Affinity and Pseudo-Affinity Membrane Chromatography for Viral Vector and Vaccine Purifications: A Review
Abstract
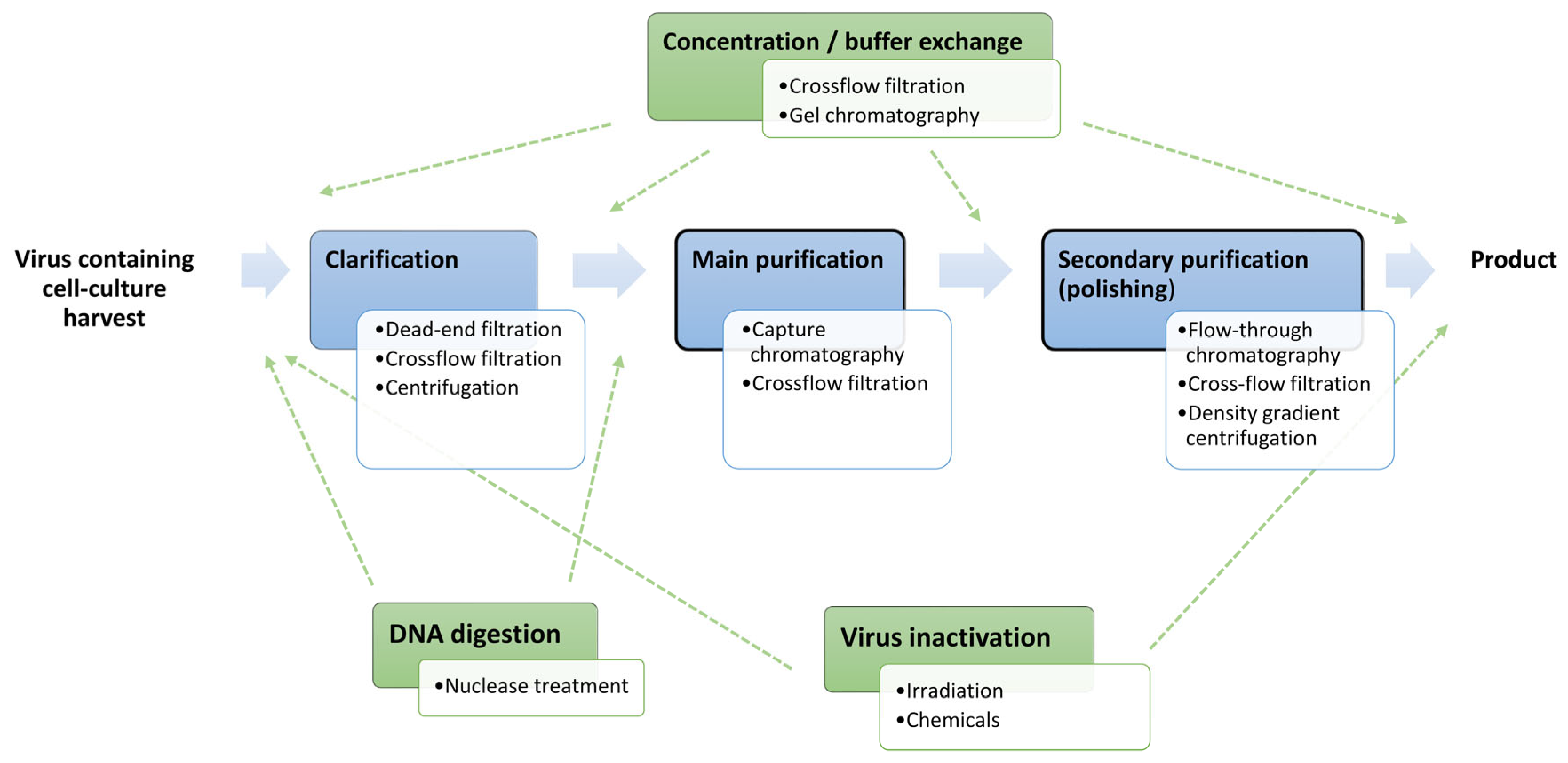
1. Introduction
2. Development of Affinity Purifications for Viruses and VLPs and Currently Established Applications
3. Affinity and Pseudo-Affinity Membranes
| Target | Ligand/Membrane Material | Commercially Available | Processed Feed Volume (Scale) | Yield/Recovery | Impurity Depletion | Reference | |
|---|---|---|---|---|---|---|---|
| [Yes or No] | [mL] | [%] | Protein [%] 1 | DNA | |||
| Adenovirus | Zn2+/cellulose | Yes (Sartorius) | 200 | 87 | <25 pg mL−1 | <0.3 ng mL−1 | [110] |
| Hepatitis C virus | Sulfated cellulose/cellulose | Yes (Sartorius) | 10 | 50 | Not determined | 78% | [21] 2 |
| Influenza A virus | Euonymus europaeusLectin/cellulose | No | 20 | 108 | 69% | 99% | [112] |
| Zn2+/cellulose | Yes (Sartorius) | 50 | 64 | 93% | 74% | [111] 3 | |
| Sulfated cellulose/cellulose | Yes (Sartorius) | 10 | 73–94 | 57–84% | 68–99% | [113] 4 | |
| Sulfated cellulose/cellulose | Yes (Sartorius) | 70 | 80 | 71% | 97.5% | [107] | |
| Sulfated cellulose/cellulose | Yes (Sartorius) | <10 | 57 | 1.2 ± 6 0.02 ngprot HAU−1 | 5.1 ± 0.2 pgDNA HAU−1 | [115] 5 | |
| Sulfated cellulose/cellulose | Yes (Sartorius) | <10 | 64 | 0.013 mgprot µgHA-1 | 0.0038 µgDNA µgHA−1 | [42] | |
| Sulfated cellulose/cellulose | Yes (Sartorius) | 10 per cycle 6 | 67.4 | 67.4 | 99.8 | [118] | |
| Influenza VLPs | Sulfated cellulose/cellulose | Yes (Sartorius) | <10 | 80 | 89% | 80% | [42] |
| Orf virus | Sulfated cellulose/cellulose | Yes (Sartorius) | 10 | 34–54 | >99% | 20–95% | [108] 7 |
| Vaccinia virus/Modified Vaccinia Ankara virus | Heparin/cellulose | No | <10 | 56 | 99% | 76% | [91] |
| Sulfated cellulose/cellulose | Yes (Sartorius) | 20 | 65 | 99% | 90% | [91] | |
| Heparin/cellulose | No | <10 | 68 | 99.9% | 80% | [96] | |
| Sulfated cellulose/cellulose | Yes (Sartorius) | <10 | 75 | 99.9% | 95% | [96] | |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penaud-Budloo, M.; Francois, A.; Clement, N.; Ayuso, E. Pharmacology of Recombinant Adeno-associated Virus Production. Mol. Ther. Methods Clin. Dev. 2018, 8, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Schnodt, M.; Buning, H. Improving the Quality of Adeno-Associated Viral Vector Preparations: The Challenge of Product-Related Impurities. Hum. Gene Ther. Methods 2017, 28, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Nestola, P.; Peixoto, C.; Silva, R.R.J.S.; Alves, P.M.; Mota, J.P.B.; Carrondo, M.J.T. Improved virus purification processes for vaccines and gene therapy. Biotechnol. Bioeng. 2015, 112, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, D.; Dieken, H.; Loewe, D.; Grein, T.A.; Salzig, D.; Czermak, P. Purification of oncolytic measles virus by cation-exchange chromatography using resin-based stationary phases. Sep. Sci. Technol. 2021, 57, 886–896. [Google Scholar] [CrossRef]
- Reiter, K.; Pereira Aguilar, P.; Grammelhofer, D.; Joseph, J.; Steppert, P.; Jungbauer, A. Separation of influenza virus-like particles from baculovirus by polymer-grafted anion exchanger. J. Sep. Sci. 2020, 43, 2270–2278. [Google Scholar] [CrossRef]
- Pato, T.P.; Souza, M.C.O.; Mattos, D.A.; Caride, E.; Ferreira, D.F.; Gaspar, L.P.; Freire, M.S.; Castilho, L.R. Purification of yellow fever virus produced in Vero cells for inactivated vaccine manufacture. Vaccine 2019, 37, 3214–3220. [Google Scholar] [CrossRef]
- Nestola, P.; Peixoto, C.; Villain, L.; Alves, P.M.; Carrondo, M.J.T.; Mota, J.P.B. Rational development of two flowthrough purification strategies for adenovirus type 5 and retro virus-like particles. J. Chromatogr. A 2015, 1426, 91–101. [Google Scholar] [CrossRef]
- Turnbull, J.; Wright, B.; Green, N.K.; Tarrant, R.; Roberts, I.; Hardick, O.; Bracewell, D.G. Adenovirus 5 recovery using nanofiber ion-exchange adsorbents. Biotechnol. Bioeng. 2019, 116, 1698–1709. [Google Scholar] [CrossRef]
- Mendes, J.P.; Silva, R.J.S.; Berg, M.; Mathiasson, L.; Peixoto, C.; Alves, P.M.; Carrondo, M.J.T. Oncolytic virus purification with periodic counter-current chromatography. Biotechnol. Bioeng. 2021, 118, 3522–3532. [Google Scholar] [CrossRef]
- Silva, R.J.S.; Mendes, J.P.; Carrondo, M.J.T.; Marques, P.M.; Peixoto, C. Continuous Chromatography Purification of Virus-Based Biopharmaceuticals: A Shortcut Design Method. Methods Mol. Biol. 2020, 2095, 367–384. [Google Scholar] [CrossRef]
- Grein, T.A.; Michalsky, R.; Vega Lopez, M.; Czermak, P. Purification of a recombinant baculovirus of Autographa californica M nucleopolyhedrovirus by ion exchange membrane chromatography. J. Virol. Methods 2012, 183, 117–124. [Google Scholar] [CrossRef]
- Sviben, D.; Forcic, D.; Ivancic-Jelecki, J.; Halassy, B.; Brgles, M. Recovery of infective virus particles in ion-exchange and hydrophobic interaction monolith chromatography is influenced by particle charge and total-to-infective particle ratio. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2017, 1054, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Y.; Zhang, Y.; Zhang, S.; Zhao, Q.; Zhu, Y.; Zou, X.; Yu, M.; Ma, G.; Su, Z. A hydrophobic interaction chromatography strategy for purification of inactivated foot-and-mouth disease virus. Protein Expr. Purif. 2015, 113, 23–29. [Google Scholar] [CrossRef]
- McNally, D.J.; Piras, B.A.; Willis, C.M.; Lockey, T.D.; Meagher, M.M. Development and Optimization of a Hydrophobic Interaction Chromatography-Based Method of AAV Harvest, Capture, and Recovery. Mol. Ther. Methods Clin. Dev. 2020, 19, 275–284. [Google Scholar] [CrossRef]
- Burden, C.S.; Jin, J.; Podgornik, A.; Bracewell, D.G. A monolith purification process for virus-like particles from yeast homogenate. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2012, 880, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Hossienizadeh, S.M.J.; Bagheri, M.; Alizadeh, M.; Rahimi, M.; Azimi, S.M.; Kamalzade, M.; Es-Haghi, A.; Ghassempour, A. Two Dimensional Anion Exchange-Size Exclusion Chromatography Combined with Mathematical Modeling for Downstream Processing of Foot and Mouth Disease Vaccine. J. Chromatogr. A 2021, 1643, 462070. [Google Scholar] [CrossRef]
- Strobel, B.; Miller, F.D.; Rist, W.; Lamla, T. Comparative Analysis of Cesium Chloride- and Iodixanol-Based Purification of Recombinant Adeno-Associated Viral Vectors for Preclinical Applications. Hum. Gene Ther. Methods 2015, 26, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Nestola, P.; Silva, R.J.; Peixoto, C.; Alves, P.M.; Carrondo, M.J.; Mota, J.P. Adenovirus purification by two-column, size-exclusion, simulated countercurrent chromatography. J. Chromatogr. A 2014, 1347, 111–121. [Google Scholar] [CrossRef]
- Transfiguracion, J.; Jaalouk, D.E.; Ghani, K.; Galipeau, J.; Kamen, A. Size-exclusion chromatography purification of high-titer vesicular stomatitis virus G glycoprotein-pseudotyped retrovectors for cell and gene therapy applications. Hum. Gene Ther. 2003, 14, 1139–1153. [Google Scholar] [CrossRef]
- Lothert, K.; Pagallies, F.; Eilts, F.; Sivanesapillai, A.; Hardt, M.; Moebus, A.; Feger, T.; Amann, R.; Wolff, M.W. A scalable downstream process for the purification of the cell culture-derived Orf virus for human or veterinary applications. J. Biotechnol. 2020, 323, 221–230. [Google Scholar] [CrossRef]
- Lothert, K.; Offersgaard, A.F.; Pihl, A.F.; Mathiesen, C.K.; Jensen, T.B.; Alzua, G.P.; Fahnoe, U.; Bukh, J.; Gottwein, J.M.; Wolff, M.W. Development of a downstream process for the production of an inactivated whole hepatitis C virus vaccine. Sci. Rep. 2020, 10, 16261. [Google Scholar] [CrossRef]
- Labisch, J.J.; Kassar, M.; Bollmann, F.; Valentic, A.; Hubbuch, J.; Pflanz, K. Steric exclusion chromatography of lentiviral vectors using hydrophilic cellulose membranes. J. Chromatogr. A 2022, 1674, 463148. [Google Scholar] [CrossRef] [PubMed]
- Labisch, J.J.; Wiese, G.P.; Pflanz, K. Steric Exclusion Chromatography for Purification of Biomolecules—A Review. Separations 2023, 10, 183. [Google Scholar] [CrossRef]
- Zhao, M.; Vandersluis, M.; Stout, J.; Haupts, U.; Sanders, M.; Jacquemart, R. Affinity chromatography for vaccines manufacturing: Finally ready for prime time? Vaccine 2019, 37, 5491–5503. [Google Scholar] [CrossRef]
- Junter, G.A.; Lebrun, L. Polysaccharide-based chromatographic adsorbents for virus purification and viral clearance. J. Pharm. Anal. 2020, 10, 291–312. [Google Scholar] [CrossRef] [PubMed]
- James, K.T.; Cooney, B.; Agopsowicz, K.; Trevors, M.A.; Mohamed, A.; Stoltz, D.; Hitt, M.; Shmulevitz, M. Novel High-throughput Approach for Purification of Infectious Virions. Sci. Rep. 2016, 6, 36826. [Google Scholar] [CrossRef] [PubMed]
- Lothert, K.; Harsy, Y.M.J.; Endres, P.; Müller, E.; Wolff, M.W. Evaluation of restricted access media for the purification of cell culture-derived Orf viruses. Eng. Life Sci. 2023, 2300009. [Google Scholar] [CrossRef]
- Steppert, P.; Mosor, M.; Stanek, L.; Burgstaller, D.; Palmberger, D.; Preinsperger, S.; Pereira Aguilar, P.; Mullner, M.; Csar, P.; Jungbauer, A. A scalable, integrated downstream process for production of a recombinant measles virus-vectored vaccine. Vaccine 2022, 40, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Lavado-Garcia, J.; Gonzalez-Dominguez, I.; Cervera, L.; Jorge, I.; Vazquez, J.; Godia, F. Molecular Characterization of the Coproduced Extracellular Vesicles in HEK293 during Virus-Like Particle Production. J. Proteome Res. 2020, 19, 4516–4532. [Google Scholar] [CrossRef]
- Lavado-Garcia, J.; Diaz-Maneh, A.; Canal-Pauli, N.; Perez-Rubio, P.; Godia, F.; Cervera, L. Metabolic engineering of HEK293 cells to improve transient transfection and cell budding of HIV-1 virus-like particles. Biotechnol. Bioeng. 2021, 118, 1649–1663. [Google Scholar] [CrossRef]
- Federico, M. From virus-like particles to engineered exosomes for a new generation of vaccines. Future Virol. 2012, 7, 473–482. [Google Scholar] [CrossRef]
- Steppert, P.; Burgstaller, D.; Klausberger, M.; Berger, E.; Aguilar, P.P.; Schneider, T.A.; Kramberger, P.; Tover, A.; Nobauer, K.; Razzazi-Fazeli, E.; et al. Purification of HIV-1 gag virus-like particles and separation of other extracellular particles. J. Chromatogr. A 2016, 1455, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Venereo-Sánchez, A.; Fulton, K.; Koczka, K.; Twine, S.; Chahal, P.; Ansorge, S.; Gilbert, R.; Henry, O.; Kamen, A. Characterization of influenza H1N1 Gag virus-like particles and extracellular vesicles co-produced in HEK-293SF. Vaccine 2019, 37, 7100–7107. [Google Scholar] [CrossRef]
- Lorenzo, E.; Miranda, L.; Godia, F.; Cervera, L. Downstream process design for Gag HIV-1 based virus-like particles. Biotechnol. Bioeng. 2023, 120, 2672–2684. [Google Scholar] [CrossRef]
- Burgess, R.R. A brief practical review of size exclusion chromatography: Rules of thumb, limitations, and troubleshooting. Protein Expr. Purif. 2018, 150, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Effio, C.L.; Hubbuch, J. Next generation vaccines and vectors: Designing downstream processes for recombinant protein-based virus-like particles. Biotechnol. J. 2015, 10, 715–727. [Google Scholar] [CrossRef]
- Vicente, T.; Mota, J.P.; Peixoto, C.; Alves, P.M.; Carrondo, M.J. Rational design and optimization of downstream processes of virus particles for biopharmaceutical applications: Current advances. Biotechnol. Adv. 2011, 29, 869–878. [Google Scholar] [CrossRef]
- Qu, W.; Wang, M.; Wu, Y.; Xu, R. Scalable downstream strategies for purification of recombinant adeno- associated virus vectors in light of the properties. Curr. Pharm. Biotechnol. 2015, 16, 684–695. [Google Scholar] [CrossRef]
- Singh, N.; Heldt, C.L. Challenges in downstream purification of gene therapy viral vectors. Curr. Opin. Chem. Eng. 2022, 35, 100780. [Google Scholar] [CrossRef]
- Mittal, M.; Banerjee, M.; Lua, L.H.L.; Rathore, A.S. Current status and future challenges in transitioning to continuous bioprocessing of virus-like particles. J. Chem. Technol. Biotechnol. 2021, 97, 2376–2385. [Google Scholar] [CrossRef]
- Moleirinho, M.G.; Silva, R.J.S.; Alves, P.M.; Carrondo, M.J.T.; Peixoto, C. Current challenges in biotherapeutic particles manufacturing. Expert. Opin. Biol. Ther. 2020, 20, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.B.; Fortuna, A.R.; Wolff, M.W.; Peixoto, C.; Alves, P.M.; Reichl, U.; Jt Carrondo, M. Purification of influenza virus-like particles using sulfated cellulose membrane adsorbers. J. Chem. Technol. Biot. 2018, 93, 1988–1996. [Google Scholar] [CrossRef]
- Loewe, D.; Dieken, H.; Grein, T.A.; Weidner, T.; Salzig, D.; Czermak, P. Opportunities to debottleneck the downstream processing of the oncolytic measles virus. Crit. Rev. Biotechnol. 2020, 40, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Kramberger, P.; Urbas, L.; Strancar, A. Downstream processing and chromatography based analytical methods for production of vaccines, gene therapy vectors, and bacteriophages. Hum. Vaccin. Immunother. 2015, 11, 1010–1021. [Google Scholar] [CrossRef]
- Lacki, K.M.; Riske, F.J. Affinity Chromatography: An Enabling Technology for Large-Scale Bioprocessing. Biotechnol. J. 2020, 15, e1800397. [Google Scholar] [CrossRef]
- Krajacic, M.; Ravnikar, M.; Strancar, A.; Gutierrez-Aguirre, I. Application of monolithic chromatographic supports in virus research. Electrophoresis 2017, 38, 2827–2836. [Google Scholar] [CrossRef] [PubMed]
- Podgornik, A.; Krajnc, N.L. Application of monoliths for bioparticle isolation. J. Sep. Sci. 2012, 35, 3059–3072. [Google Scholar] [CrossRef]
- Dimartino, S.; Herigstad, O.M.; Boi, C.; Lalli, E.; Sarti, G. Experimental and Theoretical Analysis to Assess the Use of Monolithic Columns in Process Chromatography. Chem. Eng. Trans. 2016, 49, 25–30. [Google Scholar] [CrossRef]
- Boi, C.; Malavasi, A.; Carbonell, R.G.; Gilleskie, G. A direct comparison between membrane adsorber and packed column chromatography performance. J. Chromatogr. A 2020, 1612, 460629. [Google Scholar] [CrossRef]
- Orr, V.; Zhong, L.; Moo-Young, M.; Chou, C.P. Recent advances in bioprocessing application of membrane chromatography. Biotechnol. Adv. 2013, 31, 450–465. [Google Scholar] [CrossRef]
- Muthukumar, S.; Muralikrishnan, T.; Mendhe, R.; Rathore, A.S. Economic benefits of membrane chromatography versus packed bed column purification of therapeutic proteins expressed in microbial and mammalian hosts. J. Chem. Technol. Biotechnol. 2017, 92, 59–68. [Google Scholar] [CrossRef]
- Rajamanickam, V.; Herwig, C.; Spadiut, O. Monoliths in Bioprocess Technology. Chromatography 2015, 2, 195–212. [Google Scholar] [CrossRef]
- Trilisky, E.I.; Lenhoff, A.M. Sorption processes in ion-exchange chromatography of viruses. J. Chromatogr. A 2007, 1142, 2–12. [Google Scholar] [CrossRef]
- Ghosh, R. Protein separation using membrane chromatography: Opportunities and challenges. J. Chromatogr. A 2002, 952, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Simons, J.; Hooson, S.; Abraham, D.; Carta, G. Protein and virus-like particle adsorption on perfusion chromatography media. J. Chromatogr. A 2013, 1297, 96–105. [Google Scholar] [CrossRef]
- Wu, Y.; Abraham, D.; Carta, G. Comparison of perfusion media and monoliths for protein and virus-like particle chromatography. J. Chromatogr. A 2016, 1447, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.; Rustandi, R.R.; Zheng, X.; Payne, A.; Shang, L. Applications of Surface Plasmon Resonance and Biolayer Interferometry for Virus-Ligand Binding. Viruses 2022, 14, 717. [Google Scholar] [CrossRef] [PubMed]
- Cairns, T.M.; Ditto, N.T.; Atanasiu, D.; Lou, H.; Brooks, B.D.; Saw, W.T.; Eisenberg, R.J.; Cohen, G.H. Surface Plasmon Resonance Reveals Direct Binding of Herpes Simplex Virus Glycoproteins gH/gL to gD and Locates a gH/gL Binding Site on gD. J. Virol. 2019, 93, e00289-19. [Google Scholar] [CrossRef]
- Sousa, I.T.; Taipa, M.A. Biomimetic Affinity Ligands for Protein Purification. Methods Mol. Biol. 2021, 2178, 167–199. [Google Scholar] [CrossRef]
- Lowen, A.C. Constraints, Drivers, and Implications of Influenza A Virus Reassortment. Annu. Rev. Virol. 2017, 4, 105–121. [Google Scholar] [CrossRef]
- Du, X.; King, A.A.; Woods, R.J.; Pascual, M. Evolution-informed forecasting of seasonal influenza A (H3N2). Sci. Transl. Med. 2017, 9, eaan5325. [Google Scholar] [CrossRef]
- Huddleston, J.; Barnes, J.R.; Rowe, T.; Xu, X.; Kondor, R.; Wentworth, D.E.; Whittaker, L.; Ermetal, B.; Daniels, R.S.; McCauley, J.W.; et al. Integrating genotypes and phenotypes improves long-term forecasts of seasonal influenza A/H3N2 evolution. eLife 2020, 9, e60067. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef]
- Mietzsch, M.; Smith, J.K.; Yu, J.C.; Banala, V.; Emmanuel, S.N.; Jose, A.; Chipman, P.; Bhattacharya, N.; McKenna, R.; Agbandje-McKenna, M. Characterization of AAV-Specific Affinity Ligands: Consequences for Vector Purification and Development Strategies. Mol. Ther. Methods Clin. Dev. 2020, 19, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Opitz, L.; Salaklang, J.; Buttner, H.; Reichl, U.; Wolff, M.W. Lectin-affinity chromatography for downstream processing of MDCK cell culture derived human influenza A viruses. Vaccine 2007, 25, 939–947. [Google Scholar] [CrossRef]
- Ruiz-May, E.; Catala, C.; Rose, J.K. N-glycoprotein enrichment by lectin affinity chromatography. Methods Mol. Biol. 2014, 1072, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Debray, H.; Montreuil, J. Lectin Affinity Chromatography of Glycoconjugates. In Advances in Lectin Research; Springer: Berlin/Heidelberg, Germany, 1991; pp. 51–96. [Google Scholar] [CrossRef]
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem. Rev. 2015, 115, 525–561. [Google Scholar] [CrossRef] [PubMed]
- Lavín de Juan, L.; García Recio, V.; Jiménez López, P.; Girbés Juan, T.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Pharmaceutical applications of lectins. J. Drug Deliv. Sci. Technol. 2017, 42, 126–133. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Weiss, A.A.; Iyer, S.S. Glycan-based high-affinity ligands for toxins and pathogen receptors. Med. Res. Rev. 2010, 30, 327–393. [Google Scholar] [CrossRef]
- Moreira, A.S.; Bezemer, S.; Faria, T.Q.; Detmers, F.; Hermans, P.; Sierkstra, L.; Coroadinha, A.S.; Peixoto, C. Implementation of Novel Affinity Ligand for Lentiviral Vector Purification. Int. J. Mol. Sci. 2023, 24, 3354. [Google Scholar] [CrossRef]
- Chen, N.; Kong, X.; Zhao, S.; Xiaofeng, W. Post-translational modification of baculovirus-encoded proteins. Virus Res. 2020, 279, 197865. [Google Scholar] [CrossRef]
- Florea, M.; Nicolaou, F.; Pacouret, S.; Zinn, E.M.; Sanmiguel, J.; Andres-Mateos, E.; Unzu, C.; Wagers, A.J.; Vandenberghe, L.H. High-efficiency purification of divergent AAV serotypes using AAVX affinity chromatography. Mol. Ther. Methods Clin. Dev. 2023, 28, 146–159. [Google Scholar] [CrossRef]
- Adams, B.; Bak, H.; Tustian, A.D. Moving from the bench towards a large scale, industrial platform process for adeno-associated viral vector purification. Biotechnol. Bioeng. 2020, 117, 3199–3211. [Google Scholar] [CrossRef]
- Nguyen, L.; McCord, K.A.; Bui, D.T.; Bouwman, K.M.; Kitova, E.N.; Elaish, M.; Kumawat, D.; Daskhan, G.C.; Tomris, I.; Han, L.; et al. Sialic acid-containing glycolipids mediate binding and viral entry of SARS-CoV-2. Nat. Chem. Biol. 2022, 18, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Agelidis, A.M.; Shukla, D. Cell entry mechanisms of HSV: What we have learned in recent years. Future Virol. 2015, 10, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Revol, R.; Ostbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef]
- Laureti, M.; Narayanan, D.; Rodriguez-Andres, J.; Fazakerley, J.K.; Kedzierski, L. Flavivirus Receptors: Diversity, Identity, and Cell Entry. Front. Immunol. 2018, 9, 2180. [Google Scholar] [CrossRef]
- Yin, X.; Ambardekar, C.; Lu, Y.; Feng, Z. Distinct Entry Mechanisms for Nonenveloped and Quasi-Enveloped Hepatitis E Viruses. J. Virol. 2016, 90, 4232–4242. [Google Scholar] [CrossRef]
- Koehler, M.; Delguste, M.; Sieben, C.; Gillet, L.; Alsteens, D. Initial Step of Virus Entry: Virion Binding to Cell-Surface Glycans. Annu. Rev. Virol. 2020, 7, 143–165. [Google Scholar] [CrossRef]
- Pronkin, P.G.; Tatikolov, A.S. Polymethine Dyes as Probes for Detecting the SARS-COV-2 Coronavirus: In silico Molecular Docking Study. Russ. J. Phys. Chem. B 2021, 15, 25–32. [Google Scholar] [CrossRef]
- Liu, J.; Thorp, S.C. Cell surface heparan sulfate and its roles in assisting viral infections. Med. Res. Rev. 2002, 22, 1–25. [Google Scholar] [CrossRef]
- Eilts, F.; Bauer, S.; Fraser, K.; Dordick, J.S.; Wolff, M.W.; Linhardt, R.J.; Zhang, F. The diverse role of heparan sulfate and other GAGs in SARS-CoV-2 infections and therapeutics. Carbohydr. Polym. 2023, 299, 120167. [Google Scholar] [CrossRef]
- Rabenstein, D.L. Heparin and heparan sulfate: Structure and function. Nat. Prod. Rep. 2002, 19, 312–331. [Google Scholar] [CrossRef]
- Shriver, Z.; Capila, I.; Venkataraman, G.; Sasisekharan, R. Heparin and heparan sulfate: Analyzing structure and microheterogeneity. In Heparin—A Century of Progress; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 207, pp. 159–176. [Google Scholar] [CrossRef]
- Hao, C.; Xu, H.; Yu, L.; Zhang, L. Heparin: An essential drug for modern medicine. Prog. Mol. Biol. Transl. Sci. 2019, 163, 1–19. [Google Scholar] [CrossRef]
- Nasimuzzaman, M.; Lynn, D.; van der Loo, J.C.; Malik, P. Purification of baculovirus vectors using heparin affinity chromatography. Mol. Ther. Methods Clin. Dev. 2016, 3, 16071. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.M.; Kamen, A.; Trudel, P.; Garnier, A. A novel purification strategy for retrovirus gene therapy vectors using heparin affinity chromatography. Biotechnol. Bioeng. 2005, 90, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, J.M.; Kim, A.Y.; Park, S.H.; Kim, J.S.; Kim, H.; Park, J.W.; Park, J.H.; Ko, Y.J.; Park, C.K. Application of Heparin Affinity Chromatography to Produce a Differential Vaccine without Eliciting Antibodies against the Nonstructural Proteins of the Serotype O Foot-and-Mouth Disease Viruses. Viruses 2020, 12, 1405. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.W.; Siewert, C.; Lehmann, S.; Hansen, S.P.; Djurup, R.; Faber, R.; Reichl, U. Capturing of cell culture-derived modified Vaccinia Ankara virus by ion exchange and pseudo-affinity membrane adsorbers. Biotechnol. Bioeng. 2010, 105, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Reiter, K.; Aguilar, P.P.; Wetter, V.; Steppert, P.; Tover, A.; Jungbauer, A. Separation of virus-like particles and extracellular vesicles by flow-through and heparin affinity chromatography. J. Chromatogr. A 2019, 1588, 77–84. [Google Scholar] [CrossRef]
- Aguilar, P.P.; Reiter, K.; Wetter, V.; Steppert, P.; Maresch, D.; Ling, W.L.; Satzer, P.; Jungbauer, A. Capture and purification of Human Immunodeficiency Virus-1 virus-like particles: Convective media vs porous beads. J. Chromatogr. A 2020, 1627, 461378. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhou, Z.; Wang, P.; Xi, X.; Hu, S.; Xu, R.; Du, G.; Li, J.; Chen, J.; et al. Synthesis of bioengineered heparin by recombinant yeast Pichia pastoris. Green Chem. 2022, 24, 3180–3192. [Google Scholar] [CrossRef]
- Paluck, S.J.; Nguyen, T.H.; Maynard, H.D. Heparin-Mimicking Polymers: Synthesis and Biological Applications. Biomacromolecules 2016, 17, 3417–3440. [Google Scholar] [CrossRef]
- Wolff, M.W.; Siewert, C.; Hansen, S.P.; Faber, R.; Reichl, U. Purification of cell culture-derived modified vaccinia ankara virus by pseudo-affinity membrane adsorbers and hydrophobic interaction chromatography. Biotechnol. Bioeng. 2010, 107, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, A.R.; van Teeffelen, S.; Ley, A.; Fischer, L.M.; Taft, F.; Genzel, Y.; Villain, L.; Wolff, M.W.; Reichl, U. Use of sulfated cellulose membrane adsorbers for chromatographic purification of cell cultured-derived influenza A and B viruses. Sep. Purif. Technol. 2019, 226, 350–358. [Google Scholar] [CrossRef]
- Wang, Q.; Lock, M.; Prongay, A.; Alvira, M.R.; Petkov, B.; Wilson, J.M. 90. Identification of an Adeno-Associated Virus Binding Epitope for AVB Sepharose Affinity Resin. Mol. Ther. 2015, 23, S38–S39. [Google Scholar] [CrossRef][Green Version]
- Smith, R.H.; Levy, J.R.; Kotin, R.M. A simplified baculovirus-AAV expression vector system coupled with one-step affinity purification yields high-titer rAAV stocks from insect cells. Mol. Ther. 2009, 17, 1888–1896. [Google Scholar] [CrossRef]
- Opitz, L.; Zimmermann, A.; Lehmann, S.; Genzel, Y.; Lubben, H.; Reichl, U.; Wolff, M.W. Capture of cell culture-derived influenza virus by lectins: Strain independent, but host cell dependent. J. Virol. Methods 2008, 154, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Liu, Y.; Wang, L.; Zhang, B.; Yin, D.; Zhang, Q. Monolithic macroporous hydrogels prepared from oil-in-water high internal phase emulsions for high-efficiency purification of Enterovirus 71. Chem. Eng. J. 2020, 401, 126051. [Google Scholar] [CrossRef]
- Tscheuschner, G.; Ponader, M.; Raab, C.; Weider, P.S.; Hartfiel, R.; Kaufmann, J.O.; Volzke, J.L.; Bosc-Bierne, G.; Prinz, C.; Schwaar, T.; et al. Efficient Purification of Cowpea Chlorotic Mottle Virus by a Novel Peptide Aptamer. Viruses 2023, 15, 697. [Google Scholar] [CrossRef]
- Gagnon, P.; Leskovec, M.; Prebil, S.D.; Zigon, R.; Stokelj, M.; Raspor, A.; Peljhan, S.; Strancar, A. Removal of empty capsids from adeno-associated virus preparations by multimodal metal affinity chromatography. J. Chromatogr. A 2021, 1649, 462210. [Google Scholar] [CrossRef] [PubMed]
- Kadoi, K.; Iwamoto, E.; Nakama, T. Fabrication and characterization of a cellulose monolith-like particle for virus purification. Biochem. Eng. J. 2023, 192, 108849. [Google Scholar] [CrossRef]
- Effio, C.L.; Hahn, T.; Seiler, J.; Oelmeier, S.A.; Asen, I.; Silberer, C.; Villain, L.; Hubbuch, J. Modeling and simulation of anion-exchange membrane chromatography for purification of Sf9 insect cell-derived virus-like particles. J. Chromatogr. A 2016, 1429, 142–154. [Google Scholar] [CrossRef]
- Boix-Besora, A.; Lorenzo, E.; Lavado-Garcia, J.; Godia, F.; Cervera, L. Optimization, Production, Purification and Characterization of HIV-1 GAG-Based Virus-like Particles Functionalized with SARS-CoV-2. Vaccines 2022, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Weigel, T.; Solomaier, T.; Wehmeyer, S.; Peuker, A.; Wolff, M.W.; Reichl, U. A membrane-based purification process for cell culture-derived influenza A virus. J. Biotechnol. 2016, 220, 12–20. [Google Scholar] [CrossRef]
- Lothert, K.; Pagallies, F.; Feger, T.; Amann, R.; Wolff, M.W. Selection of chromatographic methods for the purification of cell culture-derived Orf virus for its application as a vaccine or viral vector. J. Biotechnol. 2020, 323, 62–72. [Google Scholar] [CrossRef]
- Wu, C.; Soh, K.Y.; Wang, S.; Okada, T.; Nonaka-Sarukawa, M.; Uchibori, R.; Kinoshita, K.; Hayashita-Kinoh, H.; Nitahara-Kasahara, Y.; Takeda, S.; et al. Ion-exchange membrane chromatography method for rapid and efficient purification of recombinant baculovirus and baculovirus gp64 protein. Hum. Gene Ther. 2007, 18, 665–672. [Google Scholar] [CrossRef]
- Lee, D.-S.; Kim, B.-M.; Seol, D.-W. Improved purification of recombinant adenoviral vector by metal affinity membrane chromatography. Biochem. Biophys. Res. Commun. 2009, 378, 640–644. [Google Scholar] [CrossRef]
- Opitz, L.; Hohlweg, J.; Reichl, U.; Wolff, M.W. Purification of cell culture-derived influenza virus A/Puerto Rico/8/34 by membrane-based immobilized metal affinity chromatography. J. Virol. Methods 2009, 161, 312–316. [Google Scholar] [CrossRef]
- Opitz, L.; Lehmann, S.; Zimmermann, A.; Reichl, U.; Wolff, M.W. Impact of adsorbents selection on capture efficiency of cell culture derived human influenza viruses. J. Biotechnol. 2007, 131, 309–317. [Google Scholar] [CrossRef]
- Opitz, L.; Lehmann, S.; Reichl, U.; Wolff, M.W. Sulfated membrane adsorbers for economic pseudo-affinity capture of influenza virus particles. Biotechnol. Bioeng. 2009, 103, 1144–1154. [Google Scholar] [CrossRef]
- Hoffmann, M.; Snyder, N.L.; Hartmann, L. Polymers Inspired by Heparin and Heparan Sulfate for Viral Targeting. Macromolecules 2022, 55, 7957–7973. [Google Scholar] [CrossRef]
- Fortuna, A.R.; Taft, F.; Villain, L.; Wolff, M.W.; Reichl, U. Optimization of cell culture-derived influenza A virus particles purification using sulfated cellulose membrane adsorbers. Eng. Life Sci. 2018, 18, 29–39. [Google Scholar] [CrossRef] [PubMed]
- McCann, N.; O’Connor, D.; Lambe, T.; Pollard, A.J. Viral vector vaccines. Curr. Opin. Immunol. 2022, 77, 102210. [Google Scholar] [CrossRef]
- Marichal-Gallardo, P.; Pieler, M.M.; Wolff, M.W.; Reichl, U. Steric exclusion chromatography for purification of cell culture-derived influenza A virus using regenerated cellulose membranes and polyethylene glycol. J. Chromatogr. A 2017, 1483, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, A.R.; Taft, F.; Villain, L.; Wolff, M.W.; Reichl, U. Continuous purification of influenza A virus particles using pseudo-affinity membrane chromatography. J. Biotechnol. 2021, 342, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zobel-Roos, S.; Stein, D.; Strube, J. Evaluation of Continuous Membrane Chromatography Concepts with an Enhanced Process Simulation Approach. Antibodies 2018, 7, 13. [Google Scholar] [CrossRef]
- El Andari, J.; Grimm, D. Production, Processing, and Characterization of Synthetic AAV Gene Therapy Vectors. Biotechnol. J. 2021, 16, e2000025. [Google Scholar] [CrossRef]
- Bernaud, J.; Rossi, A.; Fis, A.; Gardette, L.; Aillot, L.; Buning, H.; Castelnovo, M.; Salvetti, A.; Faivre-Moskalenko, C. Characterization of AAV vector particle stability at the single-capsid level. J. Biol. Phys. 2018, 44, 181–194. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lothert, K.; Wolff, M.W. Affinity and Pseudo-Affinity Membrane Chromatography for Viral Vector and Vaccine Purifications: A Review. Membranes 2023, 13, 770. https://doi.org/10.3390/membranes13090770
Lothert K, Wolff MW. Affinity and Pseudo-Affinity Membrane Chromatography for Viral Vector and Vaccine Purifications: A Review. Membranes. 2023; 13(9):770. https://doi.org/10.3390/membranes13090770
Chicago/Turabian StyleLothert, Keven, and Michael W. Wolff. 2023. "Affinity and Pseudo-Affinity Membrane Chromatography for Viral Vector and Vaccine Purifications: A Review" Membranes 13, no. 9: 770. https://doi.org/10.3390/membranes13090770
APA StyleLothert, K., & Wolff, M. W. (2023). Affinity and Pseudo-Affinity Membrane Chromatography for Viral Vector and Vaccine Purifications: A Review. Membranes, 13(9), 770. https://doi.org/10.3390/membranes13090770







