Changes in Ion Transport across Biological Membranes Exposed to Particulate Matter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Particulate Matter
2.2. Chemicals
2.3. Cell Culture
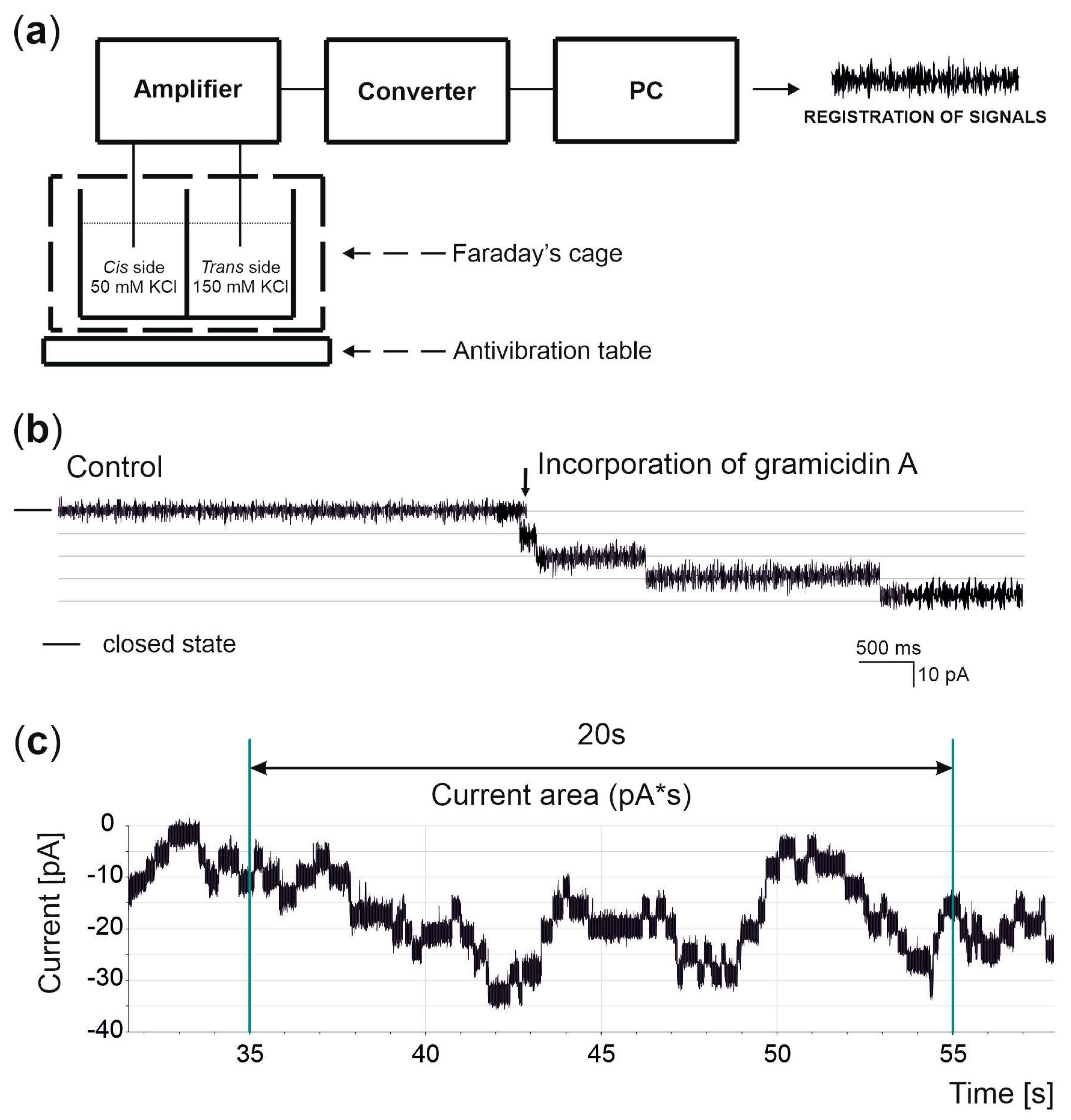
2.4. Black Lipid Membrane Technique
2.5. Data Analysis
3. Results
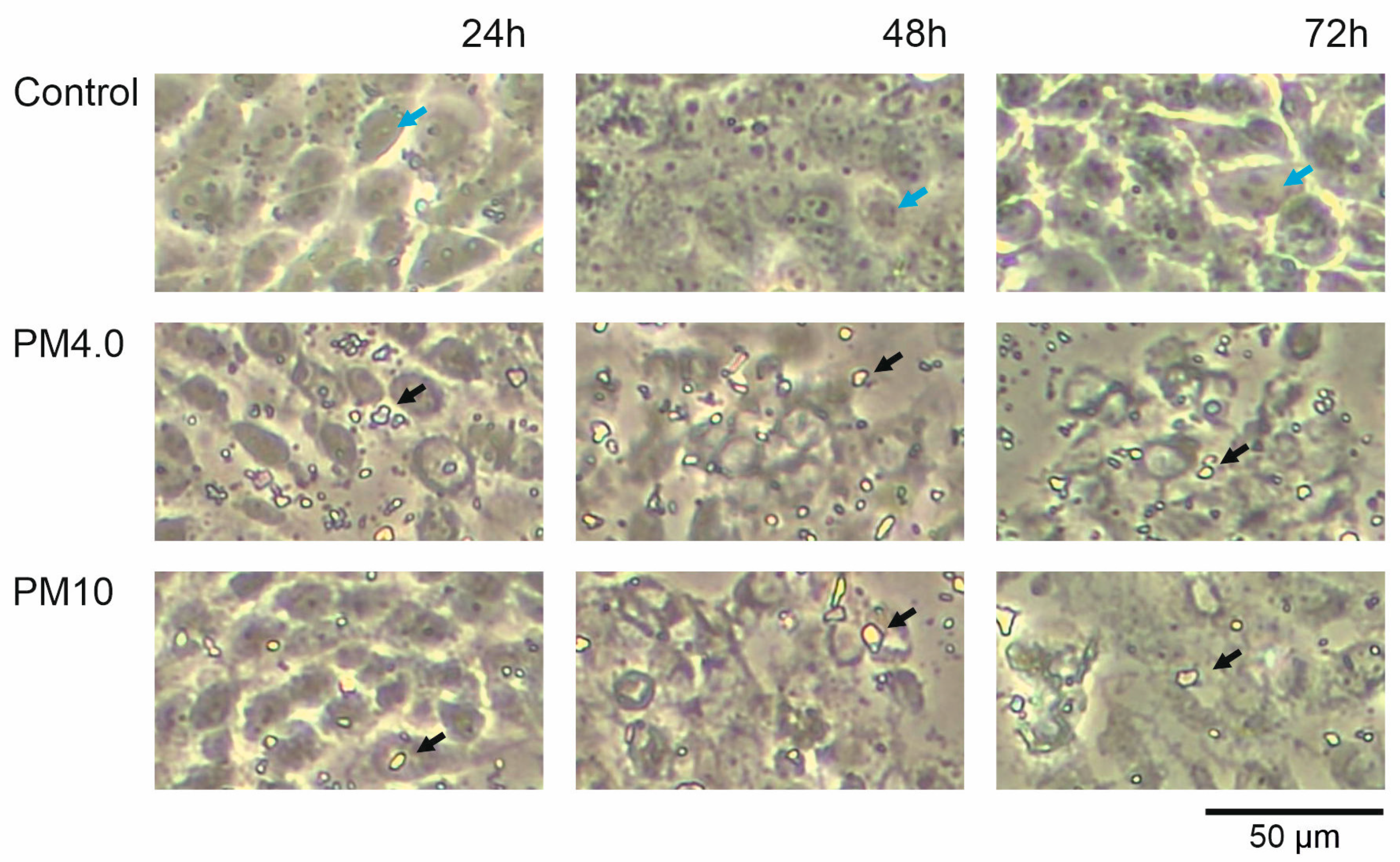
3.1. Cell Morphology
3.2. Basis of the Measurements and Analysis
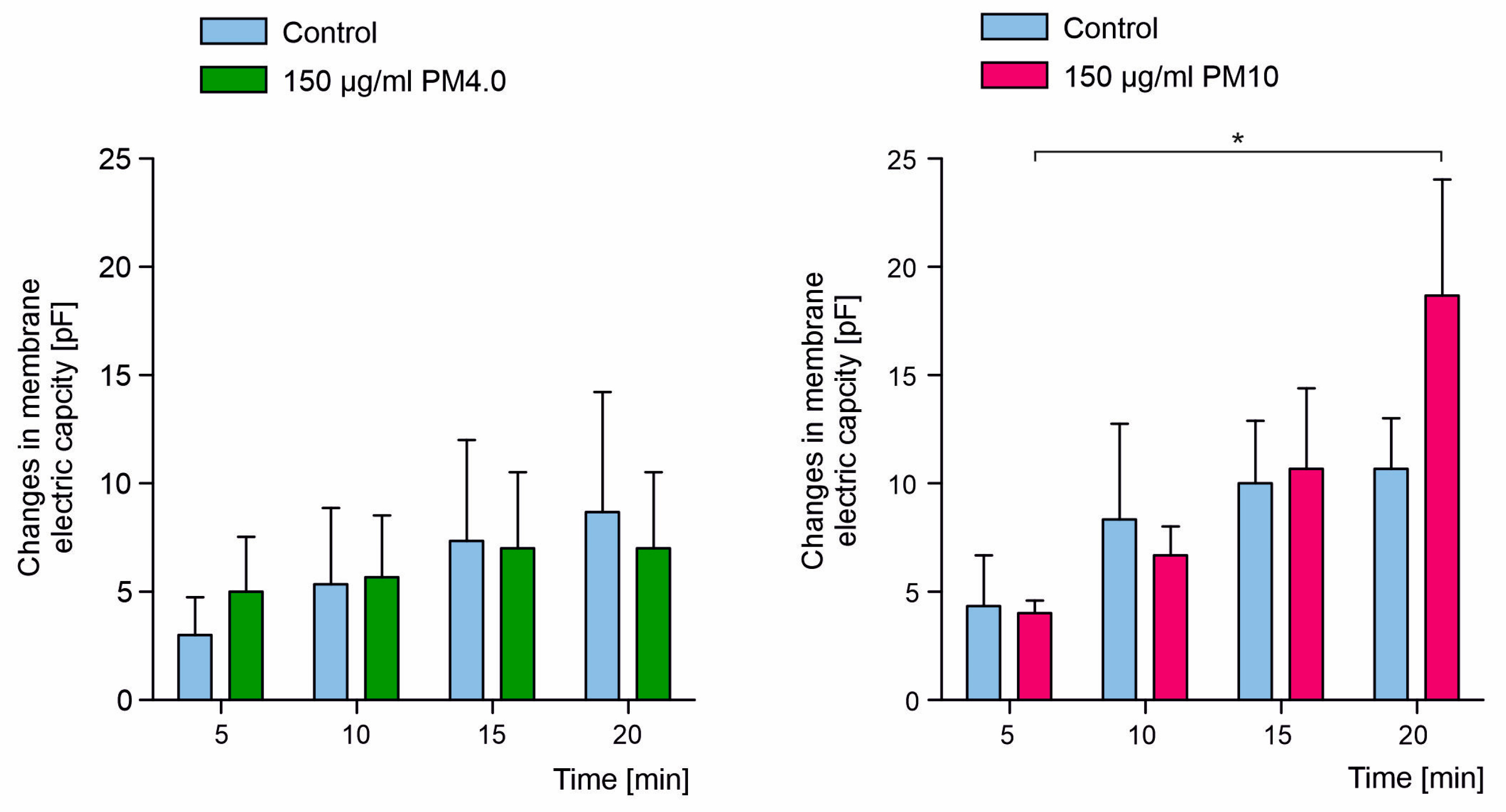
3.3. Interaction of Particulate Matter with Lipid Membranes
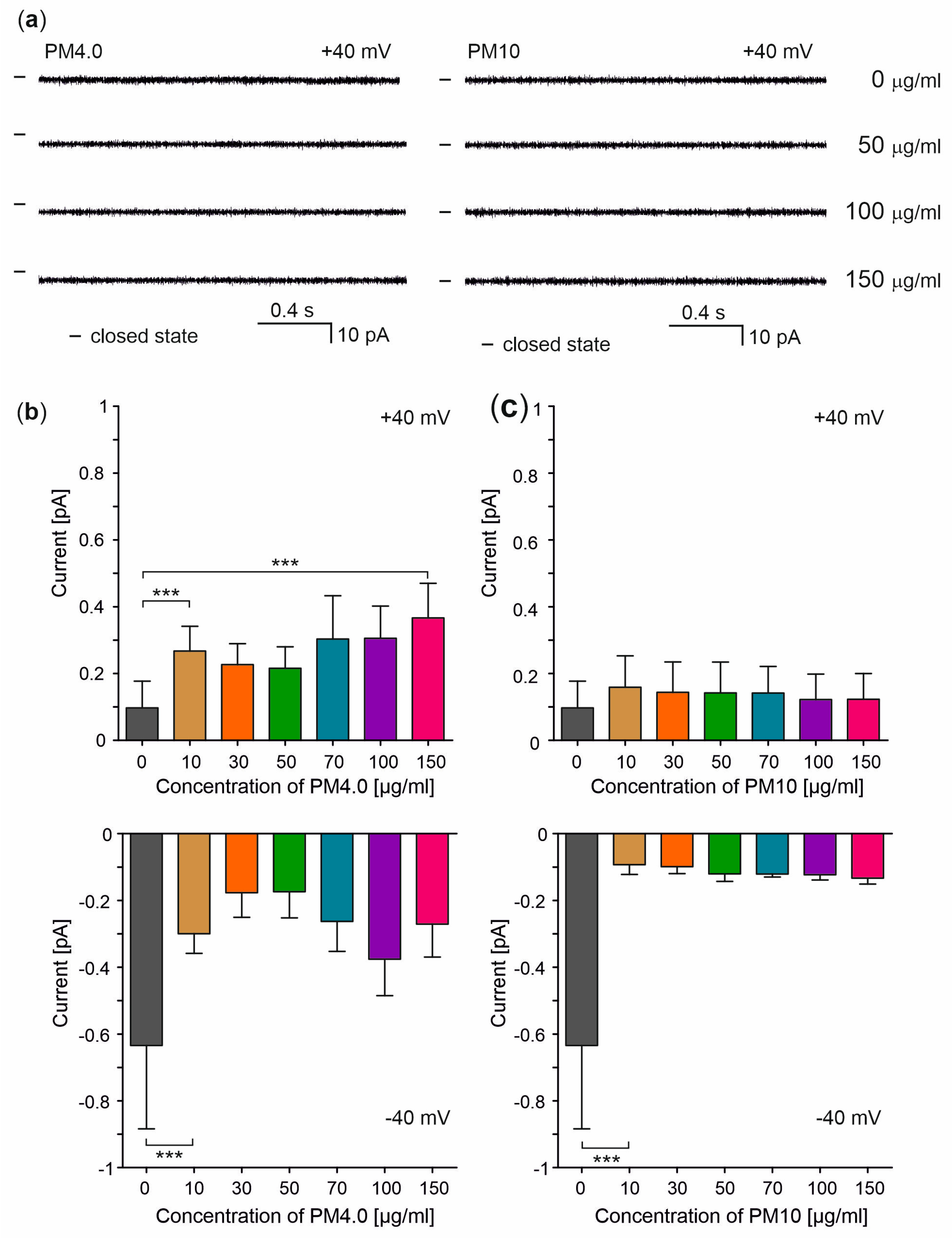
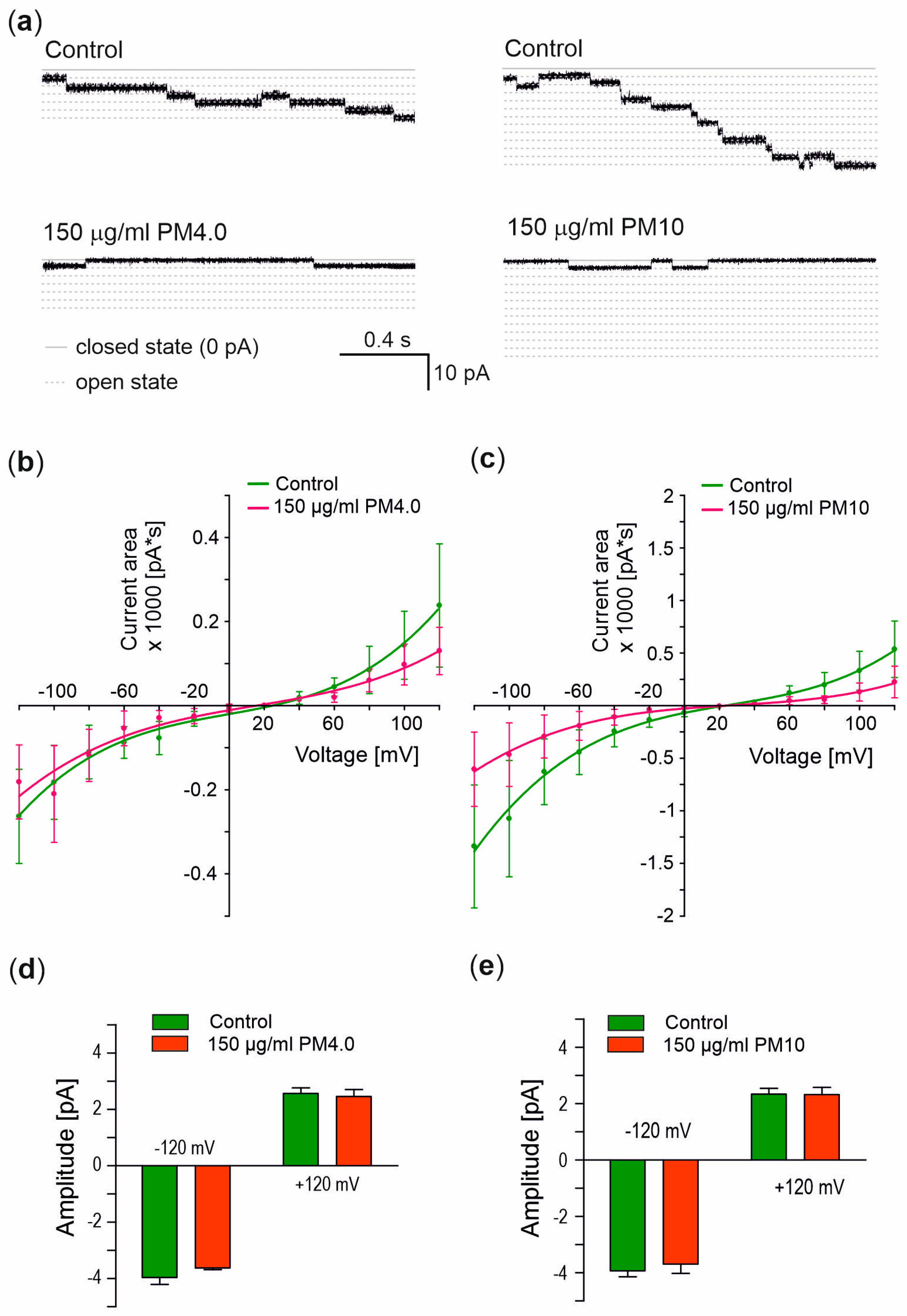
3.4. Interaction of Particulate Matter with Gramicidin A Channel
4. Discussion
- (1)
- Does PM permeabilize biological membranes?
- (2)
- How does the presence of PM affect the electrical capacity of biological membranes?
- (3)
- Does PM regulate the flow of ions through ion channels?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassee, F.R.; Héroux, M.E.; Gerlofs-Nijland, M.E.; Kelly, F.J. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 2013, 25, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Paciência, I.; Cavaleiro Rufo, J.; Moreira, A. Environmental inequality: Air pollution and asthma in children. Pediatr. Allergy Immunol. 2022, 33, e13818. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef] [PubMed]
- Mühlfeld, C.; Rothen-Rutishauser, B.; Blank, F.; Vanhecke, D.; Ochs, M.; Gehr, P. Interactions of nanoparticles with pulmonary structures and cellular responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L817–L829. [Google Scholar] [CrossRef] [PubMed]
- Oeder, S.; Dietrich, S.; Weichenmeier, I.; Schober, W.; Pusch, G.; Jörres, R.A.; Schierl, R.; Nowak, D.; Fromme, H.; Behrendt, H.; et al. Toxicity and elemental composition of particulate matter from outdoor and indoor air of elementary schools in Munich, Germany. Indoor Air 2012, 22, 148–158. [Google Scholar] [CrossRef]
- Pryor, J.T.; Cowley, L.O.; Simonds, S.E. The Physiological Effects of Air Pollution: Particulate Matter, Physiology and Disease. Front. Public. Health 2022, 10, 882569. [Google Scholar] [CrossRef]
- Dabrowska, A.; Zajac, M.; Bednarczyk, P.; Lukasiak, A. Effect of Quercetin on mitoBK(Ca) Channel and Mitochondrial Function in Human Bronchial Epithelial Cells Exposed to Particulate Matter. Int. J. Mol. Sci. 2022, 24, 638. [Google Scholar] [CrossRef]
- Milici, A.; Talavera, K. TRP Channels as Cellular Targets of Particulate Matter. Int. J. Mol. Sci. 2021, 22, 2783. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Li, G.; Zhang, Y.; Li, J.; Chen, H. Fluorescent reconstitution on deposition of PM(2.5) in lung and extrapulmonary organs. Proc. Natl. Acad. Sci. USA 2019, 116, 2488–2493. [Google Scholar] [CrossRef]
- Nguyen, J.P.; Huff, R.D.; Cao, Q.T.; Tiessen, N.; Carlsten, C.; Hirota, J.A. Effects of environmental air pollutants on CFTR expression and function in human airway epithelial cells. Toxicol. In Vitro 2021, 77, 105253. [Google Scholar] [CrossRef]
- Nehrke, K. Membrane Ion Transport in Non-Excitable Tissues. WormBook. The C. elegans Research Community. 2014. Available online: https://doi.org/10.1895/wormbook.1.174.1 (accessed on 14 July 2023). [CrossRef]
- Grewer, C.; Gameiro, A.; Mager, T.; Fendler, K. Electrophysiological characterization of membrane transport proteins. Annu. Rev. Biophys. 2013, 42, 95–120. [Google Scholar] [CrossRef]
- Prasad, K.U.; Alonso-Romanowski, S.; Venkatachalam, C.M.; Trapane, T.L.; Urry, D.W. Synthesis, characterization, and black lipid membrane studies of [7-L-alanine] gramicidin A. Biochemistry 1986, 25, 456–463. [Google Scholar] [CrossRef]
- Stefanowska, A.; Koprowski, P.; Bednarczyk, P.; Szewczyk, A.; Krysinski, P. Electrochemical studies of the mitochondrial ROMK2 potassium channel activity reconstituted into the free-standing and tethered bilayer lipid membranes. Bioelectrochemistry 2023, 151, 108372. [Google Scholar] [CrossRef]
- Kulawiak, B.; Bednarczyk, P. Reconstitution of brain mitochondria inner membrane into planar lipid bilayer. Acta Neurobiol. Exp. 2005, 65, 271–276. [Google Scholar]
- Walewska, A.; Krajewska, M.; Stefanowska, A.; Buta, A.; Bilewicz, R.; Krysiński, P.; Bednarczyk, P.; Koprowski, P.; Szewczyk, A. Methods of Measuring Mitochondrial Potassium Channels: A Critical Assessment. Int. J. Mol. Sci. 2022, 23, 1210. [Google Scholar] [CrossRef] [PubMed]
- Montal, M.; Mueller, P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Koeppe, R.E., 2nd; Roux, B. Gramicidin channels. IEEE Trans. Nanobiosci. 2005, 4, 10–20. [Google Scholar] [CrossRef]
- Sun, Z.; Barboiu, M. Artificial Gramicidins. Front. Chem. 2019, 7, 611. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.W.; Itoh, H.; Dan, S.; Inoue, M. Gramicidin A accumulates in mitochondria, reduces ATP levels, induces mitophagy, and inhibits cancer cell growth. Chem. Sci. 2022, 13, 7482–7491. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Kelkar, D.A. Ion channels and D-amino acids. J. Biosci. 2005, 30, 147–149. [Google Scholar] [CrossRef]
- Hille, B. Ionic Channels of Excitable Membranes; Sinauer: Sunderland, MD, USA, 2001. [Google Scholar]
- Schantz, M.M.; Cleveland, D.; Heckert, N.A.; Kucklick, J.R.; Leigh, S.D.; Long, S.E.; Lynch, J.M.; Murphy, K.E.; Olfaz, R.; Pintar, A.L.; et al. Development of two fine particulate matter standard reference materials (<4 mum and <10 mum) for the determination of organic and inorganic constituents. Anal. Bioanal. Chem. 2016, 408, 4257–4266. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, P.; Dołowy, K.; Szewczyk, A. Matrix Mg2+ regulates mitochondrial ATP-dependent potassium channel from heart. FEBS Lett. 2005, 579, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public. Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Engels, S.M.; Kamat, P.; Pafilis, G.S.; Li, Y.; Agrawal, A.; Haller, D.J.; Phillip, J.M.; Contreras, L.M. Particulate matter composition drives differential molecular and morphological responses in lung epithelial cells. bioRxiv 2023. [Google Scholar] [CrossRef]
- Zakharian, E. Ion channel reconstitution in lipid bilayers. Methods Enzymol. 2021, 652, 273–291. [Google Scholar] [CrossRef]
- Kozon, D.; Bednarczyk, P.; Szewczyk, A.; Jańczewski, D. Regulation of Lipid Bilayer Ion Permeability by Antibacterial Polymethyloxazoline-Polyethyleneimine Copolymers. Chembiochem 2021, 22, 1020–1029. [Google Scholar] [CrossRef]
- Pérez-Peinado, C.; Dias, S.A.; Domingues, M.M.; Benfield, A.H.; Freire, J.M.; Rádis-Baptista, G.; Gaspar, D.; Castanho, M.; Craik, D.J.; Henriques, S.T.; et al. Mechanisms of bacterial membrane permeabilization by crotalicidin (Ctn) and its fragment Ctn(15-34), antimicrobial peptides from rattlesnake venom. J. Biol. Chem. 2018, 293, 1536–1549. [Google Scholar] [CrossRef]
- Epand, R.M. Introduction to membrane lipids. Methods Mol. Biol. 2015, 1232, 1–6. [Google Scholar] [CrossRef]
- Kondrashov, O.V.; Rokitskaya, T.I.; Batishchev, O.V.; Kotova, E.A.; Antonenko, Y.N.; Akimov, S.A. Peptide-induced membrane elastic deformations decelerate gramicidin dimer-monomer equilibration. Biophys. J. 2021, 120, 5309–5321. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Xu, M.; Zhang, H.; Chen, Y.; Chung, K.F.; Adcock, I.M.; Li, F. Roles of TRPA1 and TRPV1 in cigarette smoke -induced airway epithelial cell injury model. Free Radic. Biol. Med. 2019, 134, 229–238. [Google Scholar] [CrossRef]
- Veronesi, B.; de Haar, C.; Lee, L.; Oortgiesen, M. The surface charge of visible particulate matter predicts biological activation in human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2002, 178, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Gurnev, P.A.; Bezrukov, S.M. Inversion of membrane surface charge by trivalent cations probed with a cation-selective channel. Langmuir 2012, 28, 15824–15830. [Google Scholar] [CrossRef]
- Bean, R.C.; Shepherd, W.C.; Chan, H.; Eichner, J. Discrete conductance fluctuations in lipid bilayer protein membranes. J. Gen. Physiol. 1969, 53, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lipids 2019, 221, 46–52. [Google Scholar] [CrossRef] [PubMed]
| Condition | Conductance (pS) 1 | Reversal Potential (mV) 2 |
|---|---|---|
| Control | 31.7 ± 0.5 | 26.1 ± 0.7 |
| PM4.0 | 29.1 ± 0.2 | 23.9 ± 0.9 |
| PM10 | 30.1 ± 0.6 | 24.5 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoser, J.; Dabrowska, A.; Zajac, M.; Bednarczyk, P. Changes in Ion Transport across Biological Membranes Exposed to Particulate Matter. Membranes 2023, 13, 763. https://doi.org/10.3390/membranes13090763
Hoser J, Dabrowska A, Zajac M, Bednarczyk P. Changes in Ion Transport across Biological Membranes Exposed to Particulate Matter. Membranes. 2023; 13(9):763. https://doi.org/10.3390/membranes13090763
Chicago/Turabian StyleHoser, Jakub, Adrianna Dabrowska, Miroslaw Zajac, and Piotr Bednarczyk. 2023. "Changes in Ion Transport across Biological Membranes Exposed to Particulate Matter" Membranes 13, no. 9: 763. https://doi.org/10.3390/membranes13090763
APA StyleHoser, J., Dabrowska, A., Zajac, M., & Bednarczyk, P. (2023). Changes in Ion Transport across Biological Membranes Exposed to Particulate Matter. Membranes, 13(9), 763. https://doi.org/10.3390/membranes13090763






