Effect of TiO2 on Thermal, Mechanical, and Gas Separation Performances of Polyetherimide–Polyvinyl Acetate Blend Membranes
Abstract
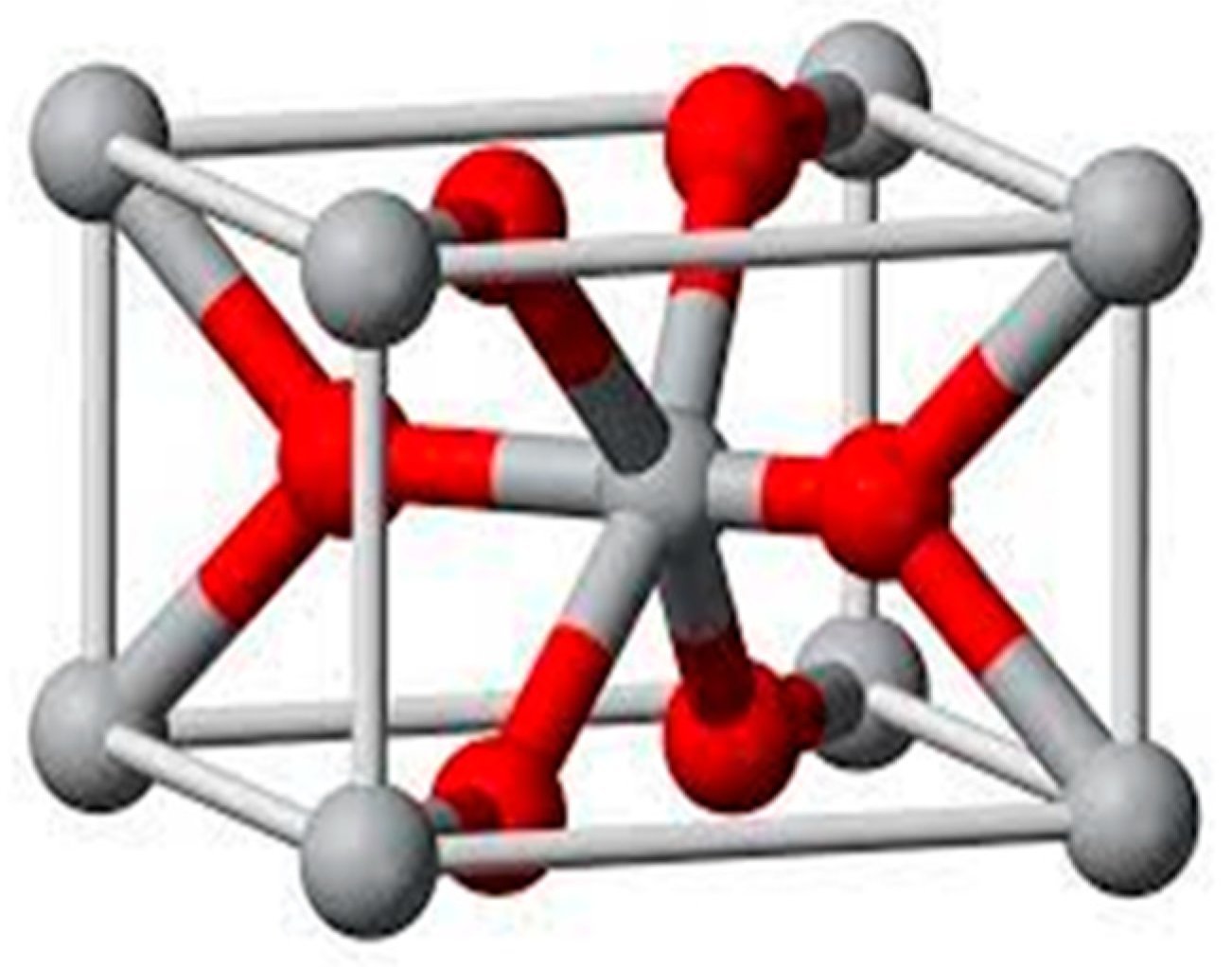
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.3. Membrane Fabrication
2.4. Characterization and Gas Permeation Tests of the Developed Membranes
2.4.1. Thermal Analysis of the Membranes
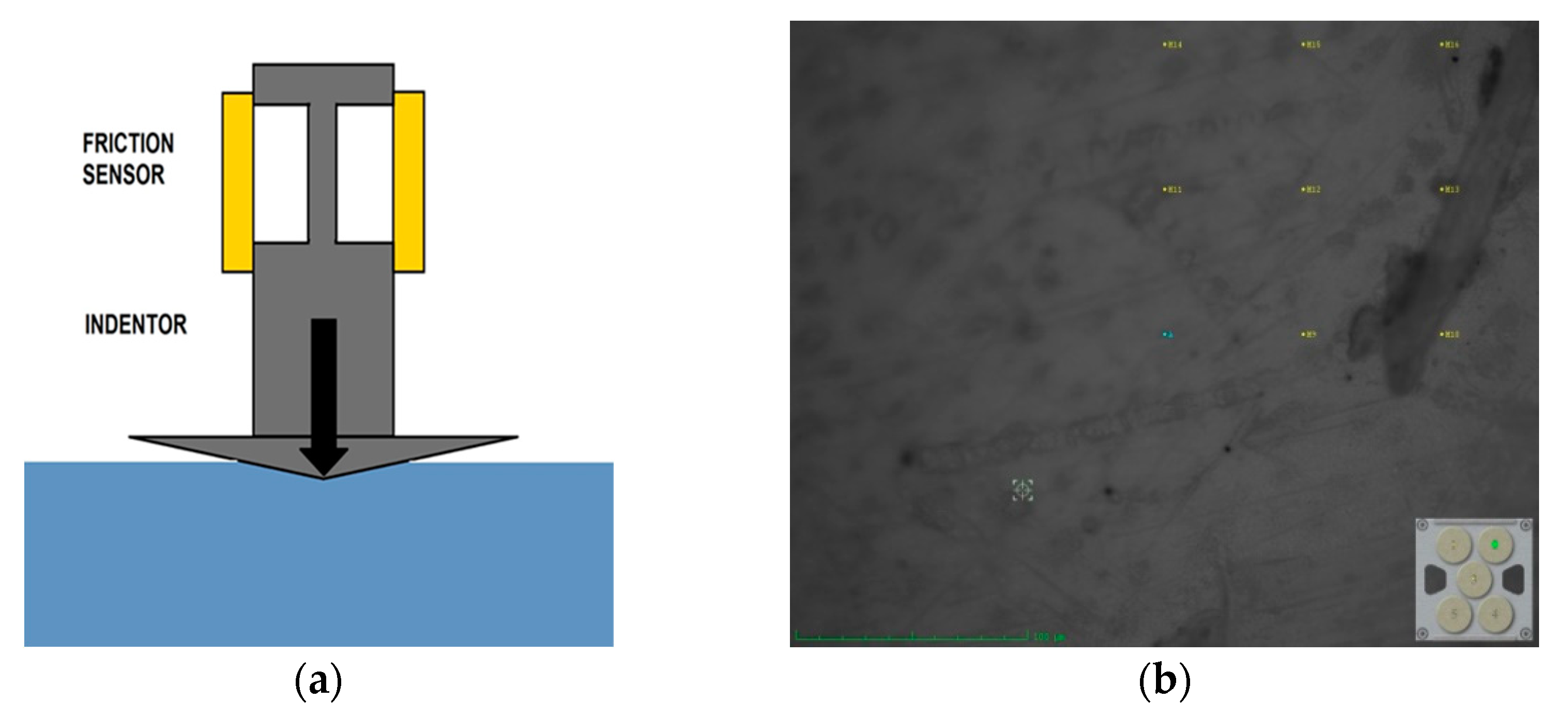
2.4.2. Nano-Indentation Technique
2.4.3. Gas Permeation Study of the Developed Membranes
3. Results and Discussion
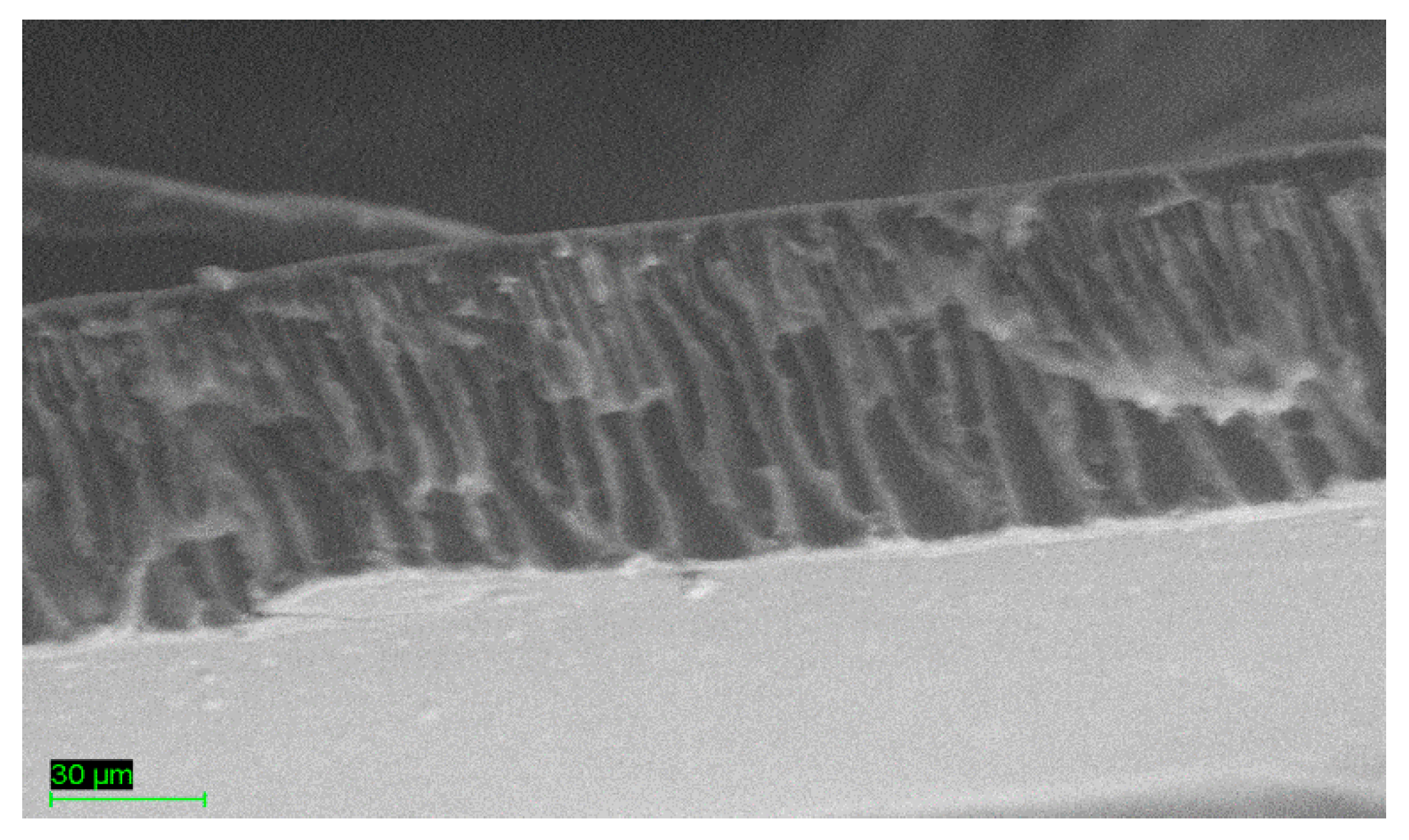
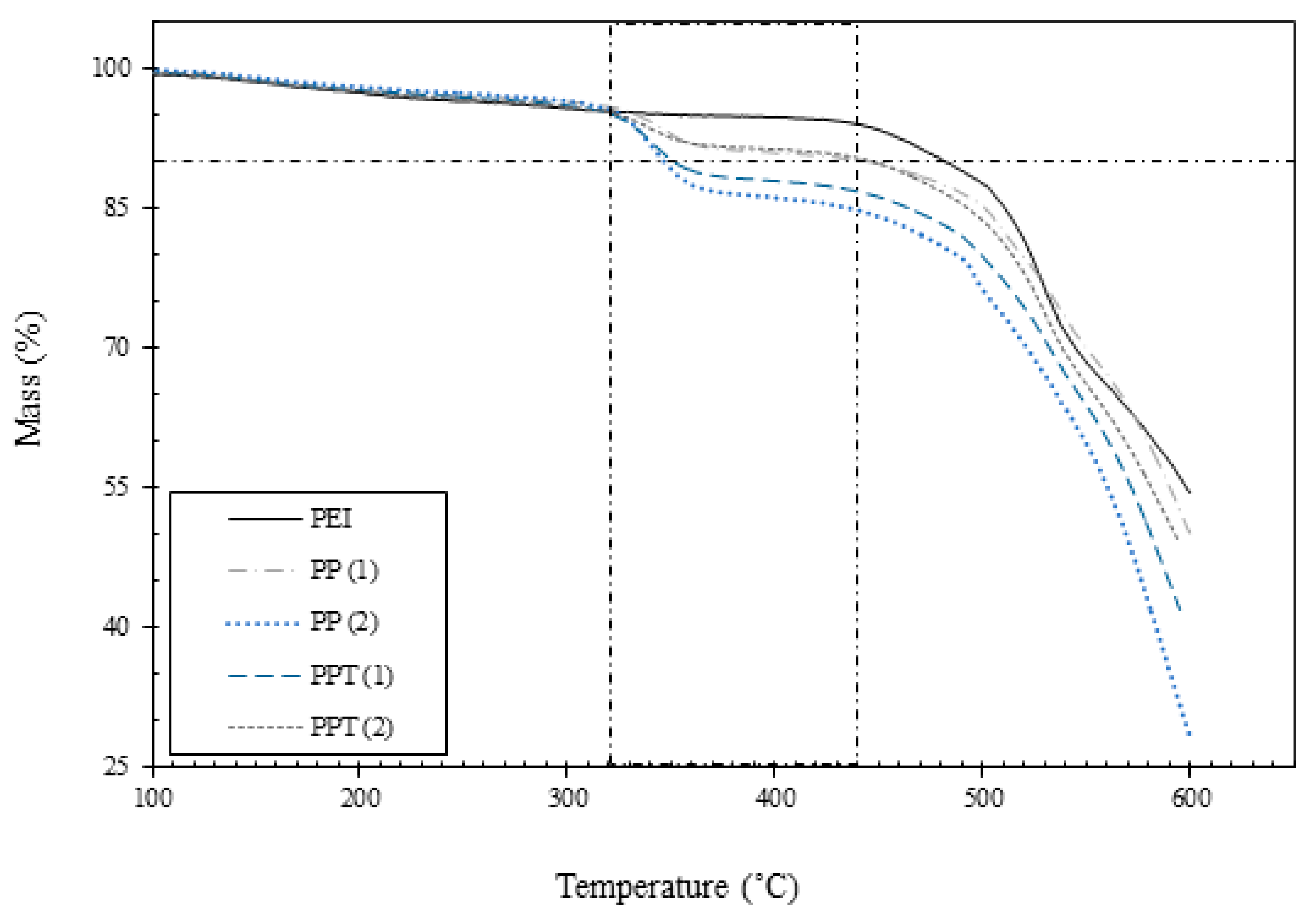
3.1. Thermal Analysis
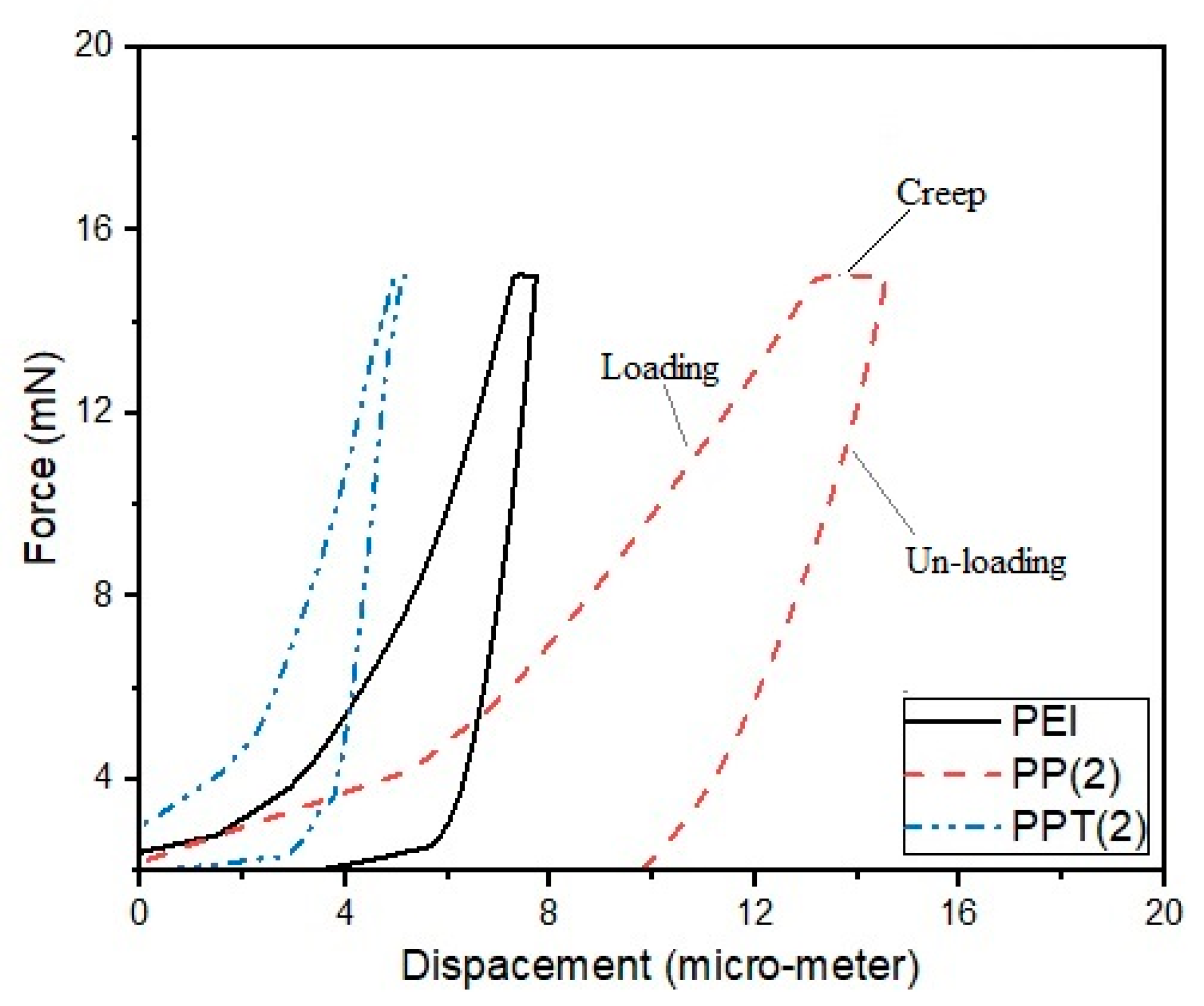
3.2. Surface Mechanical Analysis
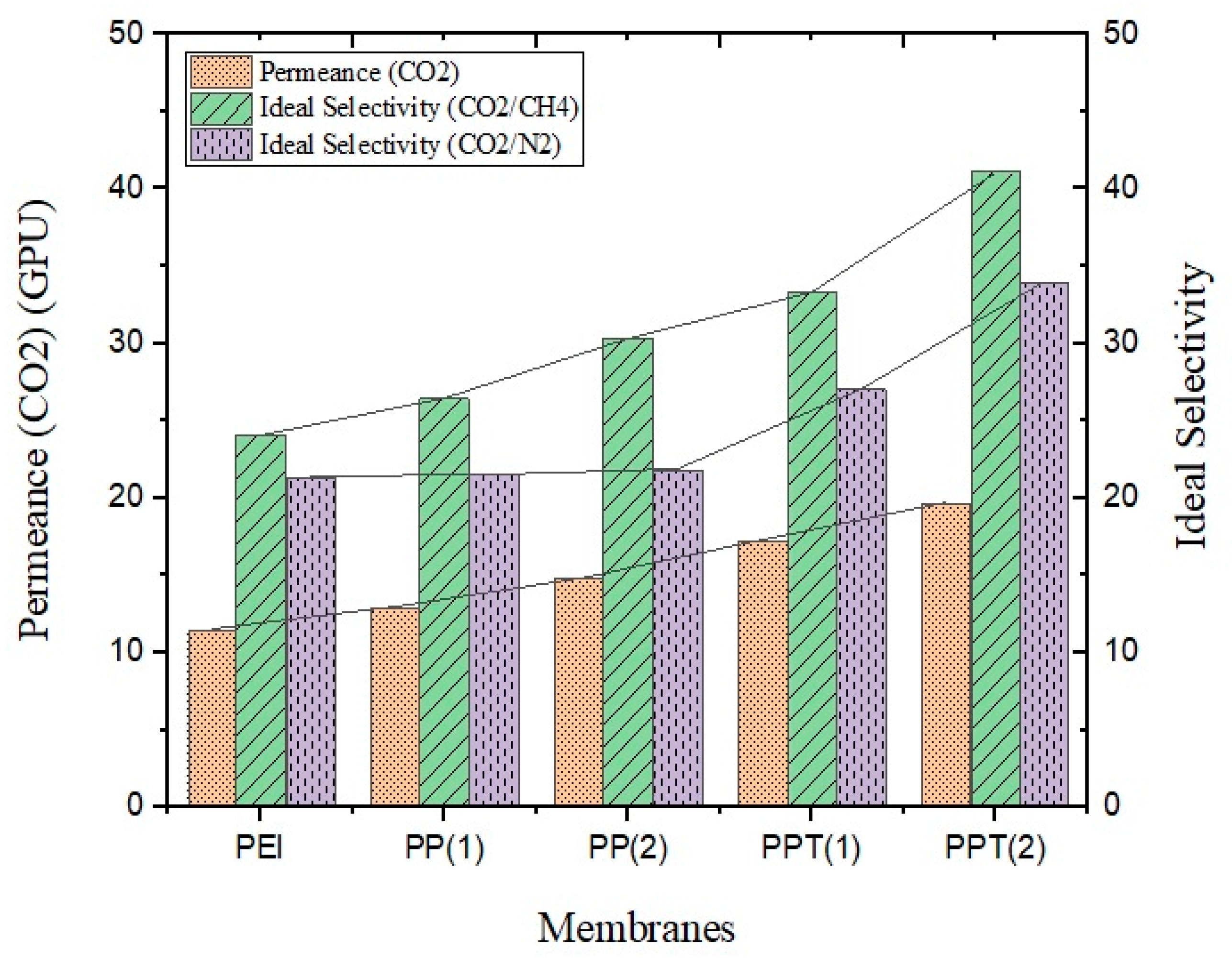
3.3. Gas Permeation Study
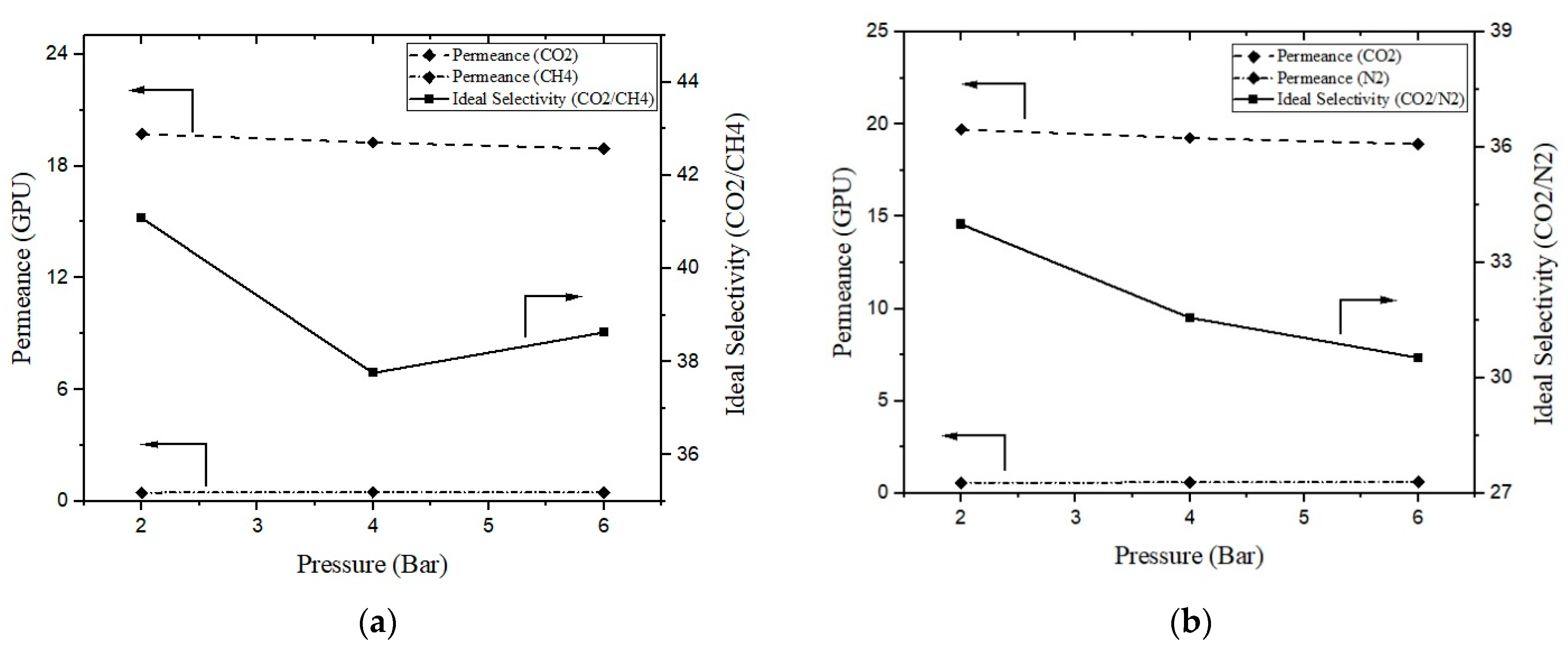
3.4. Effect of Feed Pressure
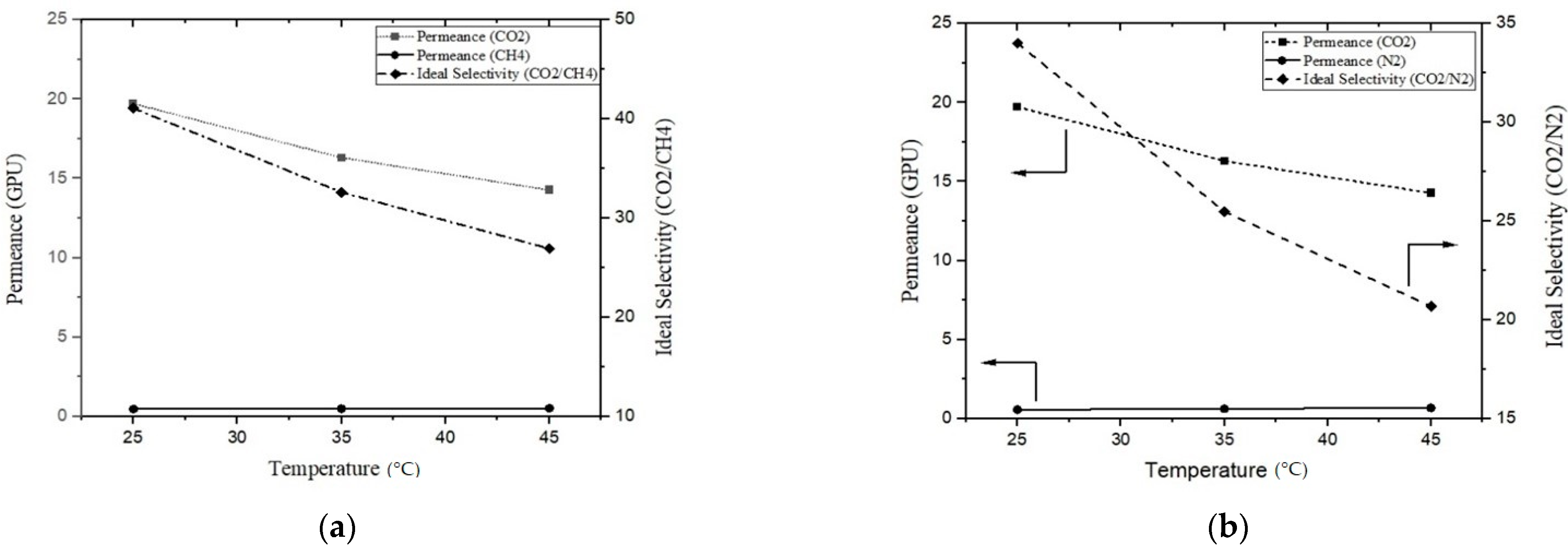
3.5. Effect of Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamil, A.; Ching, O.P.; Shariff, A.M. Polymer-nanoclay mixed matrix membranes for CO2/CH4 separation: A review. Appl. Mech. Mater. 2014, 625, 690–695. [Google Scholar] [CrossRef]
- Khurram, A.R.; Rafiq, S.; Tariq, A.; Jamil, A.; Iqbal, T.; Mahmood, H.; Mehdi, M.S.; Abdulrahman, A.; Ali, A.; Akhtar, M.S.; et al. Environmental remediation through various composite membranes moieties: Performances and thermomechanical properties. Chemosphere 2022, 309, 136613. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Macchione, M.; Drioli, E. High flux asymmetric gas separation membranes of modified poly (ether ether ketone) prepared by the dry phase inversion technique. J. Membr. Sci. 2005, 255, 167–180. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Figoli, A.; Jansen, J.C.; Drioli, E. Preparation of asymmetric PEEKWC flat membranes with different microstructures by wet phase inversion. J. Appl. Polym. Sci. 2004, 92, 576–591. [Google Scholar] [CrossRef]
- Wong, K.K.; Jawad, Z.A. A review and future prospect of polymer blend mixed matrix membrane for CO2 separation. J. Polym. Res. 2019, 26, 289. [Google Scholar] [CrossRef]
- Panapitiya, N.P.; Wijenayake, S.N.; Huang, Y.; Bushdiecker, D.; Nguyen, D.; Ratanawanate, C.; Kalaw, G.J.; Gilpin, C.J.; Musselman, I.H.; Balkus, K.J.; et al. Stabilization of immiscible polymer blends using structure directing metal organic frameworks (MOFs). Polymer 2014, 55, 2028–2034. [Google Scholar] [CrossRef]
- Kapantaidakis, G.; Kaldis, S.; Dabou, X.; Sakellaropoulos, G. Gas permeation through PSF-PI miscible blend membranes. J. Membr. Sci. 1996, 110, 239–247. [Google Scholar] [CrossRef]
- Graziano, A.; Jaffer, S.; Sain, M. Review on modification strategies of polyethylene/polypropylene immiscible thermoplastic polymer blends for enhancing their mechanical behavior. J. Elastomers Plast. 2019, 51, 291–336. [Google Scholar] [CrossRef]
- Chen, J.; Shi, Y.-Y.; Yang, J.-H.; Zhang, N.; Huang, T.; Wang, Y. Improving interfacial adhesion between immiscible polymers by carbon nanotubes. Polymer 2013, 54, 464–471. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Fu, Q. Compatibilization of immiscible poly (propylene)/polystyrene blends using clay. Macromol. Rapid Commun. 2003, 24, 231–235. [Google Scholar] [CrossRef]
- Decol, M.; Pachekoski, W.M.; Becker, D. Compatibilization and ultraviolet blocking of PLA/PCL blends via interfacial localization of titanium dioxide nanoparticles. J. Appl. Polym. Sci. 2018, 135, 44849. [Google Scholar] [CrossRef]
- Farrukh, S.; Javed, S.; Hussain, A.; Mujahid, M. Blending of TiO2 nanoparticles with cellulose acetate polymer: To study the effect on morphology and gas permeation of blended membranes. Asia-Pac. J. Chem. Eng. 2014, 9, 543–551. [Google Scholar]
- Li, J.-F.; Xu, Z.-L.; Yang, H.; Yu, L.-Y.; Liu, M. Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Appl. Surf. Sci. 2009, 255, 4725–4732. [Google Scholar] [CrossRef]
- Madaeni, S.; Badieh, M.M.S.; Vatanpour, V.; Ghaemi, N. Effect of titanium dioxide nanoparticles on polydimethylsiloxane/polyethersulfone composite membranes for gas separation. Polym. Eng. Sci. 2012, 52, 2664–2674. [Google Scholar] [CrossRef]
- Ahmad, J.; Deshmukh, K.; Hägg, M.B. Influence of TiO2 on the chemical, mechanical, and gas separation properties of polyvinyl alcohol-titanium dioxide (PVA-TiO2) nanocomposite membranes. Int. J. Polym. Anal. Charact. 2013, 18, 287–296. [Google Scholar] [CrossRef]
- Cai, X.; Li, B.; Pan, Y.; Wu, G. Morphology evolution of immiscible polymer blends as directed by nanoparticle self-agglomeration. Polymer 2012, 53, 259–266. [Google Scholar] [CrossRef]
- Hu, Q.; Marand, E.; Dhingra, S.; Fritsch, D.; Wen, J.; Wilkes, G. Poly (amide-imide)/TiO2 nano-composite gas separation membranes: Fabrication and characterization. J. Membr. Sci. 1997, 135, 65–79. [Google Scholar] [CrossRef]
- Maqsood, K.; Jamil, A.; Ahmed, A.; Sutisna, B.; Nunes, S.; Ulbricht, M. Blend membranes comprising polyetherimide and polyvinyl acetate with improved methane enrichment performance. Chemosphere 2023, 321, 138074. [Google Scholar] [CrossRef]
- Jamil, A.; Ching, O.P.; Shariff, A.M. Mixed matrix hollow fibre membrane comprising polyetherimide and modified montmorillonite with improved filler dispersion and CO2/CH4 separation performance. Appl. Clay Sci. 2017, 143, 115–124. [Google Scholar] [CrossRef]
- Abdullah, M.A.; Mukhtar, H.; Mannan, H.A.; Fong, Y.Y.; Shaharun, M.S. Polyethersulfone/polyvinyl acetate blend membrane incorporated with TiO2 nanoparticles for CO2/CH4 gas separation. Malays. J. Fundam. Appl. Sci. 2017, 13, 774–777. [Google Scholar] [CrossRef]
- Azizi, N.; Mohammadi, T.; Behbahani, R.M. Synthesis of a new nanocomposite membrane (PEBAX-1074/PEG-400/TiO2) in order to separate CO2 from CH4. J. Nat. Gas Sci. Eng. 2017, 37, 39–51. [Google Scholar] [CrossRef]
- Ahmad, J.; Hågg, M.B. Polyvinyl acetate/titanium dioxide nanocomposite membranes for gas separation. J. Membr. Sci. 2013, 445, 200–210. [Google Scholar] [CrossRef]
- Sulaiman, M.; Iqbal, T.; Yasin, S.; Mahmood, H.; Shakeel, A. Fabrication and Nanomechanical Characterization of Thermoplastic Biocomposites Based on Chemically Treated Lignocellulosic Biomass for Surface Engineering Applications. Front. Mater. 2021, 8, 408. [Google Scholar] [CrossRef]
- Jamil, A.; Zulfiqar, M.; Arshad, U.; Mahmood, S.; Iqbal, T.; Rafiq, S.; Iqbal, M.Z. Development and performance evaluation of cellulose acetate-bentonite mixed matrix membranes for CO2 separation. Adv. Polym. Technol. 2020, 2020, 8855577. [Google Scholar] [CrossRef]
- Farnam, M.; Mukhtar, H.; Shariff, A.M. An investigation on polymeric blend mixed matrix membranes of polyethersulfone/polyvinyl acetate/carbon molecular sieve for CO2/CH4 separation. J. Fundam. Appl. Sci. 2017, 9, 612–622. [Google Scholar]
- Muruganandam, N.; Paul, D. Gas sorption and transport in miscible blends of tetramethyl bisphenol-A polycarbonate and polystyrene. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 2315–2329. [Google Scholar] [CrossRef]
- Li, Y.; Chen, D.; He, X. Preparation and Characterization of Polyvinylalcohol/Polysulfone Composite Membranes for Enhanced CO2/N2 Separation. Polymers 2023, 15, 124. [Google Scholar] [CrossRef]
- Matsuyama, H.; Terada, A.; Nakagawara, T.; Kitamura, Y.; Teramoto, M. Facilitated transport of CO2 through polyethylenimine/poly (vinyl alcohol) blend membrane. J. Membr. Sci. 1999, 163, 221–227. [Google Scholar] [CrossRef]
- Khan, M.Y.; Khan, A.; Adewole, J.K.; Naim, M.; Basha, S.I.; Aziz, M.A. Biomass derived carboxylated carbon nanosheets blended polyetherimide membranes for enhanced CO2/CH4 separation. J. Nat. Gas Sci. Eng. 2020, 75, 103156. [Google Scholar] [CrossRef]
| Membrane Code | PEI (wt.%) | PVAc (wt.%) | TiO2 (wt.%) | Tg (°C) |
|---|---|---|---|---|
| PEI | 100 | - | - | 212 |
| PP(1) | 99 | 1 | - | 211 |
| PP(2) | 98 | 2 | 209 | |
| PPT(1) | 98 | 2 | 1 | 210 |
| PPT(2) | 98 | 2 | 2 | 212 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maqsood, K.; Jamil, A.; Ahmed, A.; Sutisna, B.; Nunes, S.; Ulbricht, M. Effect of TiO2 on Thermal, Mechanical, and Gas Separation Performances of Polyetherimide–Polyvinyl Acetate Blend Membranes. Membranes 2023, 13, 734. https://doi.org/10.3390/membranes13080734
Maqsood K, Jamil A, Ahmed A, Sutisna B, Nunes S, Ulbricht M. Effect of TiO2 on Thermal, Mechanical, and Gas Separation Performances of Polyetherimide–Polyvinyl Acetate Blend Membranes. Membranes. 2023; 13(8):734. https://doi.org/10.3390/membranes13080734
Chicago/Turabian StyleMaqsood, Khuram, Asif Jamil, Anas Ahmed, Burhannudin Sutisna, Suzana Nunes, and Mathias Ulbricht. 2023. "Effect of TiO2 on Thermal, Mechanical, and Gas Separation Performances of Polyetherimide–Polyvinyl Acetate Blend Membranes" Membranes 13, no. 8: 734. https://doi.org/10.3390/membranes13080734
APA StyleMaqsood, K., Jamil, A., Ahmed, A., Sutisna, B., Nunes, S., & Ulbricht, M. (2023). Effect of TiO2 on Thermal, Mechanical, and Gas Separation Performances of Polyetherimide–Polyvinyl Acetate Blend Membranes. Membranes, 13(8), 734. https://doi.org/10.3390/membranes13080734





