Carbon-Doped TiO2 Nanofiltration Membranes Prepared by Interfacial Reaction of Glycerol with TiCl4 Vapor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of GLTO Membranes via Interfacial Reaction
2.3. Membrane Characterization
2.4. Measurements of Membrane Performances
2.5. Long-Term Stability and Solvent Resistance
3. Results
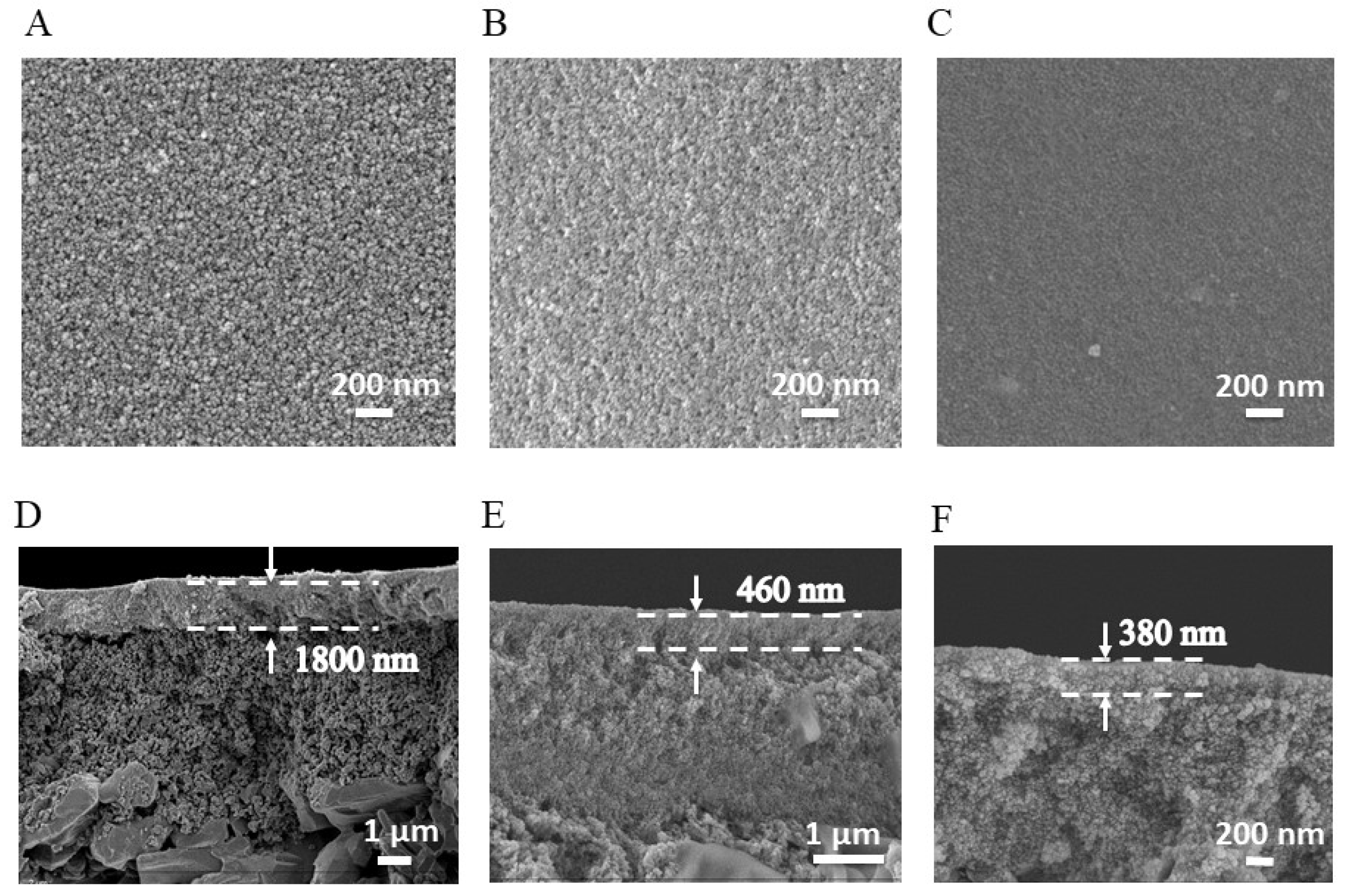
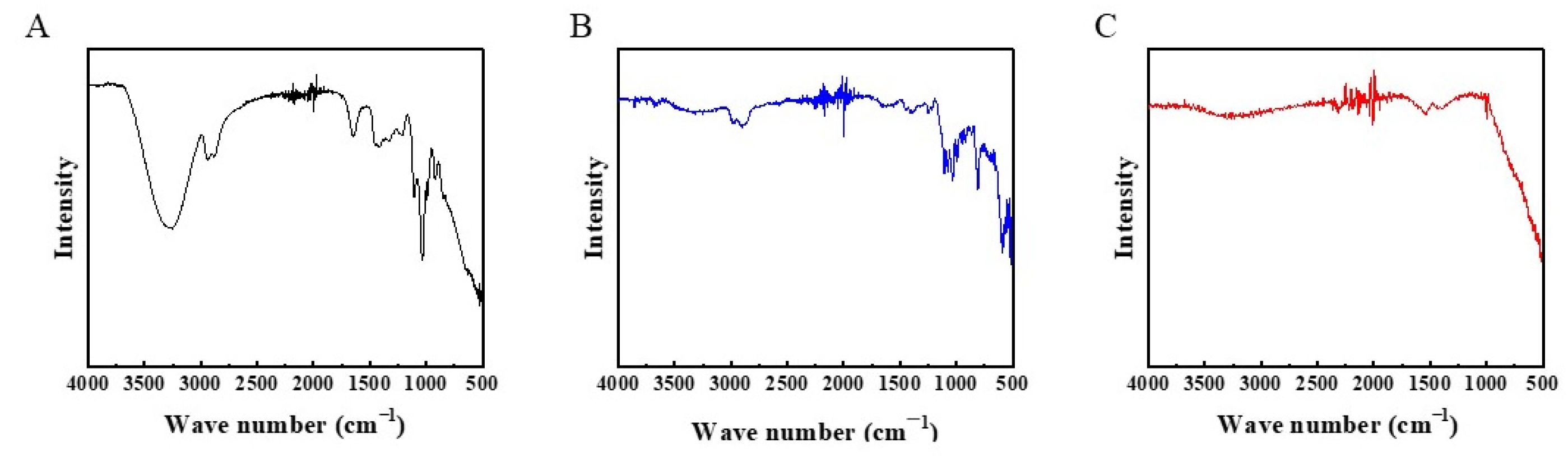
3.1. Characterizations of Membranes
3.2. Influence of Reaction Time and Organic Precursors on Membrane Performance
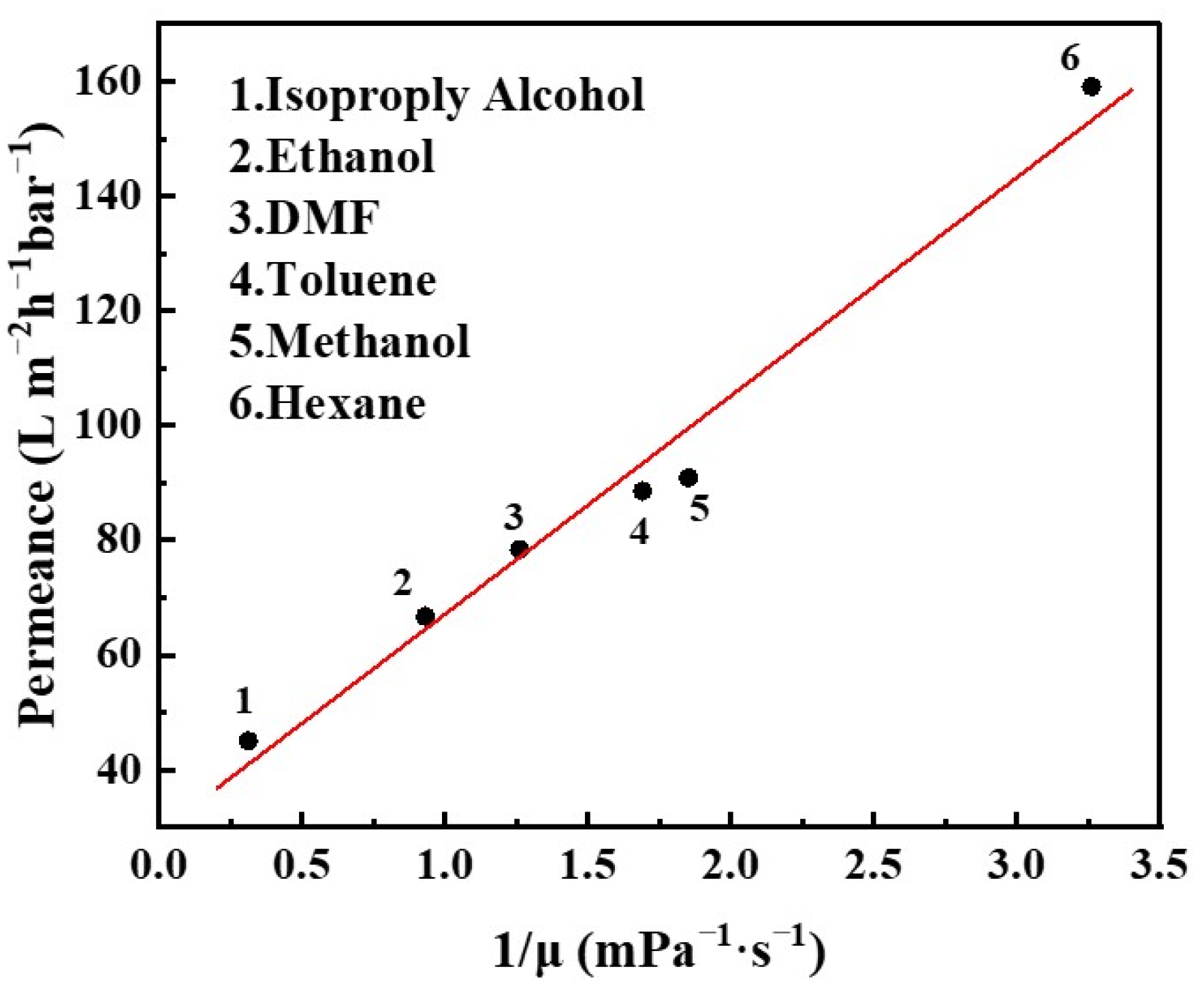
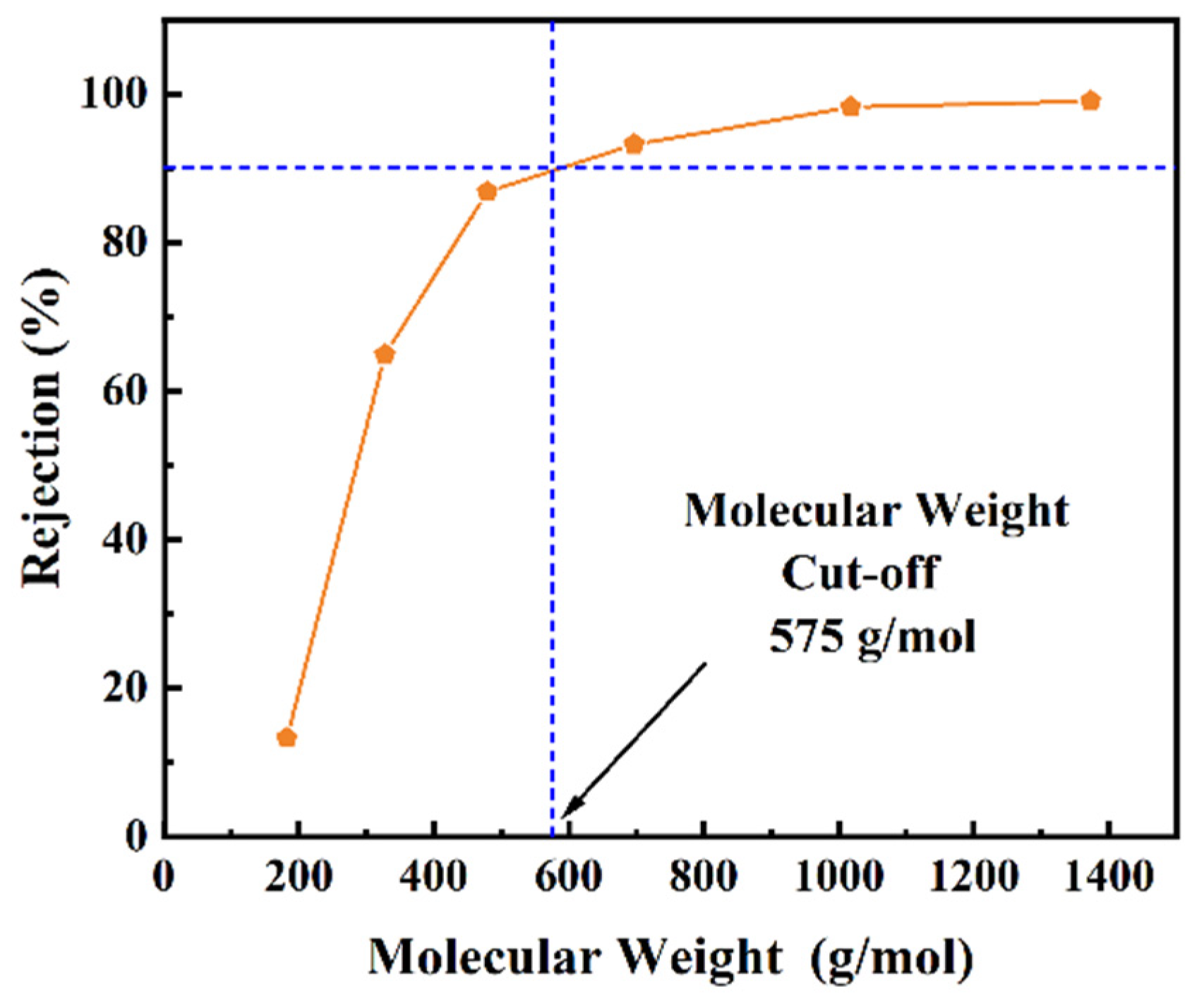
3.3. Separation Performances of GLTO Membranes
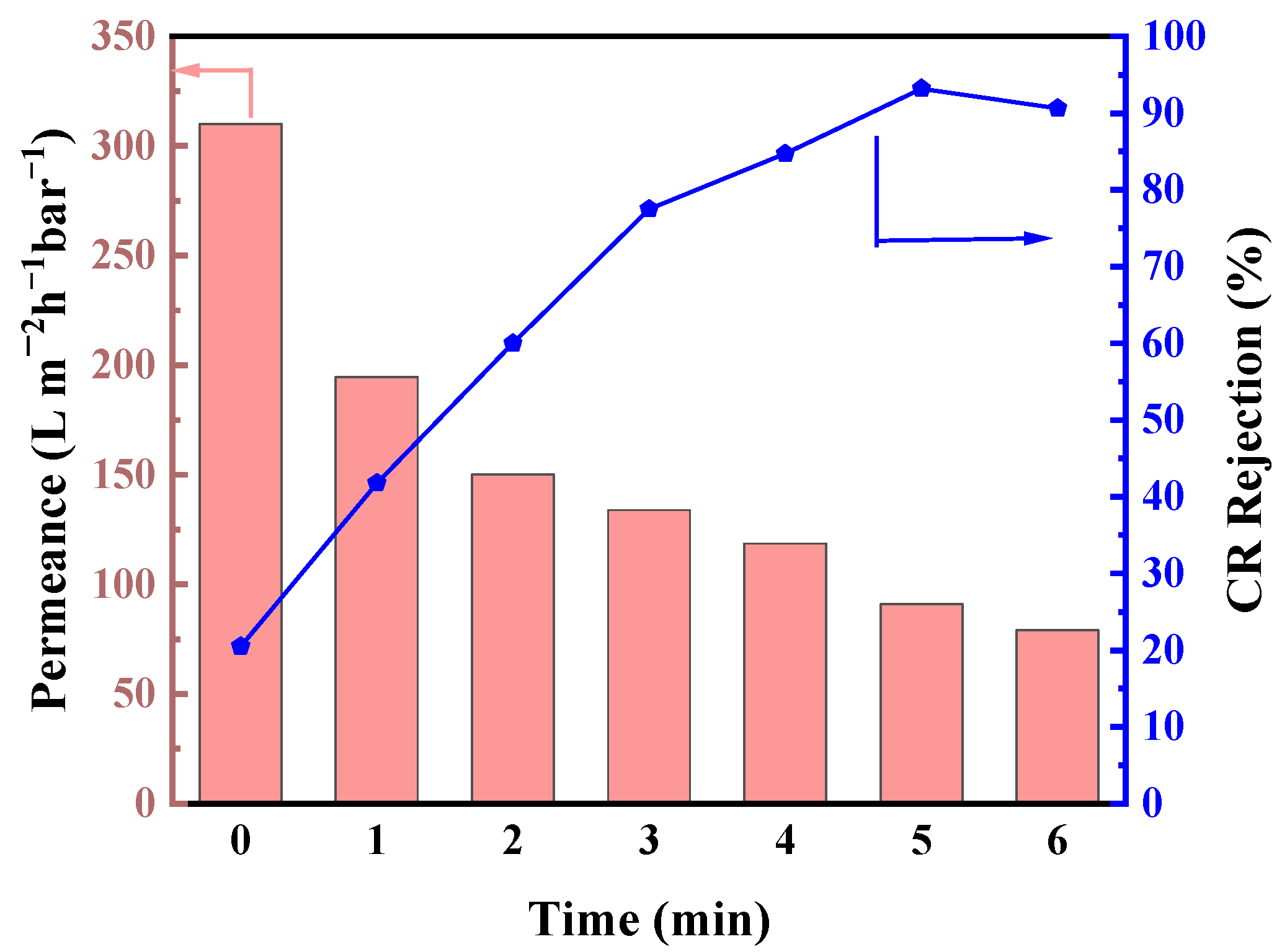
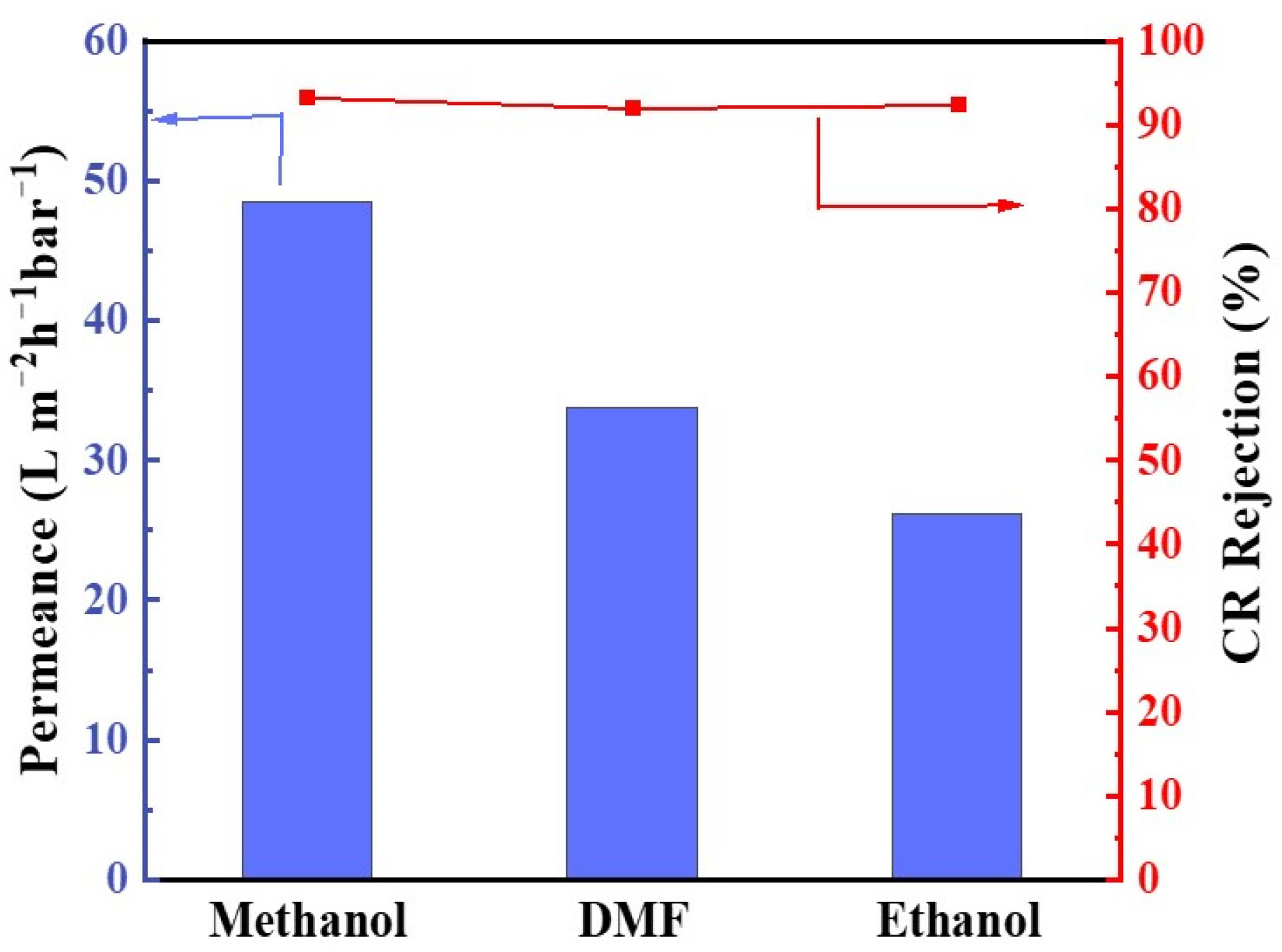
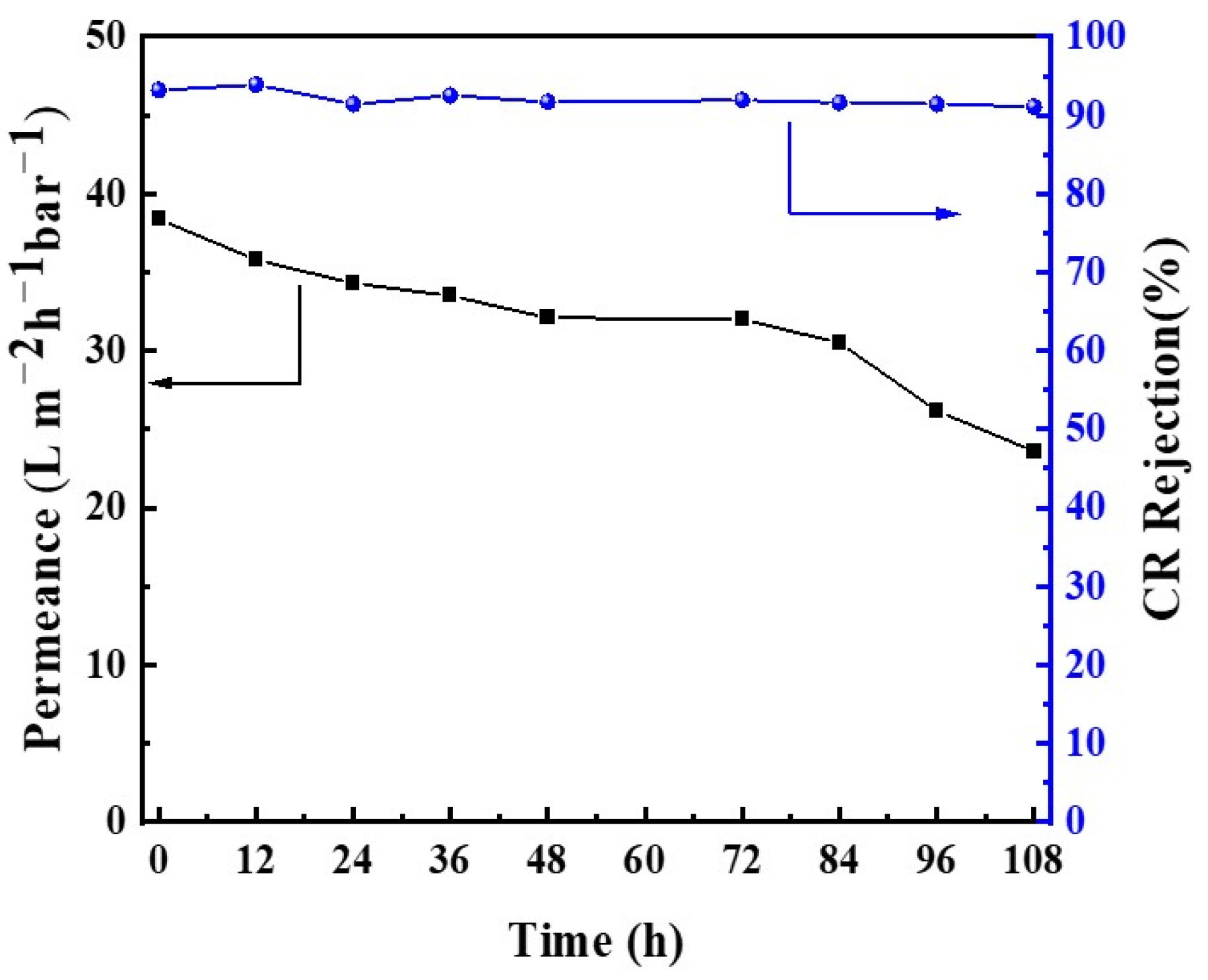
3.4. Solvent Resistance and Long-Term Stability of Membranes
3.5. Performance Comparison with Other OSN Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchetti, P.; Jimenez Solomon, M.; Szekely, F.G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.; Sirkar, K.K.; Pennisi, K.J.; Vaseghi, G.; Derdour, L.; Cohen, B. Novel perfluorinated nanofiltration membranes for isolation of pharmaceutical compounds. Sep. Purif. Technol. 2021, 258, 117944. [Google Scholar] [CrossRef]
- Shi, G.M.; Feng, Y.; Li, B.; Tham, H.M.; Lai, J.-Y.; Chung, T.-S. Recent progress of organic solvent nanofiltration membranes. Prog. Polym. Sci. 2021, 123, 101470. [Google Scholar] [CrossRef]
- Jiang, Z.; Dong, R.; Evans, A.M.; Biere, N.; Ebrahim, M.A.; Li, S.; Anselmetti, D.; Dichtel, W.R.; Livingston, A.G. Aligned macrocycle pores in ultrathin films for accurate molecular sieving. Nature 2022, 609, 58–64. [Google Scholar] [CrossRef]
- Kappert, E.J.; Raaijmakers, M.J.T.; Tempelman, K.; Cuperus, F.P.; Ogieglo, W.; Benes, N.E. Swelling of 9 polymers commonly employed for solvent-resistant nanofiltration membranes: A comprehensive dataset. J. Membr. Sci. 2019, 569, 177–199. [Google Scholar] [CrossRef]
- Shinde, D.B.; Cao, L.A.; Wonanke, D.D.; Li, X.; Kumar, S.; Liu, X.; Hedhili, M.N.; Emwas, A.-H.; Addicoat, M.; Huang, K.-W.; et al. Pore engineering of ultrathin covalent organic framework membranes for organic solvent nanofiltration and molecular sieving. Chem. Sci. 2020, 11, 5434–5440. [Google Scholar] [CrossRef]
- Li, H.K.; Kong, Z.Y.; Zhang, H.; Wang, X.L.; Xie, Y.H.; Zhao, X.Q.; Hong, Y.D. Ca2+, Fe3+ co-crosslinked tannic acid-bridged sodium alginate gel membrane for organic solvent nanofiltration. Sep. Purif. Technol. 2024, 350, 127993. [Google Scholar] [CrossRef]
- Tong, Y.H.; Luo, L.H.; Jia, R.; Han, R.; Xu, S.J.; Xu, Z.L. Whether membranes developed for organic solvent nanofiltration (OSN) tend to be hydrophilic or hydrophobic?—A review. Heliyon 2024, 10, e24330. [Google Scholar] [CrossRef]
- Mariën, H.; Vankelecom, I.F.J. Transformation of cross-linked polyimide UF membranes into highly permeable SRNF membranes via solvent annealing. J. Membr. Sci. 2017, 541, 205–213. [Google Scholar] [CrossRef]
- Li, C.; Li, S.X.; Lv, L.; Su, B.W.; Hu, M.Z. High solvent-resistant and integrally crosslinked polyimide-based composite membranes for organic solvent nanofiltration. J. Membr. Sci. 2018, 564, 10–21. [Google Scholar] [CrossRef]
- Li, S.; Dong, R.; Musteata, V.-E.; Kim, J.; Rangnekar, N.D.; Johnson, J.R.; Marshall, B.D.; Chisca, S.; Xu, J.; Hoy, S.; et al. Hydrophobic polyamide nanofilms provide rapid transport for crude oil separation. Science 2022, 377, 1555. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Zhang, M.X.; Feng, W.L.; Zhu, L.P.; Zhang, L. PIM-1 pore-filled thin film composite membranes for tunable organic solvent nanofiltration. J. Membr. Sci. 2020, 601, 117951. [Google Scholar] [CrossRef]
- He, X.; Sin, H.; Liang, B.; Ghazi, Z.A.; Khattak, A.M.; Khan, N.A.; Alanagh, H.R.; Li, L.; Lu, X.; Tang, Z. Controlling the selectivity of conjugated microporous polymer membrane for efficient organic solvent nanofiltration. Adv. Funct. Mater. 2019, 29, 1900134. [Google Scholar] [CrossRef]
- Tian, H.J.; Luo, J.Z.; Liu, X.Y.; Zong, X.P.; Xue, S. Preparation of PIM-1 thin film composite membranes with enhanced organic solvent resistance via thermal crosslinking. Sep. Purif. Technol. 2023, 323, 124431. [Google Scholar] [CrossRef]
- Wu, S.Q.; Qiu, J.H.; Wang, J.K.; Wang, L.; Tang, C.Y. Covalent organic framework membranes modified by end-capping monomers for organic solvent nanofiltration. J. Membr. Sci. 2024, 703, 122854. [Google Scholar] [CrossRef]
- Shinde, D.B.; Sheng, G.; Li, X.; Ostwal, M.; Emwas, A.H.; Huang, K.W.; Lai, Z. Crystalline 2D covalent organic framework membranes for high-flux organic solvent nanofiltration. J. Am. Chem. Soc. 2018, 140, 14342–14349. [Google Scholar] [CrossRef]
- Yuan, J.; You, X.; Zhang, R.; Yao, Z.; Liu, Q.; Cao, L.; Zhang, S.; Li, Y.; Liu, Z.; Jiang, Z.; et al. Oriented ionic covalent organic framework membranes for efficient organic solvent nanofiltration. J. Membr. Sci. 2023, 688, 122120. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High flux thin film nanocomposite membranes based on metal-organic frameworks for organic solvent nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, J.; Gascon, J.; Li, J.; Van der Bruggen, B. Metal–organic frameworks based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144. [Google Scholar] [CrossRef]
- Zeidler, S.; Puhlfürß, P.; Kätzel, U.; Voigt, I. Preparation and characterization of new low MWCO ceramic nanofiltration membranes for organic solvents. J. Membr. Sci. 2014, 470, 421–430. [Google Scholar] [CrossRef]
- Kyriakou, N.; Boorsma, E.; Aardema, G.J.; van Eck, G.R.; Drobek, M.; de Beer, S.; Nijmeijer, A.; Winnubst, L.; Pizzoccaro-Zilamy, M.A. Hybrid ceramic nanofiltration membranes prepared by impregnation and solid-state grafting of organo-phosphonic acids. J. Membr. Sci. 2023, 687, 122041. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, S.X.; Peng, Y.; Wang, E.L.; Song, W.J.; Song, J.J.; Su, B.W. Combined effect of polyelectrolyte assisted interfacial polymerization and tannic acid protective surface modification to boost organic solvent nanofiltration performance. J. Membr. Sci. 2024, 704, 122853. [Google Scholar] [CrossRef]
- Merlet, R.B.; Pizzoccaro-Zilamy, M.-A.; Nijmeijer, A.; Winnubst, L. Hybrid ceramic membranes for organic solvent nanofiltration: State-of-the-art and challenges. J. Membr. Sci. 2020, 599, 117839. [Google Scholar] [CrossRef]
- VanGestel, T.; Vandecasteele, C.; Buekenhoudt, A.; Dotremont, C.; Luyten, J.; Vander Bruggen, B.; Maes, G. Corrosion properties of alumina and titania NF membranes. J. Membr. Sci. 2003, 214, 21–29. [Google Scholar] [CrossRef]
- Benfer, S.; Popp, U.; Richter, H.; Siewert, C.; Tomandl, G. Development and characterization of ceramicnan of filtration membranes. Sep. Purif. Technol. 2001, 22, 231–237. [Google Scholar] [CrossRef]
- Sekulic, J.; Elshof, J.E.; Blank, D.H.A. Amicroporous titania membrane for nanofiltration and pervaporation. Adv. Mater. 2004, 16, 1546–1550. [Google Scholar] [CrossRef]
- George, S.M.; Yoon, B.; Dameron, A.A. Surface chemistry for molecular layer deposition of organic and hybrid organic-inorganic polymers. Acc. Chem. Res. 2009, 42, 498–508. [Google Scholar] [CrossRef]
- Meng, X. An overview of molecular layer deposition for organic and organic–inorganic hybrid materials: Mechanisms, growth characteristics, and promising applications. J. Mater. Chem. A 2017, 5, 18326–18378. [Google Scholar] [CrossRef]
- Kim, H.; Hyun, J.; Min, Y.S. Mechanistic study on molecular layer deposition of titanicone from TiCl4 and ethylene glycol. J. Phys. Chem. C 2023, 127, 2258–2265. [Google Scholar] [CrossRef]
- Song, Z.; Fathizadeh, M.; Huang, Y.; Chu, K.H.; Yoon, Y.; Wang, L.; Xu, W.L.; Yu, M. TiO2 nanofiltration membranes prepared by molecular layer deposition for water purification. J. Membr. Sci. 2016, 510, 72–78. [Google Scholar] [CrossRef]
- Kim, H.; Hyun, J.; Kim, G.; Lee, E.; Min, Y.-S. Origin of instability of titanicone grown by molecular layer deposition using TiCl4 and ethylene glycol. Chem. Mater. 2024, 36, 247–255. [Google Scholar] [CrossRef]
- Sengupta, B.; Dong, Q.B.; Khadka, R.; Behera, D.K.; Yang, R.Z.; Liu, J.; Jiang, J.; Keblinski, P.; Belfort, G.; Yu, M. Carbon-doped metal oxide interfacial nanofilms for ultrafast and precise separation of molecules. Science 2023, 381, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.T.; Tian, H.J.; Ling, H.L.; Yang, Y.L.; Luo, J.Z.; Zong, X.P.; Xue, S. Thermally rearranged OH-containing polyimide composite membranes with enhanced gas separation performance and physical aging resistance. J. Environ. Chem. Eng. 2024, 12, 112275. [Google Scholar] [CrossRef]
- Chen, G.E.; Liu, Y.J.; Xu, Z.L.; Tang, Y.J.; Huang, H.H.; Sun, L. Fabrication and characterization of a novel nanofiltration membrane by the interfacial polymerization of 1,4-diaminocyclohexane (DCH) and trimesoyl chloride (TMC). RSC Adv. 2015, 5, 40742–40752. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H. Carbon nanotubes supported mesoporous mesocrystals of anatase TiO2. Chem. Mater. 2008, 20, 2711–2718. [Google Scholar] [CrossRef]
- Shao, G.N.; Imran, S.M.; Jeon, S.J.; Engole, M.; Abbas, N.; Salman Haider, M.; Kang, S.J.; Kim, H.T. Sol gel synthesis of photoactive zirconia-titania from metal salts and investigation of their photocatalytic properties in the photodegradation of methylene blue. Powder Technol. 2014, 258, 99–109. [Google Scholar] [CrossRef]
- Yang, Q.; Su, Y.; Chi, C.; Cherian, C.T.; Huang, K.; Kravets, V.G.; Wang, F.C.; Zhang, J.C.; Pratt, A.; Grigorenko, A.N.; et al. Ultrathin graphene-based membrane with precise molecular sieving and ultrafast solvent permeation. Nat. Mater. 2017, 16, 1198–1202. [Google Scholar] [CrossRef]
- Chen, X.; Qi, T.; Zhang, Y.; Wang, T.; Qiu, M.; Cui, Z.; Fan, Y. Facile pore size tuning and characterization of nanoporous ceramic membranes for the purification of polysaccharide. J. Membr. Sci. 2020, 597, 117631. [Google Scholar] [CrossRef]
- Shen, Y.; Yao, A.; Li, J.; Hua, D.; Tan, K.B.; Zhan, G.; Rao, X. Dispersive two-dimensional MXene via potassium fulvic acid for mixed matrix membranes with enhanced organic solvent nanofiltration performance. J. Membr. Sci. 2023, 666, 121168. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Ouyang, G.; Huang, L.; Li, L.; Li, W.; Huang, W.; Li, D. Ultrathin organic solvent nanofiltration membrane with polydopamine-HKUST-1 interlayer for organic solvent separation. J. Environ. Sci. 2024, 141, 182–193. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Su, B.; Gao, X.; Gao, C. Thin film nanocomposite polyamide membrane doped with amino-functionalized graphene quantum dots for organic solvent nanofiltration. J. Membr. Sci. 2023, 685, 121960. [Google Scholar] [CrossRef]




| Sample | Interfacial Reaction Time | ||
|---|---|---|---|
| 1 min | 3 min | 5 min | |
| Organometallic hybrid film | 50.856° | 95.993° | 98.895° |
| GLTO nanofilm | 34.641° | 68.039° | 68.782° |
| Interfacial Reaction Time | Calcination Temperature | Carbon (%) | Titanium (%) | Oxygen (%) |
|---|---|---|---|---|
| 1 min | 250 °C | 52.11 | 8.96 | 38.93 |
| 3 min | 250 °C | 62.21 | 6.61 | 31.19 |
| 5 min | 250 °C | 63.80 | 5.87 | 30.33 |
| 5 min | 300 °C | 30.84 | 21.74 | 47.42 |
| Membrane | Pure Solvents Permeance (L·m−2·h−1·bar−1) | Dye Used and Its Molecular Weight (g·mol−1) | Rejection (%) | Ref. |
|---|---|---|---|---|
| GLTO | Methanol 90.9 | Congo Red (696.7) | 93.2 | This work |
| CDTO-Air-30%H2O/AAO | Methanol 125 | Congo Red (696.7) | 99 | [32] |
| TA0.125-Ca/FeSA | Methanol 107.7 | Congo Red (696.7) | 97 | [7] |
| PIM-1/Al2O3 | Methanol 2.0 | Congo Red (696.7) | 99 | [14] |
| u-Ti3C2Tx@P84 MMMsx | Methanol ≈ 7 | Congo Red (696.7) | 97 | [39] |
| PA/PDA-HKUST-10.6/PEI | Methanol 0.9 | Congo Red (696.7) | 92 | [40] |
| af-GQDs | Methanol 13.5 | Rhodamine B (479) | 99 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Luo, J.; Ling, H.; Huang, L.; Xue, S. Carbon-Doped TiO2 Nanofiltration Membranes Prepared by Interfacial Reaction of Glycerol with TiCl4 Vapor. Membranes 2024, 14, 233. https://doi.org/10.3390/membranes14110233
Zhang W, Luo J, Ling H, Huang L, Xue S. Carbon-Doped TiO2 Nanofiltration Membranes Prepared by Interfacial Reaction of Glycerol with TiCl4 Vapor. Membranes. 2024; 14(11):233. https://doi.org/10.3390/membranes14110233
Chicago/Turabian StyleZhang, Wenjing, Jiangzhou Luo, Honglei Ling, Lei Huang, and Song Xue. 2024. "Carbon-Doped TiO2 Nanofiltration Membranes Prepared by Interfacial Reaction of Glycerol with TiCl4 Vapor" Membranes 14, no. 11: 233. https://doi.org/10.3390/membranes14110233
APA StyleZhang, W., Luo, J., Ling, H., Huang, L., & Xue, S. (2024). Carbon-Doped TiO2 Nanofiltration Membranes Prepared by Interfacial Reaction of Glycerol with TiCl4 Vapor. Membranes, 14(11), 233. https://doi.org/10.3390/membranes14110233






