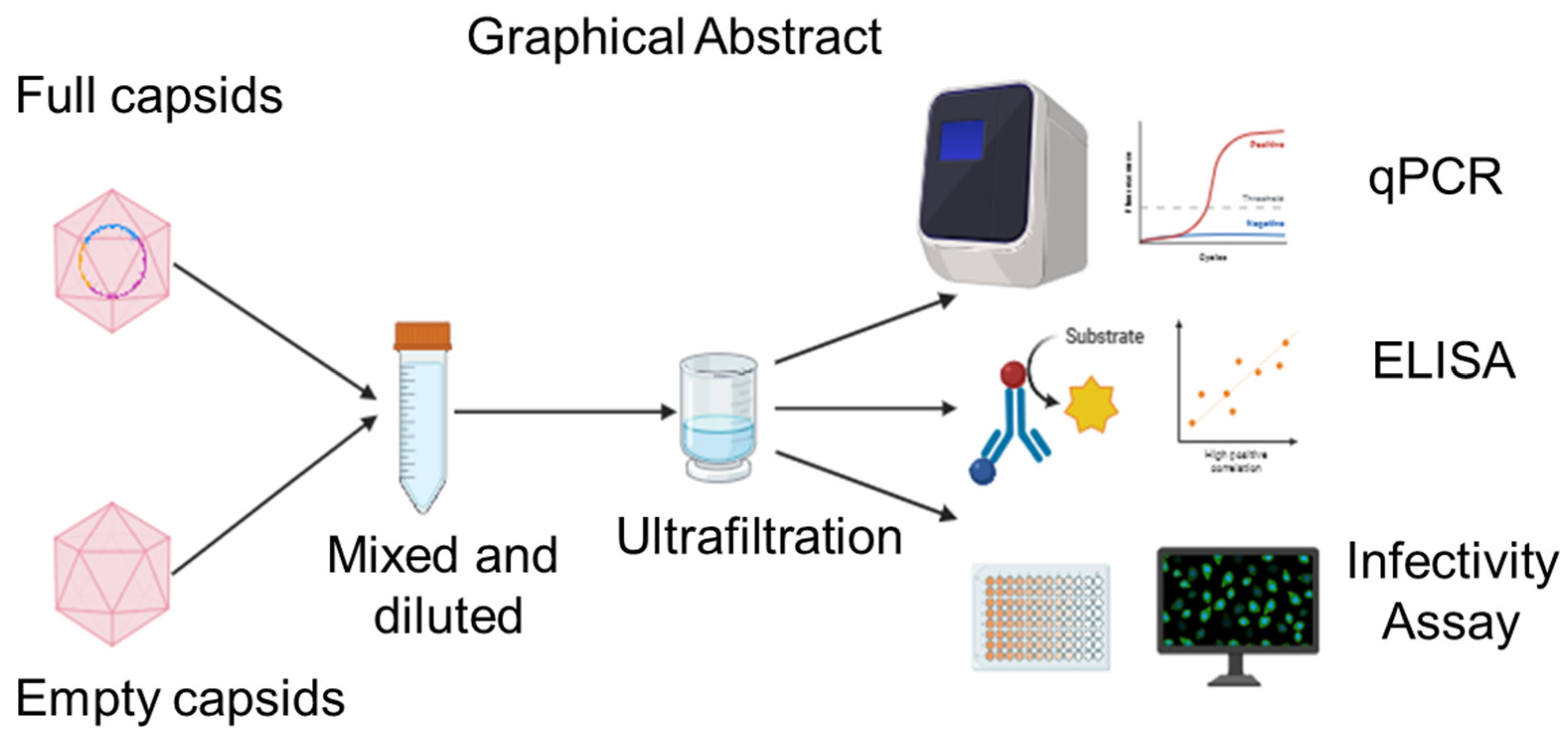
A Novel Method for Separating Full and Empty Adeno-Associated Viral Capsids Using Ultrafiltration
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Source and Storage
2.2. Selection of Buffer
2.3. Determination of Assays
2.3.1. ELISA
2.3.2. qPCR
2.3.3. Infectivity Assay
2.3.4. TEM
2.4. Dilution to Desired Ratio and Concentration
2.5. Ultrafiltration
3. Results
3.1. AAV2 Capsids Are Fully Retained by the 25 nm MCE Membrane and Pass Freely through 50 nm and 80 nm PCTE Membranes
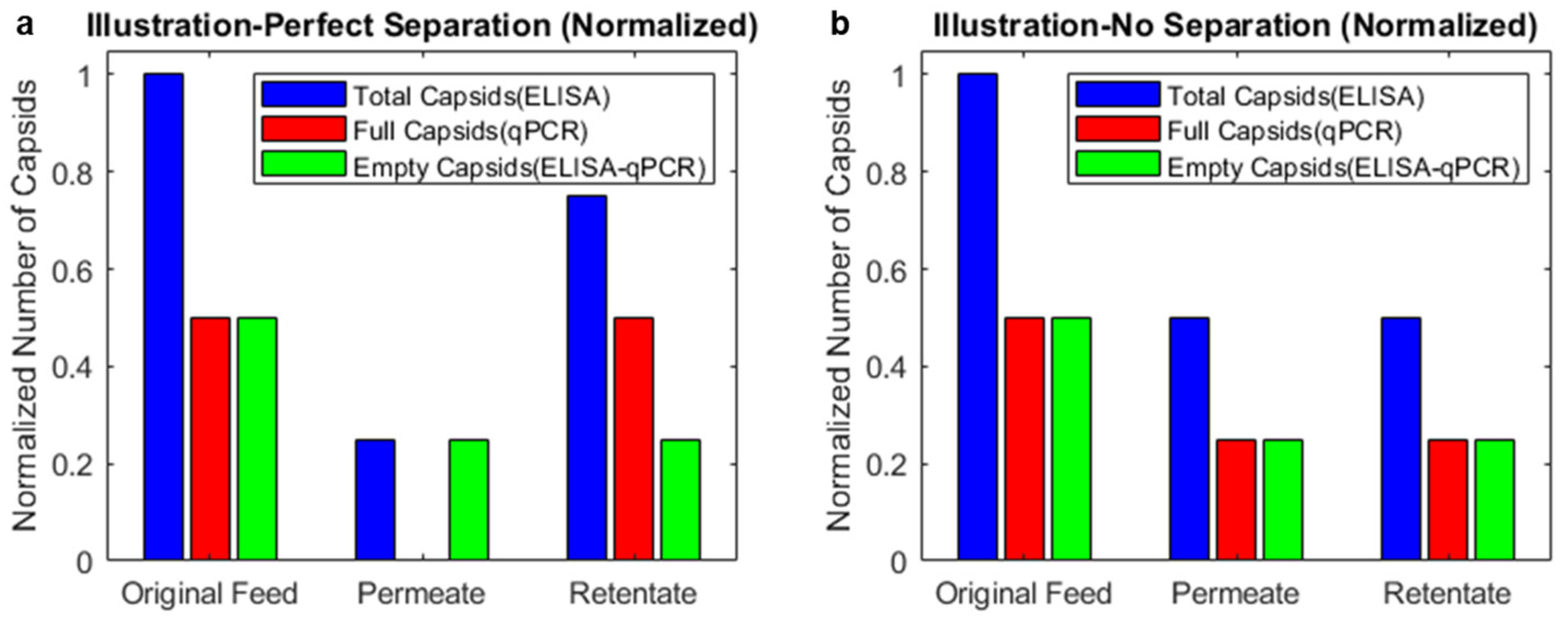
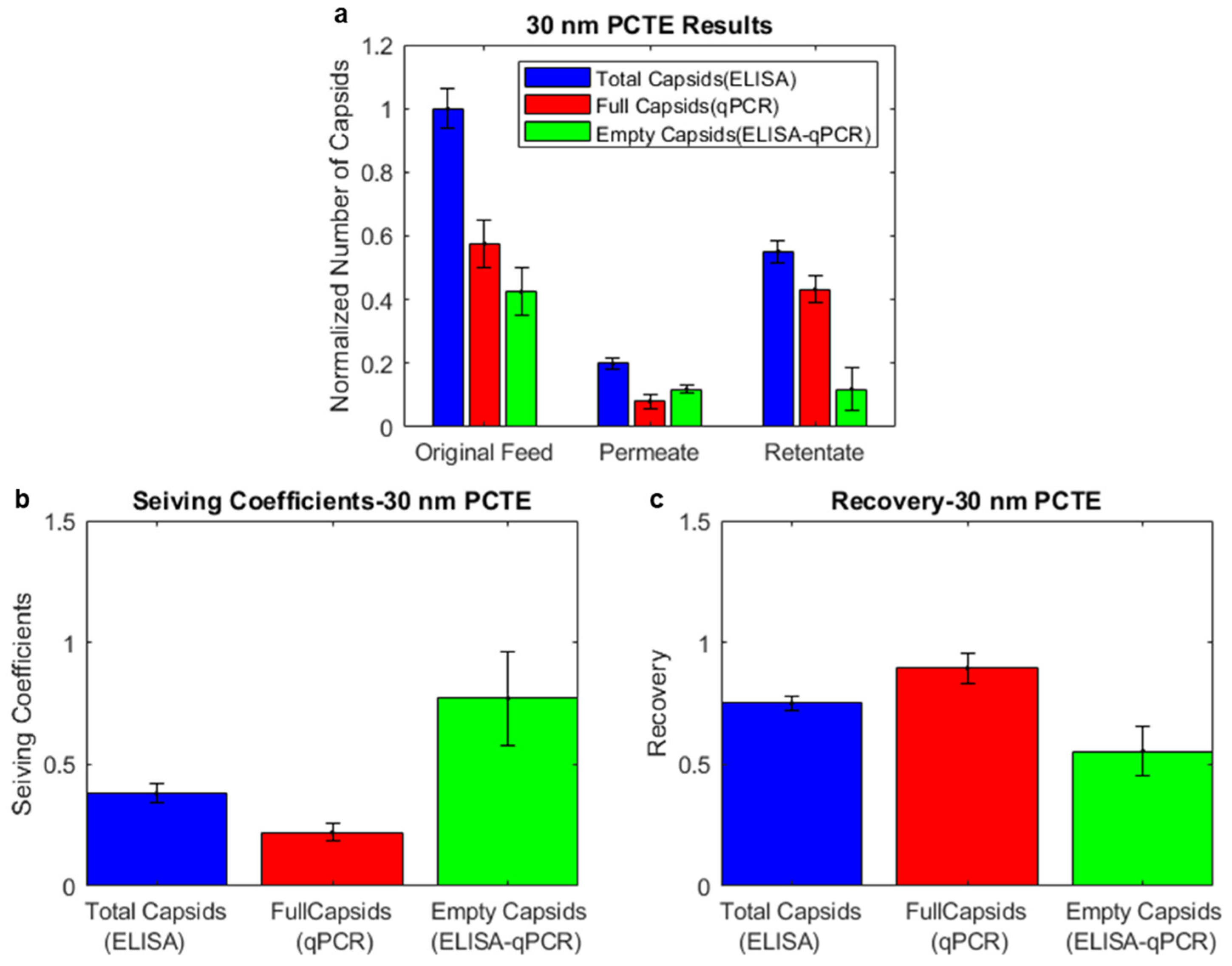
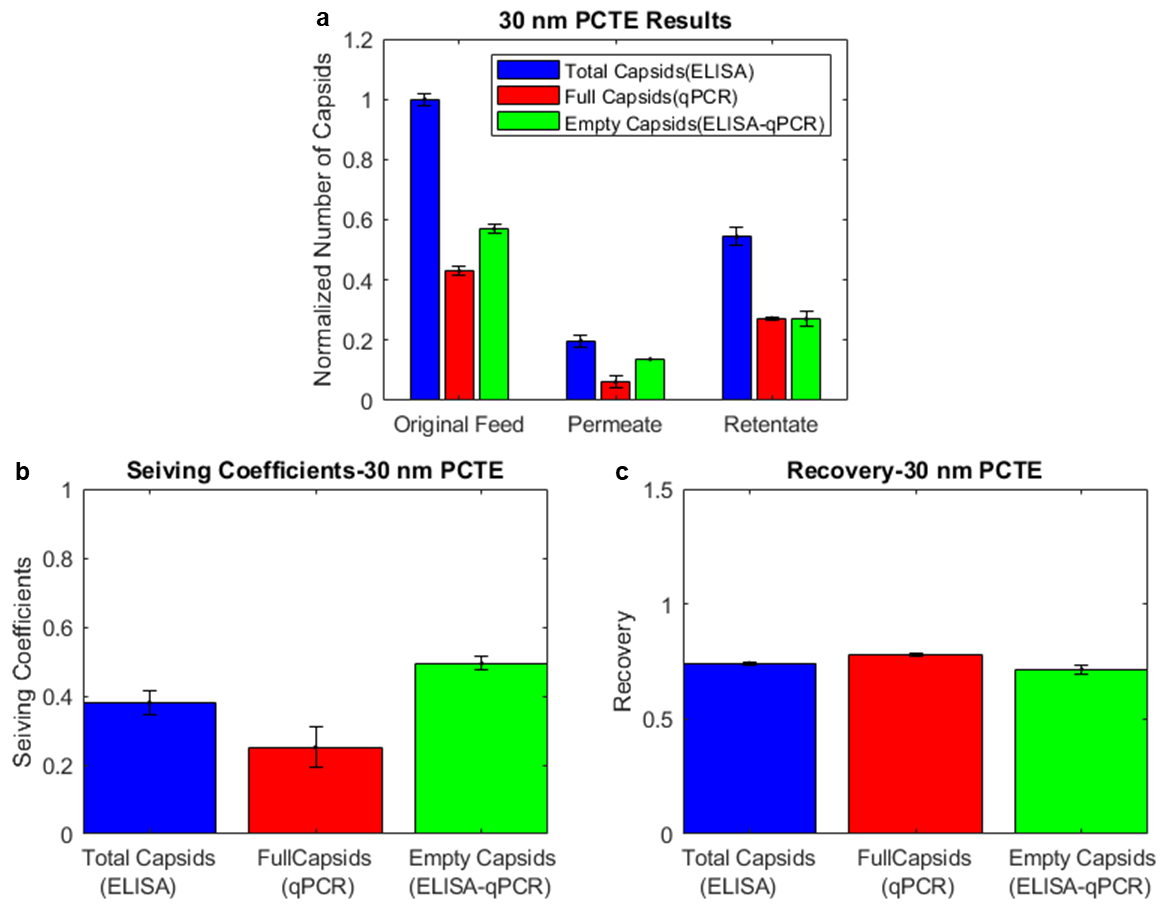
3.2. Combining ELISA and qPCR Results Indicates the Enrichment of Empty Capsids in the Permeate and Full Capsids in the Retentate for 30 nm PCTE Membranes
3.3. Combining ELISA and Infectivity Assays also Indicates Enrichment of Empty Capsids in the Permeate and Full Capsids in the Retentate for 30 nm PCTE Membranes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-Associated Virus Vector as a Platform for Gene Therapy Delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, D.A.; Shutova, M.V.; Johnston, N.R.; Smith, O.P.; Fedorin, V.V.; Kukushkin, Y.S.; van der Loo, J.C.M.; Johnstone, E.C. The Clinical Landscape for AAV Gene Therapies. Nat. Rev. Drug Discov. 2021, 20, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, D.; Barrett, D.; Nguyen-Jatkoe, L.; Millington, S.; Eckhardt, F. The State of Cell and Gene Therapy in 2023. Mol. Ther. 2023, 31, 3376–3388. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hussain, R.M.; Weng, C.Y. Voretigene Neparvovec in Retinal Diseases: A Review of the Current Clinical Evidence. Clin. Ophthalmol. 2020, 14, 3855–3869. [Google Scholar] [CrossRef] [PubMed]
- Ameri, H. Prospect of Retinal Gene Therapy Following Commercialization of Voretigene Neparvovec-Rzyl for Retinal Dystrophy Mediated by RPE65 Mutation. J. Curr. Ophthalmol. 2018, 30, 1–2. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and Safety of Voretigene Neparvovec (AAV2-hRPE65v2) in Patients with RPE65-Mediated Inherited Retinal Dystrophy: A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Ogbonmide, T.; Rathore, R.; Rangrej, S.B.; Hutchinson, S.; Lewis, M.; Ojilere, S.; Carvalho, V.; Kelly, I. Gene Therapy for Spinal Muscular Atrophy (SMA): A Review of Current Challenges and Safety Considerations for Onasemnogene Abeparvovec (Zolgensma). Cureus 2023, 15, e36197. [Google Scholar] [CrossRef]
- Tice, J.A.; Walton, S.M.; Sarker, J.; Moradi, A.; Chu, J.N.; Herce-Hagiwara, B.; Fahim, S.M.; Agboola, F.; Rind, D.; Pearson, S.D. The Effectiveness and Value of Gene Therapy for Hemophilia: A Summary from the Institute for Clinical and Economic Review’s California Technology Assessment Forum. J. Manag. Care Spec. Pharm. 2023, 29, 576–581. [Google Scholar] [CrossRef]
- Ozelo, M.C.; Mahlangu, J.; Pasi, K.J.; Giermasz, A.; Leavitt, A.D.; Laffan, M.; Symington, E.; Quon, D.V.; Wang, J.-D.; Peerlinck, K.; et al. Valoctocogene Roxaparvovec Gene Therapy for Hemophilia A. N. Engl. J. Med. 2022, 386, 1013–1025. [Google Scholar] [CrossRef]
- Manini, A.; Abati, E.; Nuredini, A.; Corti, S.; Comi, G.P. Adeno-Associated Virus (AAV)-Mediated Gene Therapy for Duchenne Muscular Dystrophy: The Issue of Transgene Persistence. Front. Neurol. 2022, 12, 814174. [Google Scholar] [CrossRef]
- Hoy, S.M. Delandistrogene Moxeparvovec: First Approval. Drugs 2023, 83, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Polanco, A.; Lee, Y.S.; Yoon, S. Critical Challenges and Advances in Recombinant Adeno-Associated Virus (rAAV) Biomanufacturing. Biotechnol. Bioeng. 2023, 120, 2601–2621. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Mallela, K.M.G.; Deorkar, N.; Brophy, G. Manufacturing Challenges and Rational Formulation Development for AAV Viral Vectors. J. Pharm. Sci. 2021, 110, 2609–2624. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Maloney, A.J.; Katsikis, G.; Nguyen, T.N.T.; Neufeld, C.; Wolfrum, J.; Barone, P.W.; Springs, S.L.; Manalis, S.R.; Sinskey, A.J.; et al. Cellular Pathways of Recombinant Adeno-Associated Virus Production for Gene Therapy. Biotechnol. Adv. 2021, 49, 107764. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.F.; Fraefel, C.; Seyffert, M. The Interplay between Adeno-Associated Virus and Its Helper Viruses. Viruses 2020, 12, 662. [Google Scholar] [CrossRef]
- Schnödt, M.; Büning, H. Improving the Quality of Adeno-Associated Viral Vector Preparations: The Challenge of Product-Related Impurities. Hum. Gene Ther. Methods 2017, 28, 101–108. [Google Scholar] [CrossRef]
- Grieger, J.C.; Soltys, S.M.; Samulski, R.J. Production of Recombinant Adeno-Associated Virus Vectors Using Suspension HEK293 Cells and Continuous Harvest of Vector From the Culture Media for GMP FIX and FLT1 Clinical Vector. Mol. Ther. 2016, 24, 287–297. [Google Scholar] [CrossRef]
- Grimm, D.; Kern, A.; Pawlita, M.; Ferrari, F.K.; Samulski, R.J.; Kleinschmidt, J.A. Titration of AAV-2 Particles via a Novel Capsid ELISA: Packaging of Genomes Can Limit Production of Recombinant AAV-2. Gene Ther. 1999, 6, 1322–1330. [Google Scholar] [CrossRef]
- Gao, K.; Li, M.; Zhong, L.; Su, Q.; Li, J.; Li, S.; He, R.; Zhang, Y.; Hendricks, G.; Wang, J.; et al. Empty Virions in AAV8 Vector Preparations Reduce Transduction Efficiency and May Cause Total Viral Particle Dose-Limiting Side Effects. Mol. Ther. Methods Clin. Dev. 2014, 1, 9. [Google Scholar] [CrossRef]
- Aebischer, M.K.; Gizardin-Fredon, H.; Lardeux, H.; Kochardt, D.; Elger, C.; Haindl, M.; Ruppert, R.; Guillarme, D.; D’Atri, V. Anion-Exchange Chromatography at the Service of Gene Therapy: Baseline Separation of Full/Empty Adeno-Associated Virus Capsids by Screening of Conditions and Step Gradient Elution Mode. Int. J. Mol. Sci. 2022, 23, 12332. [Google Scholar] [CrossRef]
- Urabe, M.; Xin, K.-Q.; Obara, Y.; Nakakura, T.; Mizukami, H.; Kume, A.; Okuda, K.; Ozawa, K. Removal of Empty Capsids from Type 1 Adeno-Associated Virus Vector Stocks by Anion-Exchange Chromatography Potentiates Transgene Expression. Mol. Ther. 2006, 13, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Hermens, W.T.J.M.C.; Brake, O.T.; Dijkhuizen, P.A.; Sonnemans, M.A.F.; Grimm, D.; Kleinschmidt, J.A.; Verhaagen, J. Purification of Recombinant Adeno-Associated Virus by Iodixanol Gradient Ultracentrifugation Allows Rapid and Reproducible Preparation of Vector Stocks for Gene Transfer in the Nervous System. Human. Gene Ther. 1999, 10, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.; Argento, C.; Pieracci, J.; Bakhshayeshi, M. Separating Empty and Full Recombinant Adeno-Associated Virus Particles Using Isocratic Anion Exchange Chromatography. Biotechnol. J. 2021, 16, e2000015. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, P.; Leskovec, M.; Prebil, S.D.; Žigon, R.; Štokelj, M.; Raspor, A.; Peljhan, S.; Štrancar, A. Removal of Empty Capsids from Adeno-Associated Virus Preparations by Multimodal Metal Affinity Chromatography. J. Chromatogr. A 2021, 1649, 462210. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Manufacturing of Adeno-Associated Viruses, for Example: AAV2. In Viral Vectors for Gene Therapy: Methods and Protocols; Merten, O.-W., Al-Rubeai, M., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; pp. 235–246. ISBN 978-1-61779-095-9. [Google Scholar]
- Mingozzi, F.; Anguela, X.M.; Pavani, G.; Chen, Y.; Davidson, R.J.; Hui, D.J.; Yazicioglu, M.; Elkouby, L.; Hinderer, C.J.; Faella, A.; et al. Overcoming Preexisting Humoral Immunity to AAV Using Capsid Decoys. Sci. Transl. Med. 2013, 5, 194ra92. [Google Scholar] [CrossRef]
- Hoffman, B.E.; Herzog, R.W. Covert Warfare Against the Immune System: Decoy Capsids, Stealth Genomes, and Suppressors. Mol. Ther. 2013, 21, 1648–1650. [Google Scholar] [CrossRef]
- Wright, J.F. AAV Empty Capsids: For Better or for Worse? Mol. Ther. 2014, 22, 1–2. [Google Scholar] [CrossRef]
- Gimpel, A.L.; Katsikis, G.; Sha, S.; Maloney, A.J.; Hong, M.S.; Nguyen, T.N.T.; Wolfrum, J.; Springs, S.L.; Sinskey, A.J.; Manalis, S.R.; et al. Analytical Methods for Process and Product Characterization of Recombinant Adeno-Associated Virus-Based Gene Therapies. Mol. Ther. Methods Clin. Dev. 2021, 20, 740–754. [Google Scholar] [CrossRef]
- Werle, A.K.; Powers, T.W.; Zobel, J.F.; Wappelhorst, C.N.; Jarrold, M.F.; Lyktey, N.A.; Sloan, C.D.K.; Wolf, A.J.; Adams-Hall, S.; Baldus, P.; et al. Comparison of Analytical Techniques to Quantitate the Capsid Content of Adeno-Associated Viral Vectors. Mol. Ther. Methods Clin. Dev. 2021, 23, 254–262. [Google Scholar] [CrossRef]
- Dobnik, D.; Kogovšek, P.; Jakomin, T.; Košir, N.; Tušek Žnidarič, M.; Leskovec, M.; Kaminsky, S.M.; Mostrom, J.; Lee, H.; Ravnikar, M. Accurate Quantification and Characterization of Adeno-Associated Viral Vectors. Front. Microbiol. 2019, 10, 1570. [Google Scholar] [CrossRef]
- Wang, Y.; Menon, N.; Shen, S.; Feschenko, M.; Bergelson, S. A qPCR Method for AAV Genome Titer with ddPCR-Level of Accuracy and Precision. Mol. Ther. Methods Clin. Dev. 2020, 19, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cooper, R.; Kiladjian, A.; Bergelson, S.; Feschenko, M. A Digestion-Free Method for Quantification of Residual Host Cell DNA in rAAV Gene Therapy Products. Mol. Ther. Methods Clin. Dev. 2019, 13, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Labisch, J.J.; Wiese, G.P.; Barnes, K.; Bollmann, F.; Pflanz, K. Infectious Titer Determination of Lentiviral Vectors Using a Temporal Immunological Real-Time Imaging Approach. PLoS ONE 2021, 16, e0254739. [Google Scholar] [CrossRef]
- Ye, M.; Keicher, M.; Gentschev, I.; Szalay, A.A. Efficient Selection of Recombinant Fluorescent Vaccinia Virus Strains and Rapid Virus Titer Determination by Using a Multi-Well Plate Imaging System. Biomedicines 2021, 9, 1032. [Google Scholar] [CrossRef]
- Meierrieks, F.; Kour, A.; Pätz, M.; Pflanz, K.; Wolff, M.W.; Pickl, A. Unveiling the Secrets of Adeno-Associated Virus: Novel High-Throughput Approaches for the Quantification of Multiple Serotypes. Mol. Ther. Methods Clin. Dev. 2023, 31, 101118. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, A.; Singh, N. Ultrafiltration Behavior of Recombinant Adeno Associated Viral Vectors Used in Gene Therapy. J. Membr. Sci. 2021, 620, 118812. [Google Scholar] [CrossRef]
- Singh, N.; Heldt, C.L. Challenges in Downstream Purification of Gene Therapy Viral Vectors. Curr. Opin. Chem. Eng. 2022, 35, 100780. [Google Scholar] [CrossRef]
- Hagen, S.; Baumann, T.; Wagner, H.J.; Morath, V.; Kaufmann, B.; Fischer, A.; Bergmann, S.; Schindler, P.; Arndt, K.M.; Müller, K.M. Modular Adeno-Associated Virus (rAAV) Vectors Used for Cellular Virus-Directed Enzyme Prodrug Therapy. Sci. Rep. 2014, 4, 3759. [Google Scholar] [CrossRef]
- Strobel, B.; Miller, F.D.; Rist, W.; Lamla, T. Comparative Analysis of Cesium Chloride- and Iodixanol-Based Purification of Recombinant Adeno-Associated Viral Vectors for Preclinical Applications. Human. Gene Ther. Methods 2015, 26, 147–157. [Google Scholar] [CrossRef]
- Zeng, C.; Moller-Tank, S.; Asokan, A.; Dragnea, B. Probing the Link among Genomic Cargo, Contact Mechanics, and Nanoindentation in Recombinant Adeno-Associated Virus 2. J. Phys. Chem. B 2017, 121, 1843–1853. [Google Scholar] [CrossRef]
- Zoratto, S.; Weiss, V.U.; Friedbacher, G.; Buengener, C.; Pletzenauer, R.; Foettinger-Vacha, A.; Graninger, M.; Allmaier, G. Adeno-Associated Virus Virus-like Particle Characterization via Orthogonal Methods: Nanoelectrospray Differential Mobility Analysis, Asymmetric Flow Field-Flow Fractionation, and Atomic Force Microscopy. ACS Omega 2021, 6, 16428–16437. [Google Scholar] [CrossRef]
- Karawdeniya, B.I.; Bandara, Y.M.N.D.Y.; Khan, A.I.; Chen, W.T.; Vu, H.-A.; Morshed, A.; Suh, J.; Dutta, P.; Kim, M.J. Adeno-Associated Virus Characterization for Cargo Discrimination through Nanopore Responsiveness. Nanoscale 2020, 12, 23721–23731. [Google Scholar] [CrossRef]
- Borujeni, E.E.; Zydney, A.L. Separation of Plasmid DNA Isoforms Using Centrifugal Ultrafiltration. BioTechniques 2012, 53, 49–56. [Google Scholar] [CrossRef]
- Latulippe, D.R.; Zydney, A.L. Separation of Plasmid DNA Isoforms by Highly Converging Flow through Small Membrane Pores. J. Colloid Interface Sci. 2011, 357, 548–553. [Google Scholar] [CrossRef]
- Li, Y.; Currie, D.; Zydney, A.L. Enhanced Purification of Plasmid DNA Isoforms by Exploiting Ionic Strength Effects during Ultrafiltration. Biotechnol. Bioeng. 2016, 113, 783–789. [Google Scholar] [CrossRef]
- VIROVEK | BAC-TO-AAV Technology For Large Scale AAV Production. Available online: https://www.virovek.com (accessed on 29 August 2024).
- Wright, J.F.; Le, T.; Prado, J.; Bahr-Davidson, J.; Smith, P.H.; Zhen, Z.; Sommer, J.M.; Pierce, G.F.; Qu, G. Identification of Factors That Contribute to Recombinant AAV2 Particle Aggregation and Methods to Prevent Its Occurrence during Vector Purification and Formulation. Mol. Ther. 2005, 12, 171–178. [Google Scholar] [CrossRef]
- Evans, R.K.; Nawrocki, D.K.; Isopi, L.A.; Williams, D.M.; Casimiro, D.R.; Chin, S.; Chen, M.; Zhu, D.-M.; Shiver, J.W.; Volkin, D.B. Development of Stable Liquid Formulations for Adenovirus-Based Vaccines. J. Pharm. Sci. 2004, 93, 2458–2475. [Google Scholar] [CrossRef]
- Qu, W.; Wang, M.; Wu, Y.; Xu, R. Scalable Downstream Strategies for Purification of Recombinant Adeno-Associated Virus Vectors in Light of the Properties. Curr. Pharm. Biotechnol. 2015, 16, 684–695. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image Analysis Software for Identifying and Quantifying Cell Phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A.; Goodman, A. CellProfiler 4: Improvements in Speed, Utility and Usability. BMC Bioinform. 2021, 22, 433. [Google Scholar] [CrossRef]
- Urabe, M.; Ding, C.; Kotin, R.M. Insect Cells as a Factory to Produce Adeno-Associated Virus Type 2 Vectors. Human. Gene Ther. 2002, 13, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Ebberink, E.H.T.M.; Ruisinger, A.; Nuebel, M.; Thomann, M.; Heck, A.J.R. Assessing Production Variability in Empty and Filled Adeno-Associated Viruses by Single Molecule Mass Analyses. Mol. Ther. Methods Clin. Dev. 2022, 27, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Uchida, N.; Posadas-Herrera, G.; Hayashita-Kinoh, H.; Tsunekawa, Y.; Hirai, Y.; Okada, T. Large-Scale Purification of Functional AAV Particles Packaging the Full Genome Using Short-Term Ultracentrifugation with a Zonal Rotor. Gene Ther. 2023, 30, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Marino, S.; Ho, C.Y. 97. Large Scale Purification of AAV with Continuous Flow Ultracentrifugation. Mol. Ther. 2016, 24, S42. [Google Scholar] [CrossRef]
- Lam, A.K.; Mulcrone, P.L.; Frabutt, D.; Zhang, J.; Chrzanowski, M.; Arisa, S.; Munoz, M.; Li, X.; Biswas, M.; Markusic, D.; et al. Comprehensive Comparison of AAV Purification Methods: Iodixanol Gradient Centrifugation vs. Immuno-Affinity Chromatography. Adv. Cell Gene Ther. 2023, 2023, 2339702. [Google Scholar] [CrossRef]
- Imiołek, M.; Fekete, S.; Kizekai, L.; Addepalli, B.; Lauber, M. Fast and Efficient Size Exclusion Chromatography of Adeno Associated Viral Vectors with 2.5 Micrometer Particle Low Adsorption Columns. J. Chromatogr. A 2024, 1714, 464587. [Google Scholar] [CrossRef]
- Fernandes, P.; Peixoto, C.; Santiago, V.M.; Kremer, E.J.; Coroadinha, A.S.; Alves, P.M. Bioprocess Development for Canine Adenovirus Type 2 Vectors. Gene Ther. 2013, 20, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Innthaler, B.; Lemmerer, M.; Pletzenauer, R.; Birner-Gruenberger, R. Biophysical Characterization of Adeno-Associated Virus Vectors Using Ion-Exchange Chromatography Coupled to Light Scattering Detectors. Int. J. Mol. Sci. 2022, 23, 12715. [Google Scholar] [CrossRef]
- McIntosh, N.L.; Berguig, G.Y.; Karim, O.A.; Cortesio, C.L.; De Angelis, R.; Khan, A.A.; Gold, D.; Maga, J.A.; Bhat, V.S. Comprehensive Characterization and Quantification of Adeno Associated Vectors by Size Exclusion Chromatography and Multi Angle Light Scattering. Sci. Rep. 2021, 11, 3012. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmah, D.; Husson, S.M. A Novel Method for Separating Full and Empty Adeno-Associated Viral Capsids Using Ultrafiltration. Membranes 2024, 14, 194. https://doi.org/10.3390/membranes14090194
Sarmah D, Husson SM. A Novel Method for Separating Full and Empty Adeno-Associated Viral Capsids Using Ultrafiltration. Membranes. 2024; 14(9):194. https://doi.org/10.3390/membranes14090194
Chicago/Turabian StyleSarmah, Deepraj, and Scott M. Husson. 2024. "A Novel Method for Separating Full and Empty Adeno-Associated Viral Capsids Using Ultrafiltration" Membranes 14, no. 9: 194. https://doi.org/10.3390/membranes14090194
APA StyleSarmah, D., & Husson, S. M. (2024). A Novel Method for Separating Full and Empty Adeno-Associated Viral Capsids Using Ultrafiltration. Membranes, 14(9), 194. https://doi.org/10.3390/membranes14090194







