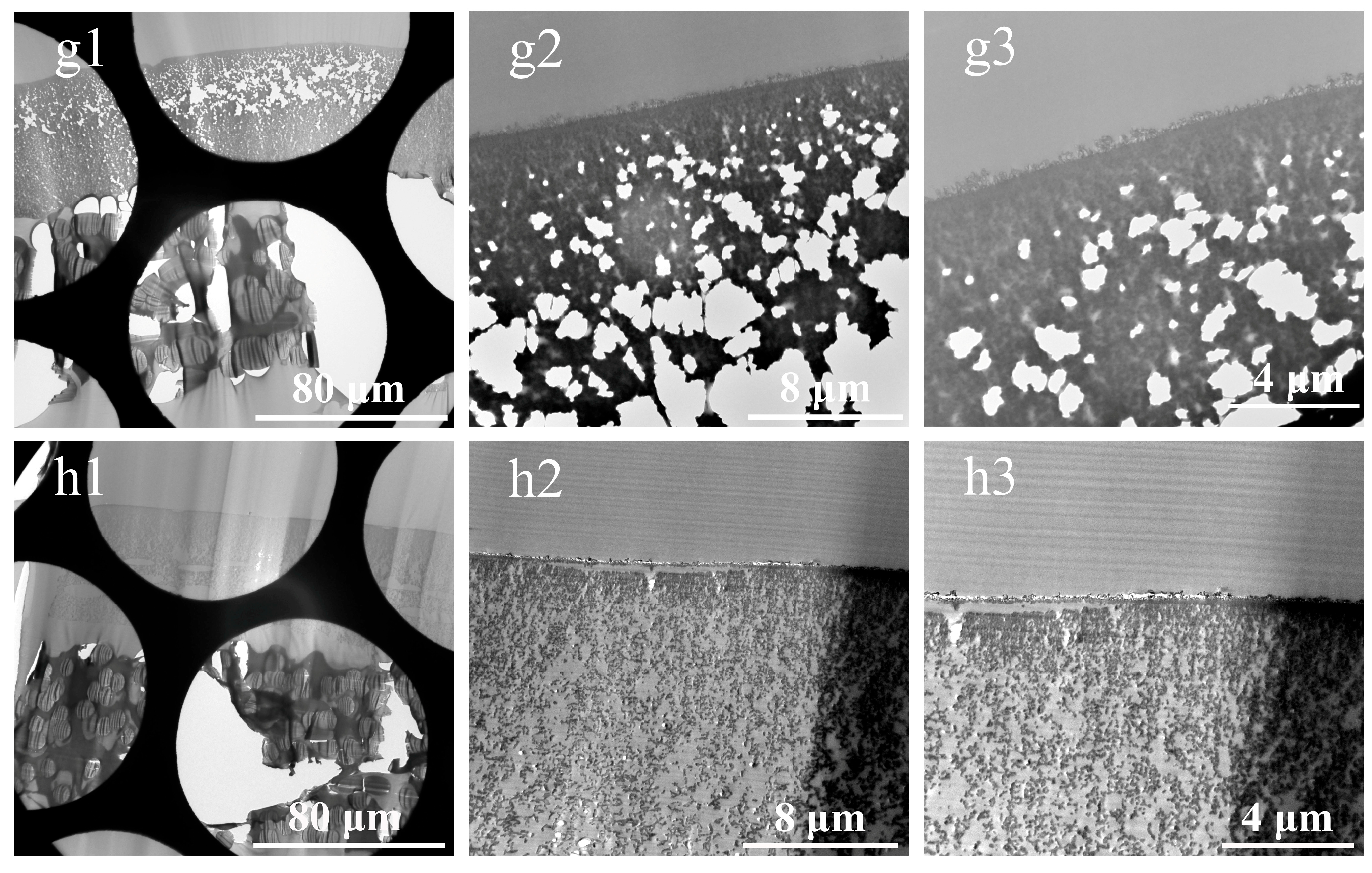
3.1. Cross-Sections of Membranes Prepared by Direct Resin-Embedding Method
- (i.)
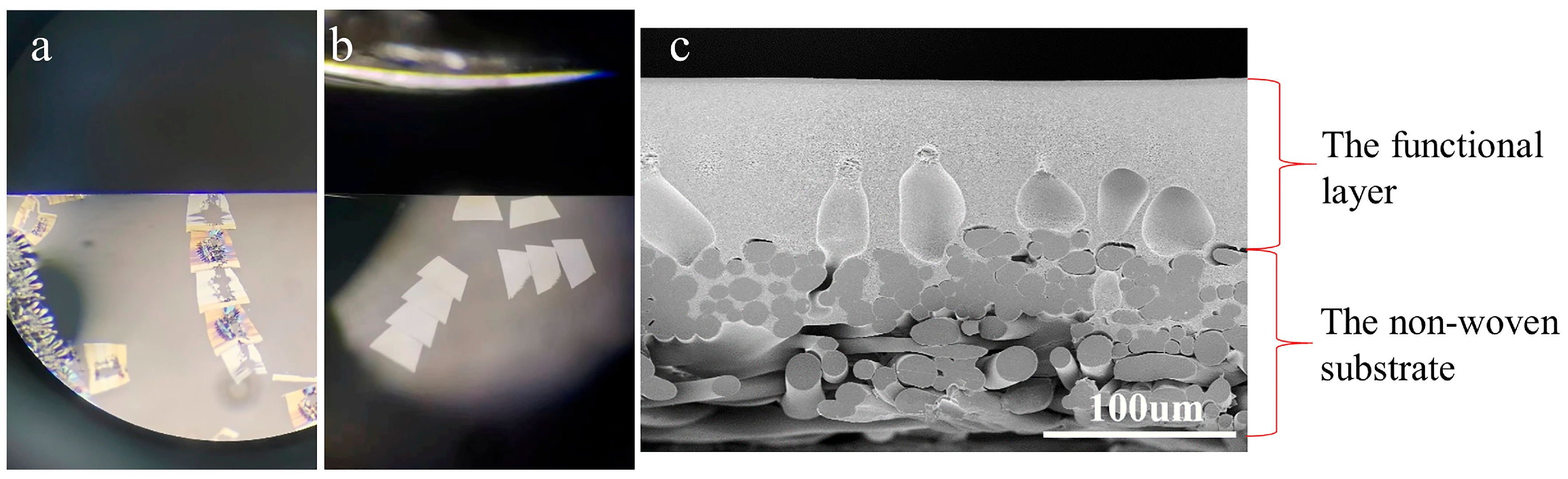
- Severe damage to the sections. This issue stems from the fact that the non-woven layer, composed of compressed PE/PET fibers with random orientations, possesses high density and negligible internal porosity, severely hindering resin penetration. The resulting embedding regions exhibited non-uniform properties, showing significant differences in hardness, toughness, and density. Consequently, the sections were prone to fragmentation, rendering the preparation of intact sections challenging. Even in partially preserved regions, localized deformation of the membrane functional layer was observed, as illustrated in Figure 5 (d1,g1).
- (ii.)
- Non-uniform thickness of functional layers. Owing to the thickness–mass contrast principle in TEM, when an electron beam passes through a sample, a fraction of the electrons undergo scattering [32]. The sample thickness exhibits a positive correlation with the number of scattered electrons, which in turn influences the brightness (grayscale) of the TEM image. This grayscale variation can be used to intuitively assess local variations in sample thickness. Therefore, in our study, the uniformity of the membrane was evaluated by analyzing the grayscale distribution in cross-sectional TEM images. As shown in Figure 5(a1–c1,h1), alternating dark and bright bands parallel to the knife edge are visible on the sections, corresponding to regions of greater and lesser thickness, respectively. This phenomenon was attributed to the vibration between the knife and the sample, induced by the poor cutting performance of embedding blocks resulting from the density and hardness of the non-woven substrates.
- (iii.)
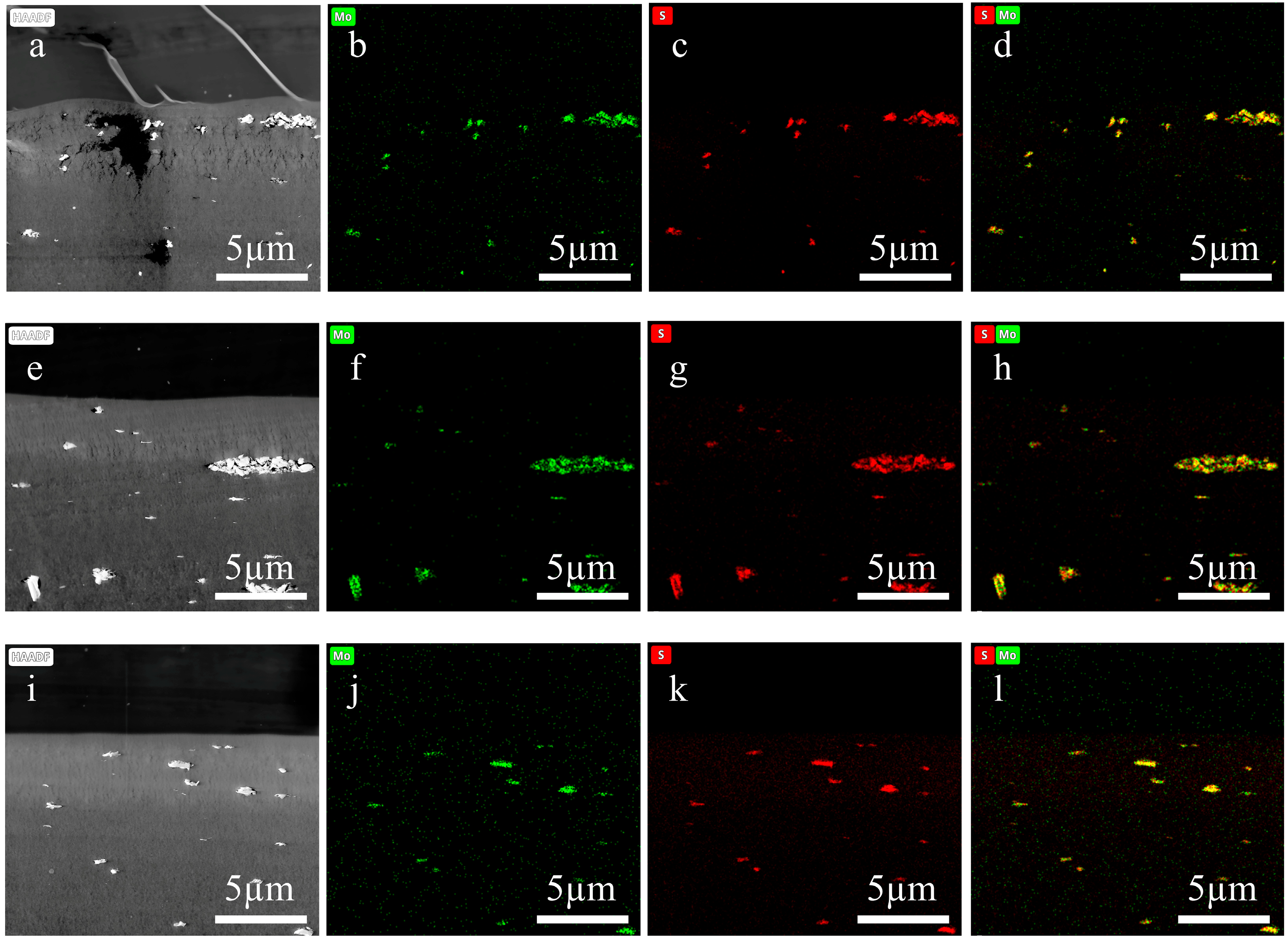
- Presence of voids within functional layers, as evidenced in Figure 5(a1–a3,c1–h3). On the one hand, the presence of the fiber layers increased overall membrane thickness, exacerbating the difficulty of resin impregnation and causing voids due to insufficient penetration. On the other hand, uneven section thickness rendered excessively thin areas prone to void formation.
- (iv.)
- Pronounced knife marks in partial sections, as displayed in Figure 5(c2,c3,d2,d3,e2,e3,f2,f3). The red arrows indicate the knife marks. Besides the possible presence of hard particles within the samples, small notches on the knife edge occurred when sectioning harder or thicker regions, resulting in knife marks. These artifacts compromised the thickness uniformity of the sections and obscured membrane structures.
- (v.)
- Sample orientation deviation. During embedding using this method, the samples were directly immersed in resin without physical fixation. During subsequent processing, the sample positions were prone to displacement due to temperature variations and resin polymerization shrinkage. This misalignment led to deviations in sample orientation. Consequently, precise perpendicular alignment of the ultramicrotome knife relative to the membrane’s extension direction was unattainable. Therefore, the thickness of membrane layers determined by TEM deviated from the actual values.
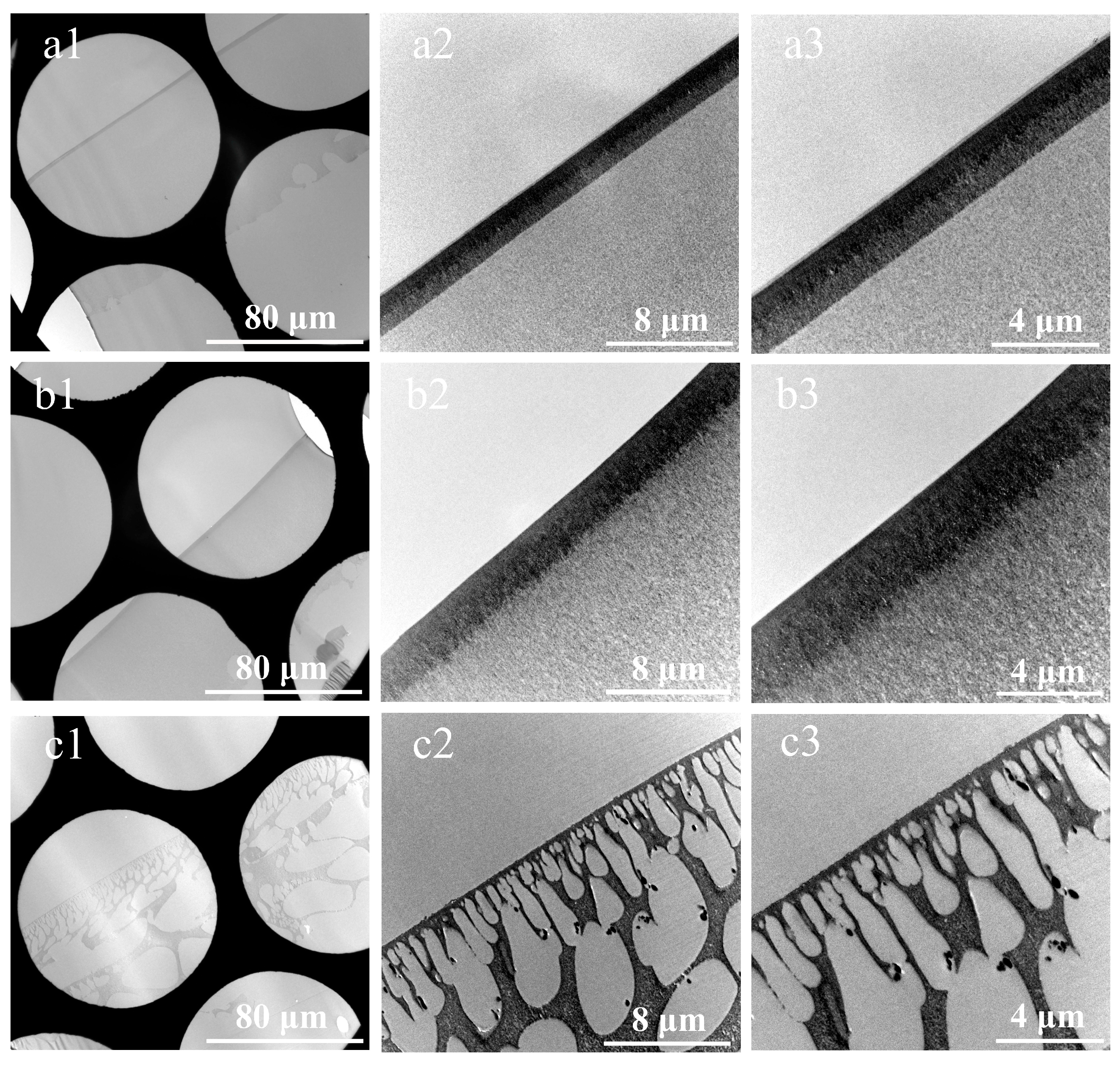
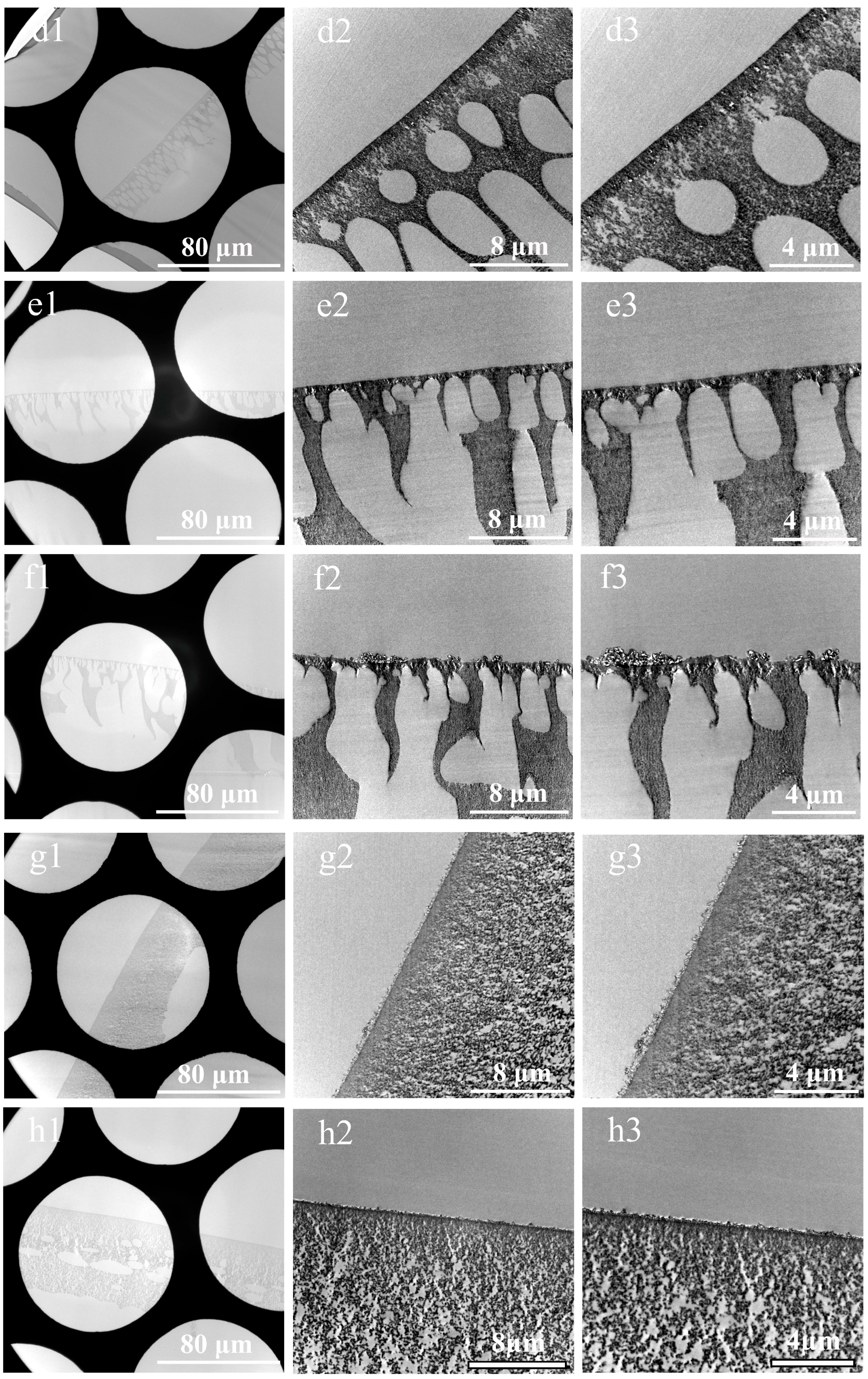
3.2. Cross-Sections of Membranes Prepared by Substrate Stripping and Double-Orientation Embedding Technique
3.3. Advantages and Disadvantages Comparison with Existing Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sidhikku Kandath Valappil, R.; Ghasem, N.; Al-Marzouqi, M. Current and future trends in polymer membrane-based gas separation technology: A comprehensive review. J. Ind. Eng. Chem. 2021, 98, 103–129. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, W.; Chou, S.; Dai, P.; Huang, X. Patterned membranes for improving hydrodynamic properties and mitigating membrane fouling in water treatment: A review. Water Res. 2023, 236, 119943. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Vatanpour, V.; Khoshnam, M.; Zargar, M. Recent advancements in the application of new monomers and membrane modification techniques for the fabrication of thin film composite membranes: A review. React. Funct. Polym. 2021, 166, 105015. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mehejabin, F.; Momtahin, A.; Tasannum, N.; Faria, N.T.; Mofijur, M.; Hoang, A.T.; Vo, D.N.; Mahlia, T.M.I. Strategies to improve membrane performance in wastewater treatment. Chemosphere 2022, 306, 135527. [Google Scholar] [CrossRef]
- Zahid, M.; Rashid, A.; Akram, S.; Rehan, Z.A.; Razzaq, W. A comprehensive review on polymeric nano-composite membranes for water treatment. J. Membr. Sci. Technol. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Bera, S.P.; Godhaniya, M.; Kothari, C. Emerging and advanced membrane technology for wastewater treatment: A review. J. Basic Microbiol. 2021, 62, 245–259. [Google Scholar] [CrossRef]
- Divya, S.; Oh, T.H. Polymer nanocomposite membrane for wastewater treatment: A critical review. Polymers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Kurihara, M.; Wang, R. Seawater desalination by reverse osmosis: Current development and future challenges in membrane fabrication—A review. J. Membr. Sci. 2021, 629, 119292. [Google Scholar] [CrossRef]
- Li, D.; Yan, Y.; Wang, H. Recent advances in polymer and polymer composite membranes for reverse and forward osmosis processes. Prog. Polym. Sci. 2016, 61, 104–155. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Impact of support layer pore size on performance of thin film composite membranes for forward osmosis. J. Membr. Sci. 2015, 483, 25–33. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef]
- Saleh, E.A.M.; Kumar, A.; Alghazali, T.; Ganesan, S.; Shankhyan, A.; Sharma, G.C.; Naidu, K.S.; Rahbari-Sisakht, M. Recent advances in thin film composite (TFC) membranes development: Materials and modification methods. Environ. Sci. Water Res. Technol. 2025, 11, 1059–1085. [Google Scholar] [CrossRef]
- Rahbari-Sisakht, M.; Mousavian, S.; Ariana, M.A.; Ismail, A.F. Thin-film nanocomposite membranes for water treatment: Evolution, applications, challenges, and advancement strategies. J. Environ. Chem. Eng. 2025, 13, 116392. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Aktij, S.A.; Dabaghian, Z.; Firouzjaei, M.D.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Abolhassani, M.; Greenlee, L.F.; Esfahani, A.R.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Membrane Modification. In Nanotechnology in Membrane Processes; Zhiming, M., Salamo, G., Bellucci, S., Eds.; Springer: New York, NY, USA, 2021; pp. 135–170. [Google Scholar]
- Guo, Q. Polymer Morphology: Principles, Characterization, and Processing; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 37–53. [Google Scholar]
- Khulbe, K.C.; Matsuura, T. Synthetic membrane characterization—A review: Part I. Membr. Technol. 2017, 2017, 7–12. [Google Scholar]
- Khulbe, K.C.; Matsuura, T. Membrane Characterization. In Nanotechnology in Membrane Processes; Zhiming, M., Salamo, G., Bellucci, S., Eds.; Springer: New York, NY, USA, 2021; pp. 88–134. [Google Scholar]
- Xue, Q.; Meng, W.; Fang, Q.; Zhu, J.; Zhang, K. Water Nanochannels in MXene Polyamide Nanofiltration Membranes: Implications for Permeability. Acs Appl. Nano Mater. 2023, 6, 11282–11290. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Xue, W.; Zhao, G.; Ding, W.; Zhang, K.; Wang, S.; Li, Y. Effect of carbon nanotube nanochannel on the separation performance of thin-film nanocomposite (TFN) membranes. Desalination 2023, 546, 116216. [Google Scholar] [CrossRef]
- Kuei, B.; Aplan, M.P.; Litofsky, J.H.; Gomez, E.D. New opportunities in transmission electron microscopy of polymers. Mater. Sci. Eng. R Rep. 2020, 139, 100516. [Google Scholar] [CrossRef]
- Liu, C.; Shi, L.; Zhang, J.; Du, Z.; Ding, L.; Han, Q.; Song, Z.; Lu, X.; Wu, C. Nanofiltration membranes with properly enhanced pore size and hydrophilicity by sulfobutyl ether β-cyclodextrin co-monomer for efficient antibiotic desalination. Sep. Purif. Technol. 2025, 377, 134417. [Google Scholar] [CrossRef]
- Xue, Q.; Meng, W.; Zhu, J.; Fang, Q.; Zhang, K. Constructing carboxylated MXene interlayer in polyamide nanofiltration membrane for enhancing perm-selectivity and antifouling performance. J. Membr. Sci. 2023, 683, 121860. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, K.; Li, F.; Wei, B.; Peng, J.; Yuan, B.; Li, P.; Hou, Y.; Sun, H.; Xia, D.; et al. Tailored dendrimer interlayer based on Marangoni convection for high-performance reverse osmosis membranes. J. Membr. Sci. 2024, 695, 122455. [Google Scholar] [CrossRef]
- Yin, J.; Yang, Y.; Hu, Z.; Deng, B. Attachment of silver nanoparticles (AgNPs) onto thin-film composite (TFC) membranes through covalent bonding to reduce membrane biofouling. J. Membr. Sci. 2013, 441, 73–82. [Google Scholar] [CrossRef]
- Li, J.-B.; Zhu, C.-Y.; Guo, B.-B.; Liu, C.; Xin, J.-H.; Zhang, C.; Wu, J.; Zhang, L.; Yang, H.-C.; Xu, Z.-K. Ultrahigh-permeance polyamide thin-film composite membranes enabled by interfacial polymerization on a macro-porous substrate toward organic solvent nanofiltration. J. Membr. Sci. 2024, 693, 122342. [Google Scholar] [CrossRef]
- Michler, G.H. Electron Microscopy of Polymers; Springer Laboratory Manuals in Polymer Science: New York, NY, USA, 2008; pp. 199–217. [Google Scholar]
- Tang, C.Y.; Yang, Z. Transmission electron microscopy (TEM). In Membrane Characterization; Hilal, N., Ismail, A.F., Matsuura, T., Oatley-Radcliffe, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 145–159. [Google Scholar]
- Zhou, Z.Y.; Wang, Q.; Qin, Y.W.; Hu, Y.X. Internal Concentration Polarization in the Polyamide Active Layer of Thin-Film Composite Membranes. Environ. Sci. Technol. 2023, 57, 5999–6007. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Pores in Membrane. In Nanotechnology in Membrane Processes; Zhiming, M., Salamo, G., Bellucci, S., Eds.; Springer: New York, NY, USA, 2021; pp. 20–21. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications; Wiley Online Library: Hoboken, NJ, USA, 2023; pp. 1–13. [Google Scholar]
- Wen, J.G. Transmission Electron Microscopy. In Practical Materials Characterization; Sardela, M., Ed.; Springer: New York, NY, USA, 2014; pp. 189–230. [Google Scholar]
- Michler, G.H. Compact Introduction to Electron Microscopy: Techniques, State, Applications, Perspectives; Springer: New York, NY, USA, 2023; pp. 37–41. [Google Scholar]
- Castro-Muñoz, R.; González-Melgoza, L.L.; García-Depraect, O. Ongoing progress on novel nanocomposite membranes for the separation of heavy metals from contaminated water. Chemosphere 2021, 270, 129421. [Google Scholar] [CrossRef]
- Yu, Q.; Zhou, Y.; Gao, C. UiO-66 regulated thin-film nanocomposite membranes for water treatment. Desalination 2024, 587, 117917. [Google Scholar] [CrossRef]
- Tayel, A.; Abdelaal, A.B.; Esawi, A.M.K.; Ramadan, A.R. Thin-Film Nanocomposite (TFN) Membranes for Water Treatment Applications: Characterization and Performance. Membranes 2023, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, Y.; Zheng, J.; Wang, X.; Xia, S.; Van der Bruggen, B. A critical review on thin-film nanocomposite membranes enabled by nanomaterials incorporated in different positions and with diverse dimensions: Performance comparison and mechanisms. J. Membr. Sci. 2022, 661, 120952. [Google Scholar] [CrossRef]
- Franco-Clavijo, N.; Cespedes, S.; Farinha, A.; Witkamp, G.J.; Picioreanu, C.; Vrouwenvelder, J.S.; Blankert, B. Optical coherence tomography for early detection, visualization and characterization of growth and deposition-driven scaling in reverse osmosis. Sep. Purif. Technol. 2025, 378, 134664. [Google Scholar] [CrossRef]
- Goi, Y.K.; Liang, Y.Y. A general modeling framework for FO spiral-wound membrane and its fouling impact on FO-RO desalination system. Desalination 2025, 593, 118236. [Google Scholar] [CrossRef]
- Chen, L.; Qiu, G. One simple physical embedding technique for the polymer film to be cryoultramicrotomed. J. Microsc. Ultrastruct. 2014, 2, 117–120. [Google Scholar] [CrossRef]
| Method | Section Integrity | Section Deformation | Thickness Uniformity | Membrane Orientation | Layer Delamination |
|---|---|---|---|---|---|
| Direct resin-embedding method | Low | Present | Poor | Imprecise | Low propensity |
| Cryoultramicrotomy | Low | Present | Poor | Precise | High propensity |
| Method proposed in this study | High | Absent | Good | Precise | Low propensity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Zhang, Z.; Li, Y.; Liu, S.; Zhang, X. A New Method for Preparing Cross-Sections of Polymer Composite Membranes for TEM Characterization by Substrate Stripping and Double-Orientation Embedding. Membranes 2025, 15, 288. https://doi.org/10.3390/membranes15100288
Ren H, Zhang Z, Li Y, Liu S, Zhang X. A New Method for Preparing Cross-Sections of Polymer Composite Membranes for TEM Characterization by Substrate Stripping and Double-Orientation Embedding. Membranes. 2025; 15(10):288. https://doi.org/10.3390/membranes15100288
Chicago/Turabian StyleRen, Hongyun, Zixing Zhang, Yi Li, Shulan Liu, and Xian Zhang. 2025. "A New Method for Preparing Cross-Sections of Polymer Composite Membranes for TEM Characterization by Substrate Stripping and Double-Orientation Embedding" Membranes 15, no. 10: 288. https://doi.org/10.3390/membranes15100288
APA StyleRen, H., Zhang, Z., Li, Y., Liu, S., & Zhang, X. (2025). A New Method for Preparing Cross-Sections of Polymer Composite Membranes for TEM Characterization by Substrate Stripping and Double-Orientation Embedding. Membranes, 15(10), 288. https://doi.org/10.3390/membranes15100288







