Development and Prospective Applications of 3D Membranes as a Sensor for Monitoring and Inducing Tissue Regeneration
Abstract
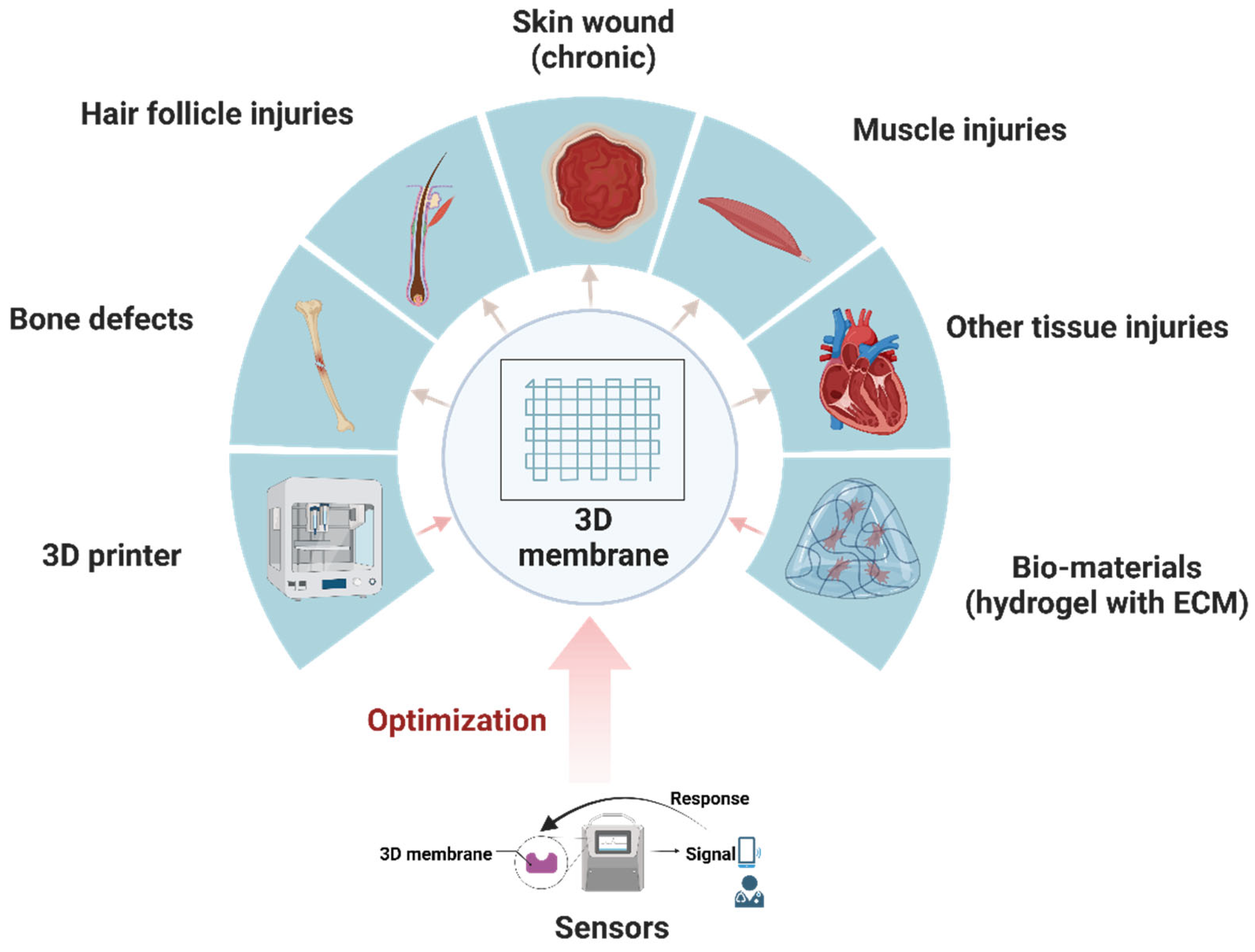
1. Introduction
2. 3D Printing
2.1. Approaches of 3D Printing
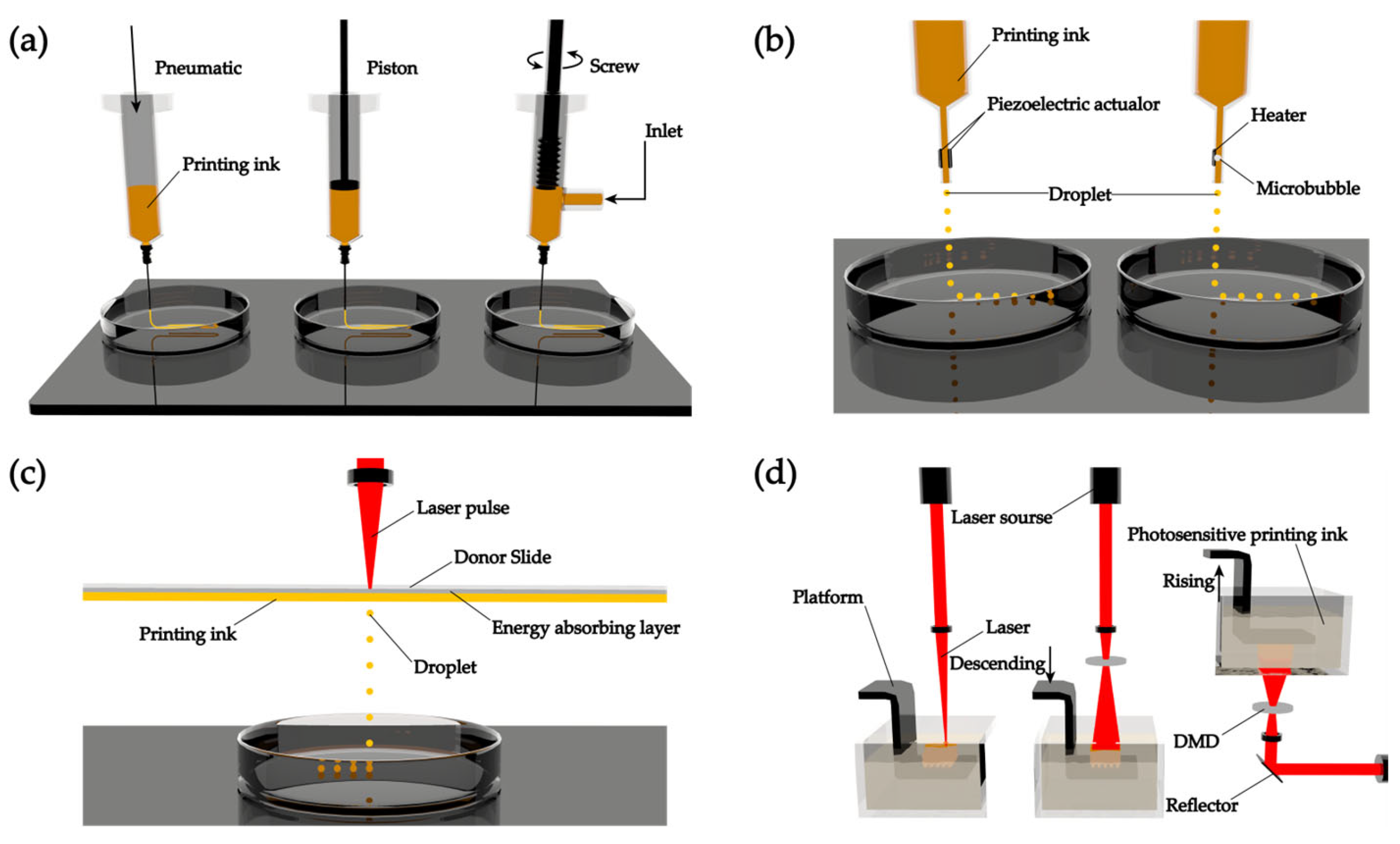
2.1.1. Extrusion-Based 3D Printing
2.1.2. Droplet-Based 3D printing
2.1.3. Laser-Assisted 3D Printing
2.1.4. Stereolithography-Based 3D Printing
2.2. Printing Inks
2.3. 3D Membranes in Different Tissues and Organs
2.3.1. Skin Membranes
2.3.2. Serosal Membranes
2.3.3. Tubular Tissue Membranes
2.3.4. Connective Tissue Membranes
2.3.5. Other Tissue Membranes
2.4. Limitations of 3D Printing
3. Sensors
3.1. Traditional Sensors for 3D Membranes
3.1.1. Traditional Sensors for Regenerative Skin Membranes
3.1.2. Traditional Sensors for Other Usages
3.2. Novel Sensors for 3D Membranes
3.2.1. Novel Sensors for Regenerative Skin Membranes
3.2.2. Novel Sensors for Regenerating Other Tissues and Organs
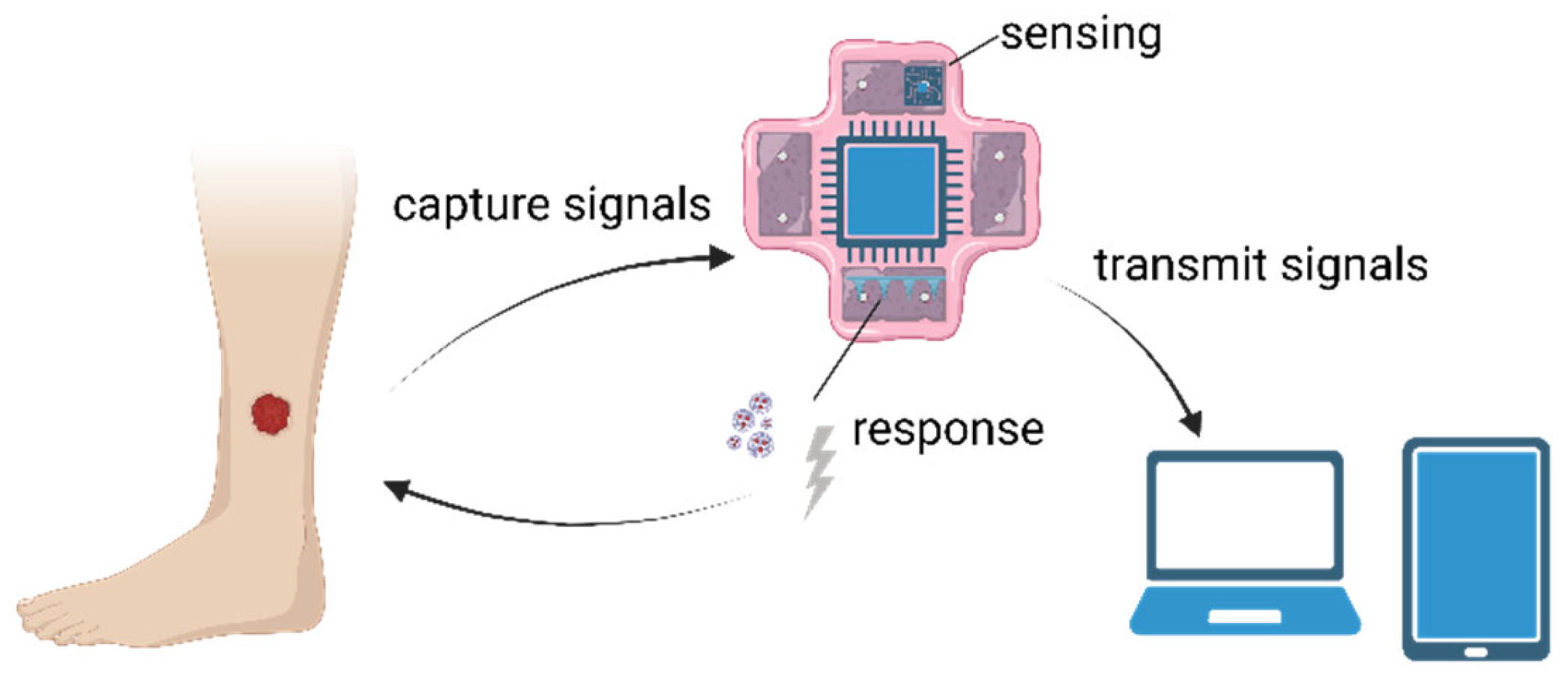
3.3. Development and Applications of 3D Membrane-Binding Sensors
3.3.1. Development of 3D Membrane-Binding Sensors
3.3.2. Applications in Skin
3.3.3. Applications in Other Tissues and Organs
4. Conclusions and Perspectives
4.1. Advantages and Limitations
4.2. Conclusions and Prospectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three dimensional |
| 4D | Four dimensional |
| dECM | Decellularized extracellular matrix |
| micro-CAL | Microscale computed axial lithography |
| pO2 | Partial pressure of oxygen |
| AD | Atopic dermatitis |
| CAD | Computer-aided manufacturing |
| CAM | Computer-aided design |
| DMD | Digital micromirror device |
| ECM | Extracellular matrix |
| FBG | Fiber Bragg grating technology |
| GO | Graphene oxide |
| MI | Myocardial infarction |
| PEG | Polyethylene glyco |
| PF127 | Pluronic F127 |
| PLA | Polylactic acid |
| PU | Polyurethane |
| PVA | Polyvinyl alcohol |
| TPP | Two-photon polymerization |
References
- Jia, J.; Gong, S.; Zhang, A.; Jiang, L.; Yao, Y. Stiffening of the gluteal muscle increased the intramuscular stress: An in-silico implication of deep tissue injury. Heliyon 2023, 9, e13459. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hur, Y.H.; Cai, X.; Cong, Q.; Yang, Y.; Xu, C.; Bilate, A.M.; Gonzales, K.A.U.; Parigi, S.M.; Cowley, C.J.; et al. A tissue injury sensing and repair pathway distinct from host pathogen defense. Cell 2023, 186, 2127–2143.e22. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yue, Z.; Xu, M.; Zhang, M.; Shen, X.; Ma, Z.; Li, J.; Xie, X. Macrophages play a key role in tissue repair and regeneration. PeerJ 2022, 10, e14053. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Koepke, L.S.; Lopez, M.T.; Tong, X.; Ambrosi, T.H.; Gulati, G.S.; Marecic, O.; Wang, Y.; Ransom, R.C.; Hoover, M.Y.; et al. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 2020, 26, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Jafferany, M.; Pastolero, P. Psychiatric and Psychological Impact of Chronic Skin Disease. Prim. Care Companion CNS Disord. 2018, 20, 17nr02247. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair. Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound Healing and Skin Regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef]
- Jimenez, F.; Vogel, J.E.; Avram, M. CME article Part II. Hair transplantation: Surgical technique. J. Am. Acad. Dermatol. 2021, 85, 818–829. [Google Scholar] [CrossRef]
- Blanca-Lopez, N.; Perez-Alzate, D.; Canto, G.; Blanca, M. Practical approach to the treatment of NSAID hypersensitivity. Expert. Rev. Clin. Immunol. 2017, 13, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Sharifuzzaman, M.; Chhetry, A.; Zahed, M.A.; Yoon, S.H.; Park, C.I.; Zhang, S.; Chandra Barman, S.; Sharma, S.; Yoon, H.; Park, J.Y. Smart bandage with integrated multifunctional sensors based on MXene-functionalized porous graphene scaffold for chronic wound care management. Biosens. Bioelectron. 2020, 169, 112637. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.L.; Achten, J.; Knight, R.; Bruce, J.; Dutton, S.J.; Madan, J.; Dritsaki, M.; Parsons, N.; Fernandez, M.; Grant, R.; et al. Effect of Incisional Negative Pressure Wound Therapy vs Standard Wound Dressing on Deep Surgical Site Infection After Surgery for Lower Limb Fractures Associated with Major Trauma: The WHIST Randomized Clinical Trial. JAMA 2020, 323, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Geanaliu-Nicolae, R.E.; Andronescu, E. Blended Natural Support Materials-Collagen Based Hydrogels Used in Biomedicine. Materials 2020, 13, 5641. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, I.E.; Fujimoto, M.; Vencovsky, J.; Aggarwal, R.; Holmqvist, M.; Christopher-Stine, L.; Mammen, A.L.; Miller, F.W. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Primers 2021, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Koukoura, A.; Tsianos, G.I.; Gargavanis, A.A.; Nielsen, A.A.; Vassiliadis, E. Organ donation in the US and Europe: The supply vs demand imbalance. Transpl. Rev. 2021, 35, 100585. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Seidi, F.; Youssefi Azarfam, M.; Khodadadi Yazdi, M.; Erfani, A.; Barani, M.; Chauhan, N.P.S.; Rabiee, N.; Kuang, T.; Kucinska-Lipka, J.; et al. Biopolymer-based composites for tissue engineering applications: A basis for future opportunities. Compos. Part. B Eng. 2023, 258, 110701. [Google Scholar] [CrossRef]
- Lanza, R.; Langer, R.; Vacanti, J.P. (Eds.) Principles of Tissue Engineering, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Cheema, U. Position Paper Progress in the development of biomimetic engineered human tissues. J. Tissue Eng. 2023, 14. [Google Scholar] [CrossRef]
- Xue, W.; Du, J.; Li, Q.; Wang, Y.; Lu, Y.; Fan, J.; Yu, S.; Yang, Y. Preparation, Properties, and Application of Graphene-Based Materials in Tissue Engineering Scaffolds. Tissue Eng. Part. B Rev. 2022, 28, 1121–1136. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Tandon, B.; Dalton, P.D. Chapter 11—Scaffold design and fabrication. In Tissue Engineering, 3rd ed.; De Boer, J., Blitterswijk, C.A.V., Uquillas, J.A., Malik, N., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 355–385. [Google Scholar]
- Serrano-Aroca, A.; Cano-Vicent, A.; Sabater, I.S.R.; El-Tanani, M.; Aljabali, A.; Tambuwala, M.M.; Mishra, Y.K. Scaffolds in the microbial resistant era: Fabrication, materials, properties and tissue engineering applications. Mater. Today Bio 2022, 16, 100412. [Google Scholar] [CrossRef]
- Chang, P.; Li, S.; Sun, Q.; Guo, K.; Wang, H.; Li, S.; Zhang, L.; Xie, Y.; Zheng, X.; Liu, Y. Large full-thickness wounded skin regeneration using 3D-printed elastic scaffold with minimal functional unit of skin. J. Tissue Eng. 2022, 13, 20417314211063022. [Google Scholar] [CrossRef] [PubMed]
- Verisqa, F.; Cha, J.R.; Nguyen, L.; Kim, H.W.; Knowles, J.C. Digital Light Processing 3D Printing of Gyroid Scaffold with Isosorbide-Based Photopolymer for Bone Tissue Engineering. Biomolecules 2022, 12, 1692. [Google Scholar] [CrossRef] [PubMed]
- Joung, D.; Lavoie, N.S.; Guo, S.Z.; Park, S.H.; Parr, A.M.; McAlpine, M.C. 3D Printed Neural Regeneration Devices. Adv. Funct. Mater. 2019, 30, 1906237. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Huang, J.; Wu, J.; Du, J. Electrospinning nanofibers to 1D, 2D, and 3D scaffolds and their biomedical applications. Nano Res. 2021, 15, 787–804. [Google Scholar] [CrossRef]
- Alave Reyes-Furrer, A.; De Andrade, S.; Bachmann, D.; Jeker, H.; Steinmann, M.; Accart, N.; Dunbar, A.; Rausch, M.; Bono, E.; Rimann, M.; et al. Matrigel 3D bioprinting of contractile human skeletal muscle models recapitulating exercise and pharmacological responses. Commun. Biol. 2021, 4, 1183. [Google Scholar] [CrossRef] [PubMed]
- Yi, N.; Cui, H.; Zhang, L.G.; Cheng, H. Integration of biological systems with electronic-mechanical assemblies. Acta Biomater. 2019, 95, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Healing chronic wounds with a wireless smart bandage with integrated sensors and stimulators. Nat. Biotechnol. 2023, 41, 622–623. [CrossRef]
- Mostafalu, P.; Lenk, W.; Dokmeci, M.R.; Ziaie, B.; Khademhosseini, A.; Sonkusale, S.R. Wireless Flexible Smart Bandage for Continuous Monitoring of Wound Oxygenation. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 670–677. [Google Scholar] [CrossRef]
- Olejnik, A.; Semba, J.A.; Kulpa, A.; Dańczak-Pazdrowska, A.; Rybka, J.D.; Gornowicz-Porowska, J. 3D Bioprinting in Skin Related Research: Recent Achievements and Application Perspectives. ACS Synth. Biol. 2022, 11, 26–38. [Google Scholar] [CrossRef]
- Netzlaff, F.; Lehr, C.M.; Wertz, P.W.; Schaefer, U.F. The human epidermis models EpiSkin, SkinEthic and EpiDerm: An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm. 2005, 60, 167–178. [Google Scholar] [CrossRef]
- Arrabito, G.; Ferrara, V.; Bonasera, A.; Pignataro, B. Artificial Biosystems by Printing Biology. Small 2020, 16, e1907691. [Google Scholar] [CrossRef] [PubMed]
- Maschmeyer, I.; Kakava, S. Organ-on-a-Chip. Adv. Biochem. Eng. Biotechnol. 2022, 179, 311–342. [Google Scholar] [PubMed]
- Welss, T.; Basketter, D.A.; Schröder, K.R. In vitro skin irritation: Facts and future. State of the art review of mechanisms and models. Toxicol. Vitr. 2004, 18, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.L.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The status, challenges, and future of additive manufacturing in engineering. Comput. Aided Des. 2015, 69, 65–89. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part. B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef]
- Lam, E.H.Y.; Yu, F.; Zhu, S.; Wang, Z. 3D Bioprinting for Next-Generation Personalized Medicine. Int. J. Mol. Sci. 2023, 24, 6357. [Google Scholar] [CrossRef]
- Mohan, T.S.; Datta, P.; Nesaei, S.; Ozbolat, V.; Ozbolat, I.T. 3D Coaxial Bioprinting: Process Mechanisms, Bioinks and Applications. Prog. Biomed. Eng. 2022, 4, 022003. [Google Scholar]
- Naghieh, S.; Chen, X. Printability-A key issue in extrusion-based bioprinting. J. Pharm. Anal. 2021, 11, 564–579. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Xiaorui, L.; Fuyin, Z.; Xudong, W.; Xuezheng, G.; Shudong, Z.; Hui, L.; Dandan, D.; Yubing, L.; Lizhen, W.; Yubo, F. 1Biomaterial inks for extrusion-based 3D bioprinting: Property, classification, modification, and selection. Int. J. Bioprint 2023, 9, 649. [Google Scholar]
- You, F.; Eames, B.F.; Chen, X. Application of Extrusion-Based Hydrogel Bioprinting for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2017, 18, 1597. [Google Scholar] [CrossRef] [PubMed]
- Kotlarz, M.; Ferreira, A.M.; Gentile, P.; Russell, S.J.; Dalgarno, K. Droplet-based bioprinting enables the fabrication of cell–hydrogel–microfibre composite tissue precursors. Bio-Des. Manuf. 2022, 5, 512–528. [Google Scholar] [CrossRef]
- Lee, S.-G.; Lee, S.; Bae, H.-K.; Lee, K.Y.; Park, C.; Kim, M.s.; Lee, D.H.; Chung, H.M.; Kim, C.Y. Evaluation of the therapeutic efficacy of human skin equivalents manufactured through droplet-based bioprinting/nebulization technology. Mol. Cell. Toxicol. 2023. [Google Scholar] [CrossRef]
- Zhang, S.; Li, G.; Man, J.; Zhang, S.; Li, J.; Li, J.; Li, D. Fabrication of Microspheres from High-Viscosity Bioink Using a Novel Microfluidic-Based 3D Bioprinting Nozzle. Micromachines 2020, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and its Future Research Trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Grosfeld, E.V.; Zhigarkov, V.S.; Alexandrov, A.I.; Minaev, N.V.; Yusupov, V.I. Theoretical and Experimental Assay of Shock Experienced by Yeast Cells during Laser Bioprinting. Int. J. Mol. Sci. 2022, 23, 9823. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef]
- Ventura, R.D. An Overview of Laser-assisted Bioprinting (LAB) in Tissue Engineering Applications. Med. Lasers 2021, 10, 76–81. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.; Ma, H.; Chapa-Villarreal, F.A.; Lobo, A.O.; Zhang, Y.S. Stereolithography apparatus and digital light processing-based 3D bioprinting for tissue fabrication. iScience 2023, 26, 106039. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kumar, H.; Tian, Z.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K. Visible Light Photoinitiation of Cell-Adhesive Gelatin Methacryloyl Hydrogels for Stereolithography 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 26859–26869. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, C.; Nong, H.; Xu, K.; Wu, X.; Zhong, W.; Xing, M.; Wang, L. Development of an Asymmetric Hydrophobic/Hydrophilic Ultrathin Graphene Oxide Membrane as Actuator and Conformable Patch for Heart Repair. Adv. Funct. Mater. 2023, 33, 2300866. [Google Scholar] [CrossRef]
- Jing, X.; Fu, H.X.; Yu, B.J.; Sun, M.Y.; Wang, L.Y. Two-photon polymerization for 3D biomedical scaffolds: Overview and updates. Front. Bioeng. Biotechnol. 2022, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Toombs, J.T.; Luitz, M.; Cook, C.C.; Jenne, S.; Li, C.C.; Rapp, B.E.; Kotz-Helmer, F.; Taylor, H.K. Volumetric additive manufacturing of silica glass with microscale computed axial lithography. Science 2022, 376, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Mei, Q.; Rao, J.; Bei, H.P.; Liu, Y.; Zhao, X. 3D Bioprinting Photo-Crosslinkable Hydrogels for Bone and Cartilage Repair. Int. J. Bioprint 2021, 7, 367. [Google Scholar] [CrossRef]
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv. Mater. 2015, 27, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Gudapati, H.; Ozbolat, I.T. The Role of Concentration on Drop Formation and Breakup of Collagen, Fibrinogen, and Thrombin Solutions during Inkjet Bioprinting. Langmuir 2020, 36, 15373–15385. [Google Scholar] [CrossRef] [PubMed]
- Kerouredan, O.; Hakobyan, D.; Remy, M.; Ziane, S.; Dusserre, N.; Fricain, J.C.; Delmond, S.; Thebaud, N.B.; Devillard, R. In situ prevascularization designed by laser-assisted bioprinting: Effect on bone regeneration. Biofabrication 2019, 11, 045002. [Google Scholar] [CrossRef] [PubMed]
- Moncal, K.K.; Ozbolat, V.; Datta, P.; Heo, D.N.; Ozbolat, I.T. Thermally-controlled extrusion-based bioprinting of collagen. J. Mater. Sci. Mater. Med. 2019, 30, 55. [Google Scholar] [CrossRef] [PubMed]
- de Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater. 2020, 117, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Petta, D.; Armiento, A.R.; Grijpma, D.; Alini, M.; Eglin, D.; D’Este, M. 3D bioprinting of a hyaluronan bioink through enzymatic-and visible light-crosslinking. Biofabrication 2018, 10, 044104. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gong, Y.; Zhang, K.; Ke, S.; Wang, Y.; Wang, J.; Wang, H. Recent Advances in Decellularized Matrix-Derived Materials for Bioink and 3D Bioprinting. Gels 2023, 9, 195. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, S.; Yang, J.; Qiu, J.; Jiao, X.; Yue, X.; Ke, X.; Yang, G.; Zhang, L. Application of 3D-bioprinted nanocellulose and cellulose derivative-based bio-inks in bone and cartilage tissue engineering. Int. J. Bioprint 2023, 9, 637. [Google Scholar] [CrossRef]
- Piras, C.C.; Fernandez-Prieto, S.; De Borggraeve, W.M. Nanocellulosic materials as bioinks for 3D bioprinting. Biomater. Sci. 2017, 5, 1988–1992. [Google Scholar] [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for Tissue Repair and Organ Three-Dimensional (3D) Bioprinting. Micromachines 2019, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; Garcia, J.M.; O’Connell, G.D. Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Kim, G.J.; Kim, H.W.; Lee, J.; Zhang, X.; Kang, M.G.; Seo, J.W.; Cha, J.M.; Park, H.J.; Lee, M.Y.; et al. Kappa-Carrageenan-Based Dual Crosslinkable Bioink for Extrusion Type Bioprinting. Polymers 2020, 12, 2377. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Piluso, S.; Skvortsov, G.A.; Altunbek, M.; Afghah, F.; Khani, N.; Koc, B.; Patterson, J. 3D bioprinting of molecularly engineered PEG-based hydrogels utilizing gelatin fragments. Biofabrication 2021, 13, 045008. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.T.; Hsu, S.H. Double-Network Polyurethane-Gelatin Hydrogel with Tunable Modulus for High-Resolution 3D Bioprinting. ACS Appl. Mater. Interfaces 2019, 11, 32746–32757. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Hsieh, F.Y.; Tseng, C.S.; Hsu, S.H. Preparation and characterization of a biodegradable polyurethane hydrogel and the hybrid gel with soy protein for 3D cell-laden bioprinting. J. Mater. Chem. B 2016, 4, 6694–6705. [Google Scholar] [CrossRef]
- Setayeshmehr, M.; Hafeez, S.; van Blitterswijk, C.; Moroni, L.; Mota, C.; Baker, M.B. Bioprinting Via a Dual-Gel Bioink Based on Poly(Vinyl Alcohol) and Solubilized Extracellular Matrix towards Cartilage Engineering. Int. J. Mol. Sci. 2021, 22, 3901. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, R.; Sun, D.; Zhou, J.; Li, M.; Zhang, Y.; Wang, Y. Design and evaluation of sodium alginate/polyvinyl alcohol blend hydrogel for 3D bioprinting cartilage scaffold: Molecular dynamics simulation and experimental method. J. Mater. Res. Technol. 2022, 17, 66–78. [Google Scholar] [CrossRef]
- Tümer, E.H.; Erbil, H.Y. Extrusion-Based 3D Printing Applications of PLA Composites: A Review. Coatings 2021, 11, 390. [Google Scholar] [CrossRef]
- Shamma, R.N.; Sayed, R.H.; Madry, H.; El Sayed, N.S.; Cucchiarini, M. Triblock Copolymer Bioinks in Hydrogel Three-Dimensional Printing for Regenerative Medicine: A Focus on Pluronic F127. Tissue Eng. Part. B Rev. 2022, 28, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Da, L.C.; Huang, Y.Z.; Xie, H.Q.; Zheng, B.H.; Huang, Y.C.; Du, S.R. Membranous Extracellular Matrix-Based Scaffolds for Skin Wound Healing. Pharmaceutics 2021, 13, 1796. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.L.; Qi, J.T.Z.; Yeong, W.Y.; Naing, M.W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018, 10, 025005. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Chua, D.A.C.; Tang, J.R.J.; Bonnard, C.; Leavesley, D.; Liang, K. Design of hydrogel-based scaffolds for in vitro three-dimensional human skin model reconstruction. Acta Biomater. 2022, 153, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.C.; Davoodi, P.; Vijayavenkataraman, S.; Tian, Y.; Ng, W.C.; Fuh, J.Y.H.; Robinson, K.S.; Wang, C.H. 3D bioprinting of skin tissue: From pre-processing to final product evaluation. Adv. Drug Deliv. Rev. 2018, 132, 270–295. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Correia, I.J. Production and characterization of a novel asymmetric 3D printed construct aimed for skin tissue regeneration. Colloids Surf. B Biointerfaces 2019, 181, 994–1003. [Google Scholar] [CrossRef]
- Poerio, A.; Guibert, B.; Leroux, M.M.; Mano, J.F.; Cleymand, F.; Jehl, J.P. Mechanical Characterization of 3D-Printed Patterned Membranes for Cardiac Tissue Engineering: An Experimental and Numerical Study. Biomedicines 2023, 11, 963. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, C.; Xu, T.; Lin, S.; Wang, Z.; Zhu, Y. Subaqueous Bioprinting: A Novel Strategy for Fetal Membrane Repair with 7-Axis Robot-Assisted Minimally Invasive Surgery. Adv. Funct. Mater. 2022, 32, 2207496. [Google Scholar] [CrossRef]
- Sung, K.; Patel, N.R.; Ashammakhi, N.; Nguyen, K.L. 3-Dimensional Bioprinting of Cardiovascular Tissues: Emerging Technology. JACC Basic. Transl. Sci. 2021, 6, 467–482. [Google Scholar] [CrossRef]
- Farhat, W.; Chatelain, F.; Marret, A.; Faivre, L.; Arakelian, L.; Cattan, P.; Fuchs, A. Trends in 3D bioprinting for esophageal tissue repair and reconstruction. Biomaterials 2021, 267, 120465. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Nam, H.; Jang, J.; Lee, S.J. 3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs. Bioengineering 2020, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Topfer, E.; Pasotti, A.; Telopoulou, A.; Italiani, P.; Boraschi, D.; Ewart, M.A.; Wilde, C. Bovine colon organoids: From 3D bioprinting to cryopreserved multi-well screening platforms. Toxicol. Vitr. 2019, 61, 104606. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wang, Z. Research Progress of Three-Dimensional Bioprinting Artificial Cardiac Tissue. Tissue Eng. Regen. Med. 2023, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Suntornnond, R.; Tan, E.Y.S.; An, J.; Chua, C.K. A highly printable and biocompatible hydrogel composite for direct printing of soft and perfusable vasculature-like structures. Sci. Rep. 2017, 7, 16902. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, J.; Ma, J.; Wang, T.; Zhao, X.; Zhu, D.; Jin, W.; Zhang, K.; Sun, X.; Shen, Y.; et al. Three-dimensional bioprinted BMSCs-laden highly adhesive artificial periosteum containing gelatin-dopamine and graphene oxide nanosheets promoting bone defect repair. Biofabrication 2023, 15, 025010. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Chalisserry, E.P.; Mondal, S.; Oh, J.; Nam, S.Y. Silicon-substituted hydroxyapatite reinforced 3D printed gelatin membrane for guided bone regeneration. Mater. Lett. 2021, 304, 130670. [Google Scholar] [CrossRef]
- Sufaru, I.G.; Macovei, G.; Stoleriu, S.; Martu, M.A.; Luchian, I.; Kappenberg-Nitescu, D.C.; Solomon, S.M. 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes 2022, 12, 902. [Google Scholar] [CrossRef]
- Huang, C.-C. Tuning gelatin–alginate bioink properties by introducing new decellularized elastic cartilage scaffolds for bioinspired composite membranes in orthopedics. Polym. Bull. 2022, 80, 3279–3291. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Salehi, S.; Costantini, M.; Suthiwanich, K.; Ebrahimi, M.; Sadeghian, R.B.; Fujie, T.; Shi, X.; Cannata, S.; Gargioli, C.; et al. 3D Bioprinting in Skeletal Muscle Tissue Engineering. Small 2019, 15, e1805530. [Google Scholar] [CrossRef]
- Larson, N.M.; Mueller, J.; Chortos, A.; Davidson, Z.S.; Clarke, D.R.; Lewis, J.A. Rotational multimaterial printing of filaments with subvoxel control. Nature 2023, 613, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Liu, F.W.; Wang, N.; Yue, G.C.; Wang, X.Y.; Cai, B.L.; Hao, Y.K.; Li, Y.W.; Guo, F.Y.; Zhang, Z.Y.; et al. Bioinspired stretchable helical nanofiber yarn scaffold for locomotive tissue dynamic regeneration. Matter 2022, 5, 4480–4501. [Google Scholar] [CrossRef]
- Kuo, C.; Wilson, E.; Rhodes, K.; Fuson, A.; Fisher, J.; Cleary, K.; Reilly, B. Automated Bioprinting of Customized Tissue Engineered Grafts for Tympanic Membrane Perforation Repair. Tissue Eng. Part. A 2016, 22, S29. [Google Scholar]
- Orash Mahmoud Salehi, A.; Heidari-Keshel, S.; Poursamar, S.A.; Zarrabi, A.; Sefat, F.; Mamidi, N.; Behrouz, M.J.; Rafienia, M. Bioprinted Membranes for Corneal Tissue Engineering: A Review. Pharmaceutics 2022, 14, 2797. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Edgar, T.Y.S.; Yeong, W.Y.; Laude, A. Hybrid three-dimensional (3D) bioprinting of retina equivalent for ocular research. Int. J. Bioprint 2017, 3, 008. [Google Scholar] [CrossRef] [PubMed]
- Micko, K.; Papcun, P.; Zolotova, I. Review of IoT Sensor Systems Used for Monitoring the Road Infrastructure. Sensors 2023, 23, 4469. [Google Scholar] [CrossRef] [PubMed]
- Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012. [Google Scholar] [CrossRef]
- Massari, L.; Fransvea, G.; D’Abbraccio, J.; Filosa, M.; Terruso, G.; Aliperta, A.; D’Alesio, G.; Zaltieri, M.; Schena, E.; Palermo, E.; et al. Functional mimicry of Ruffini receptors with fibre Bragg gratings and deep neural networks enables a bio-inspired large-area tactile-sensitive skin. Nat. Mach. Intell. 2022, 4, 425–435. [Google Scholar] [CrossRef]
- Kalasin, S.; Sangnuang, P.; Surareungchai, W. Intelligent Wearable Sensors Interconnected with Advanced Wound Dressing Bandages for Contactless Chronic Skin Monitoring: Artificial Intelligence for Predicting Tissue Regeneration. Anal. Chem. 2022, 94, 6842–6852. [Google Scholar] [CrossRef]
- Yong, U.; Kim, D.; Kim, H.; Hwang, D.G.; Cho, S.; Nam, H.; Kim, S.; Kim, T.; Jeong, U.; Kim, K.; et al. Biohybrid 3D Printing of a Tissue-Sensor Platform for Wireless, Real-Time, and Continuous Monitoring of Drug-Induced Cardiotoxicity. Adv. Mater. 2023, 35, 2208983. [Google Scholar] [CrossRef]
- Pinho, T.S.; Cunha, C.B.; Lanceros-Méndez, S.; Salgado, A.J. Electroactive Smart Materials for Neural Tissue Regeneration. ACS Appl. Bio Mater. 2021, 4, 6604–6618. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R.; Lim, H.R.; Rigo, B.; Yeo, W.H. Fully implantable wireless batteryless vascular electronics with printed soft sensors for multiplex sensing of hemodynamics. Sci. Adv. 2022, 8, eabm1175. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Lou, D.; Li, S.; Wang, G.; Qiao, B.; Dong, S.; Ma, L.; Gao, C.; Wu, Z. Smart Flexible Electronics-Integrated Wound Dressing for Real-Time Monitoring and On-Demand Treatment of Infected Wounds. Adv. Sci. 2020, 7, 1902673. [Google Scholar] [CrossRef] [PubMed]
- Eskilson, O.; Zattarin, E.; Berglund, L.; Oksman, K.; Hanna, K.; Rakar, J.; Sivler, P.; Skog, M.; Rinklake, I.; Shamasha, R.; et al. Nanocellulose composite wound dressings for real-time pH wound monitoring. Mater. Today Bio 2023, 19, 100574. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Elsherif, M.; AlQattan, B.; Salih, A.; Lee, S.M.; Yetisen, A.K.; Park, S.; Butt, H. 3D Printed Contact Lenses. ACS Biomater. Sci. Eng. 2021, 7, 794–803. [Google Scholar] [CrossRef]
- Tavares, C.; Leitao, C.; Lo Presti, D.; Domingues, M.F.; Alberto, N.; Silva, H.; Antunes, P. Respiratory and heart rate monitoring using an FBG 3D-printed wearable system. Biomed. Opt. Express 2022, 13, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Zafaripour, G.; Yazdchi, M.; Alizadeh, A.a.; Ghadiri Nejad, M.; Abasi Dehkordi, D.; Semirumi, D.T. Fabrication and evaluation of 3D bio-scaffold wound dressings for monitoring of chronic pH wounds using fuzzy logic analysis. Mater. Sci. Eng. B 2023, 294, 116542. [Google Scholar] [CrossRef]
- Jørgensen, L.B.; Halekoh, U.; Jemec, G.B.E.; Sørensen, J.A.; Yderstræde, K.B. Monitoring Wound Healing of Diabetic Foot Ulcers Using Two-Dimensional and Three-Dimensional Wound Measurement Techniques: A Prospective Cohort Study. Adv. Wound Care 2020, 9, 553–563. [Google Scholar] [CrossRef]
- Tsegay, F.; Hisham, M.; Elsherif, M.; Schiffer, A.; Butt, H. 3D Printing of pH Indicator Auxetic Hydrogel Skin Wound Dressing. Molecules 2023, 28, 1339. [Google Scholar] [CrossRef]
- Cramer, M.N.; Gagnon, D.; Laitano, O.; Crandall, C.G. Human temperature regulation under heat stress in health, disease, and injury. Physiol. Rev. 2022, 102, 1907–1989. [Google Scholar] [CrossRef]
- Frykberg, R.G. Topical Wound Oxygen Therapy in the Treatment of Chronic Diabetic Foot Ulcers. Medicina 2021, 57, 917. [Google Scholar] [CrossRef] [PubMed]
- Roussakis, E.; Ortines, R.V.; Pinsker, B.L.; Mooers, C.T.; Evans, C.L.; Miller, L.S.; Calderón-Colón, X. Theranostic biocomposite scaffold membrane. Biomaterials 2019, 212, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Dreifke, M.B.; Jayasuriya, A.A.; Jayasuriya, A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 651–662. [Google Scholar] [CrossRef] [PubMed]
- El Saboni, Y.; Hunt, J.A.; Stanley, J.; Moffatt, C.; Wei, Y. Development of a textile based protein sensor for monitoring the healing progress of a wound. Sci. Rep. 2022, 12, 7972. [Google Scholar] [CrossRef]
- Anitha Pavithran, A.; Ramamoorthy, L.; Bs, S.; Murugesan, R.; Mj, K. Comparison of Fingertip vs Palm Site Sampling on Pain Perception, and Variation in Capillary Blood Glucose Level among Patients with Diabetes Mellitus. J. Caring Sci. 2020, 9, 182–187. [Google Scholar] [PubMed]
- Knapp, P.E.; Showers, K.M.; Phipps, J.C.; Speckman, J.L.; Sternthal, E.; Freund, K.M.; Ash, A.S.; Apovian, C.M. Self-monitoring of blood glucose with finger tip versus alternative site sampling: Effect on glycemic control in insulin-using patients with type 2 diabetes. Diabetes Technol. Ther. 2009, 11, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Q.; Luo, X.; Yang, L.; Cui, Y. Continuous monitoring of diabetes with an integrated microneedle biosensing device through 3D printing. Microsyst. Nanoeng. 2021, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.K.; Park, S.A.; Sim, D.S.; Jeong, M.H.; Lee, D.W. 3D-Printed Biodegradable Polymeric Stent Integrated with A Battery-Less Pressure Sensor for Biomedical Applications. In Proceedings of the 19th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), Kaohsiung, Taiwan, 18–22 June 2017; pp. 47–50. [Google Scholar]
- Polley, C.; Distler, T.; Detsch, R.; Lund, H.; Springer, A.; Boccaccini, A.R.; Seitz, H. 3D Printing of Piezoelectric Barium Titanate-Hydroxyapatite Scaffolds with Interconnected Porosity for Bone Tissue Engineering. Materials 2020, 13, 1773. [Google Scholar] [CrossRef]
- Ozgok Kangal, M.K.; Regan, J.P. Wound Healing; StatPearls, StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Song, J.W.; Ryu, H.; Bai, W.; Xie, Z.; Vázquez-Guardado, A.; Nandoliya, K.; Avila, R.; Lee, G.; Song, Z.; Kim, J.; et al. Bioresorbable, wireless, and battery-free system for electrotherapy and impedance sensing at wound sites. Sci. Adv. 2023, 9, eade4687. [Google Scholar] [CrossRef]
- Jiang, Y.; Trotsyuk, A.A.; Niu, S.; Henn, D.; Chen, K.; Shih, C.C.; Larson, M.R.; Mermin-Bunnell, A.M.; Mittal, S.; Lai, J.C.; et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 2023, 41, 652–662. [Google Scholar] [CrossRef]
- Phan, H.P. Implanted Flexible Electronics: Set Device Lifetime with Smart Nanomaterials. Micromachines 2021, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Adv. Health Mater. 2017, 6, 1700718. [Google Scholar] [CrossRef] [PubMed]
- Mirani, B.; Hadisi, Z.; Pagan, E.; Dabiri, S.M.H.; van Rijt, A.; Almutairi, L.; Noshadi, I.; Armstrong, D.G.; Akbari, M. Smart Dual-Sensor Wound Dressing for Monitoring Cutaneous Wounds. Adv. Health Mater. 2023, 12, e2203233. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Xiao, L.; Liu, Q.; Kwon, S.Y.; Zhang, Y.; Sharma, P.R.; Jin, L.; Li, X.; Xu, B. 3D Printed Microheater Sensor-Integrated, Drug-Encapsulated Microneedle Patch System for Pain Management. Adv. Health Mater. 2019, 8, e1901170. [Google Scholar] [CrossRef] [PubMed]
- Keum, D.H.; Kim, S.K.; Koo, J.; Lee, G.H.; Jeon, C.; Mok, J.W.; Mun, B.H.; Lee, K.J.; Kamrani, E.; Joo, C.K.; et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 2020, 6, eaba3252. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, J.X.; Lin, M.X.; Xiao, S.; Liu, H.; Zhou, P. Ionic flexible force sensors and their potential applications. J. Mater. Chem. C 2021, 9, 16378–16390. [Google Scholar] [CrossRef]
- Gholami, M.; Napier, C.; Patino, A.G.; Cuthbert, T.J.; Menon, C. Fatigue Monitoring in Running Using Flexible Textile Wearable Sensors. Sensors 2020, 20, 5573. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Guo, C.; Li, H.; Yang, H.; Xiong, F.; Chen, D. The Progress of Research into Flexible Sensors in the Field of Smart Wearables. Sensors 2022, 22, 5089. [Google Scholar] [CrossRef]
- Ma, J.; Wang, P.; Chen, H.; Bao, S.; Chen, W.; Lu, H. Highly Sensitive and Large-Range Strain Sensor with a Self-Compensated Two-Order Structure for Human Motion Detection. ACS Appl. Mater. Interfaces 2019, 11, 8527–8536. [Google Scholar] [CrossRef]
- Bernasconi, R.; Pizzetti, F.; Rossetti, A.; Butler, B.; Levi, M.; Pane, S.; Rossi, F.; Magagnin, L. Layer-by-Layer Fabrication of Hydrogel Microsystems for Controlled Drug Delivery from Untethered Microrobots. Front. Bioeng. Biotechnol. 2021, 9, 692648. [Google Scholar] [CrossRef]
- Martins, A.; Fonseca, I.; Farinha, J.T.; Reis, J.; Cardoso, A.J.M. Online Monitoring of Sensor Calibration Status to Support Condition-Based Maintenance. Sensors 2023, 23, 2402. [Google Scholar] [CrossRef] [PubMed]
- Sawayama, J.; Okitsu, T.; Nakamata, A.; Kawahara, Y.; Takeuchi, S. Hydrogel Glucose Sensor with In Vivo Stable Fluorescence Intensity Relying on Antioxidant Enzymes for Continuous Glucose Monitoring. iScience 2020, 23, 101243. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Sun, S.; Wang, X.; Shi, K.; Wang, Z.; Ma, X.; Zhang, W.; Bao, G.; Tian, Y.; Zhang, Z.; et al. Nondestructive identification of softness via bioinspired multisensory electronic skins integrated on a robotic hand. NPJ Flex. Electron. 2022, 6, 45. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, S.; Mei, D.; Li, J.; Zhang, J.; Yang, S.; Guan, S. Application of 3D Printing Technology in Bone Tissue Engineering: A Review. Curr. Drug Deliv. 2021, 18, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Tamay, D.G.; Dursun Usal, T.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Afzali Naniz, M.; Askari, M.; Zolfagharian, A.; Afzali Naniz, M.; Bodaghi, M. 4D printing: A cutting-edge platform for biomedical applications. Biomed. Mater. 2022, 17, 062001. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. Additive manufacturing for 4D applications. In 4D Printing; Singh, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. xiii–xxviii. [Google Scholar]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2020, 101, 26–42. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Y.; Wang, M. In situ delivery of rhBMP-2 in surface porous shape memory scaffolds developed through cryogenic 3D plotting. Mater. Lett. 2017, 189, 140–143. [Google Scholar] [CrossRef]
- Tang, Y.; Dai, B.; Su, B.; Shi, Y. Recent Advances of 4D Printing Technologies Toward Soft Tactile Sensors. Front. Mater. 2021, 8, 658046. [Google Scholar] [CrossRef]
- Lai, J.; Li, J.; Wang, M. 3D Printed porous tissue engineering scaffolds with the self-folding ability and controlled release of growth factor. MRS Commun. 2020, 10, 579–586. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, W.; Castro, N.J.; Nowicki, M.; Zhou, X.; Cui, H.; Fisher, J.P.; Zhang, L.G. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 2016, 6, 27226. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Wu, C.; He, Y.; Lu, F. Skin-associated adipocytes in skin barrier immunity: A mini-review. Front. Immunol. 2023, 14, 1116548. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Michael, S.; Bharti, K.; Ferrer, M.; Song, M.J. A biofabricated vascularized skin model of atopic dermatitis for preclinical studies. Biofabrication 2020, 12, 035002. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, S.; Vadnere, G.P. Relevance and Perspectives of Experimental Wound Models in Wound Healing Research. Asian J. Pharm. Clin. Res. 2017, 10, 57–62. [Google Scholar] [CrossRef][Green Version]
- Abaci, H.E.; Guo, Z.; Doucet, Y.; Jackow, J.; Christiano, A. Next generation human skin constructs as advanced tools for drug development. Exp. Biol. Med. 2017, 242, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Lukács, B.; Bajza, Á.; Kocsis, D.; Csorba, A.; Antal, I.; Iván, K.; Laki, A.J.; Erdő, F. Skin-on-a-Chip Device for Ex Vivo Monitoring of Transdermal Delivery of Drugs-Design, Fabrication, and Testing. Pharmaceutics 2019, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ma, H.; Xu, F.; Wang, X.; Sun, W. Microfluidics in cardiovascular disease research: State of the art and future outlook. Microsyst. Nanoeng. 2021, 7, 19. [Google Scholar] [CrossRef]
- Sun, W.; Luo, Z.; Lee, J.; Kim, H.J.; Lee, K.; Tebon, P.; Feng, Y.; Dokmeci, M.R.; Sengupta, S.; Khademhosseini, A. Organ-on-a-Chip for Cancer and Immune Organs Modeling. Adv. Health Mater. 2019, 8, e1801363. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanizamani, F.; Moulahoum, H.; Guler Celik, E.; Timur, S. Material Design in Implantable Biosensors toward Future Personalized Diagnostics and Treatments. Appl. Sci. 2023, 13, 4630. [Google Scholar] [CrossRef]
- Zhao, P.; Dang, Z.; Liu, M.; Guo, D.; Luo, R.; Zhang, M.; Xie, F.; Zhang, X.; Wang, Y.; Pan, S.; et al. Molecular hydrogen promotes wound healing by inducing early epidermal stem cell proliferation and extracellular matrix deposition. Inflamm. Regen. 2023, 43, 22. [Google Scholar] [CrossRef]

| Type | Biomaterials | Crosslinking | 3D Printing Methods | Refs. |
|---|---|---|---|---|
| Animal-sourced natural ECM materials | Collagen | Thermal | Extrusion-based, droplet-based, laser-assisted | [64,65,66] |
| Fibrinogen | Enzymatic | Extrusion-based, droplet-based | [67] | |
| Hyaluronan | Photic, enzymatic | Extrusion-based | [68] | |
| Decellularized extracellular matrix (dECM) | Photic, thermal, pH | Extrusion-based, droplet-based | [69] | |
| Gelatin | Photic, thermal, enzymatic, ionic | Extrusion-based, droplet-based, stereolithography-based | [70] | |
| Non-animal-derived natural hydrogels | Cellulose | Photic, thermal, enzymatic, ionic, pH | Extrusion-based, droplet-based | [71,72] |
| Alginate | Photic, thermal, ionic | Extrusion-based, droplet-based, laser-assisted | [73] | |
| Chitosan | Thermal, enzymatic, ionic, pH | Extrusion-based | [74] | |
| Agarose | Thermal | Extrusion-based, droplet-based | [59,75] | |
| Carrageenan | Photic, thermal, ionic | Extrusion-based | [76,77] | |
| Synthetic hydrogels | Polyethylene glycol (PEG) | Photic | Extrusion-based, droplet-based, stereolithography-based | [59,78] |
| Polyurethane (PU) | Thermal | Extrusion-based | [79,80] | |
| Polyvinyl alcohol (PVA) | Chemic, ionic | Extrusion-based | [81,82] | |
| Polylactic acid (PLA) | Thermal | Extrusion-based | [83] | |
| Pluronic F127 (PF127) | Thermal | Extrusion-based | [84] |
| Advantages | Limitations |
|---|---|
| High individualization, flexibility, and repeatability in manufacturing | Difficulty in selecting biocompatible materials |
| BN provides real-time monitoring and active wound care treatment with minimal physician intervention at wound sites | Biosafety: ethical issues and electronic reagents |
| Better regenerative effects | High costs and long healing times |
| Enables large-scale fabrication | Difficulty in remodeling blood vessels and nerve tissues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Chen, J.; Zhao, P.; Liu, M.; Xie, F.; Ma, X. Development and Prospective Applications of 3D Membranes as a Sensor for Monitoring and Inducing Tissue Regeneration. Membranes 2023, 13, 802. https://doi.org/10.3390/membranes13090802
Wu H, Chen J, Zhao P, Liu M, Xie F, Ma X. Development and Prospective Applications of 3D Membranes as a Sensor for Monitoring and Inducing Tissue Regeneration. Membranes. 2023; 13(9):802. https://doi.org/10.3390/membranes13090802
Chicago/Turabian StyleWu, Hanning, Jiawen Chen, Pengxiang Zhao, Mengyu Liu, Fei Xie, and Xuemei Ma. 2023. "Development and Prospective Applications of 3D Membranes as a Sensor for Monitoring and Inducing Tissue Regeneration" Membranes 13, no. 9: 802. https://doi.org/10.3390/membranes13090802
APA StyleWu, H., Chen, J., Zhao, P., Liu, M., Xie, F., & Ma, X. (2023). Development and Prospective Applications of 3D Membranes as a Sensor for Monitoring and Inducing Tissue Regeneration. Membranes, 13(9), 802. https://doi.org/10.3390/membranes13090802






